Abstract
MS (multiple sclerosis) is a chronic autoimmune and neurodegenerative pathology of the CNS (central nervous system) affecting approx. 2.5 million people worldwide. Current and emerging DMDs (disease-modifying drugs) predominantly target the immune system. These therapeutic agents slow progression and reduce severity at early stages of MS, but show little activity on the neurodegenerative component of the disease. As the latter determines permanent disability, there is a critical need to pursue alternative modalities. VIP (vasoactive intestinal peptide) and PACAP (pituitary adenylate cyclase-activating peptide) have potent anti-inflammatory and neuroprotective actions, and have shown significant activity in animal inflammatory disease models including the EAE (experimental autoimmune encephalomyelitis) MS model. Thus, their receptors have become candidate targets for inflammatory diseases. Here, we will discuss the immunomodulatory and neuroprotective actions of VIP and PACAP and their signalling pathways, and then extensively review the structure–activity relationship data and biophysical interaction studies of these peptides with their cognate receptors.
Keywords: autoimmunity, drug-design, experimental autoimmune encephalomyelitis (EAE), neuroprotection, PACAP, VIP
Abbreviations: AC, adenylate cyclase; AD, Alzheimer's disease; ADNF, activity-dependent neurotrophic factor; ADNP, activity-dependent neuroprotective protein; APC, antigen-presenting cells; BM, bone marrow; Bz-Phe, benzophenone-Phe; CCR, CC chemokine receptor, ; CIA, collagen-induced arthritis; CNS, central nervous system; DC, dendritic cell; DMD, disease-modifying drug; DSS, dextran sodium sulfate; EAE, experimental autoimmune encephalomyelitis; FDA, Food and Drug Administration; GPCR, G-protein-coupled receptor; IFN, interferon; IL-6, interleukin 6; JNK, c-Jun N-terminal kinase; KO, knockout; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein 1; MIP-2, macrophage inflammatory protein 2; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; MSC, mesenchymal stem cell; NAP, neutrophil-activating protein; NMDA, N-methyl-d-aspartate; PACAP, pituitary adenylate cyclase-activating peptide; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; PMCAO, permanent middle cerebral artery occlusion; PPMS, primary progressive form of MS; RAMP, receptor-activity-modifying protein; RANTES, regulated upon activation, normal T-cell expressed and secreted; SAPK, stress-activated protein kinase; SCG, superior cervical ganglion; SPMS, secondary progressive form of MS; S-SCAM, synaptic scaffolding molecule; STAT, signal transducer and activator of transcription; TGFβ, transforming growth factor β; TM, transmembrane; TNFα, tumour necrosis factor α; Treg, regulatory T; VIP, vasoactive intestinal peptide; WT, wild-type
INTRODUCTION
MS (multiple sclerosis) is a chronic autoimmune demyelinating and neurodegenerative disorder of the CNS (central nervous system), afflicting over two-and-a-half million people worldwide, with women affected three times more frequently than men. MS is characterized by an extensive and complex immune response, demyelination, glial scarring (sclerosis), perivascular leucocyte infiltration, and oligodendrocyte and axonal loss (Peterson and Fujinami, 2007). Being the most common cause of neurological disability in young adults, it represents a prototypic autoimmune inflammatory disorder of the CNS. Although the agent(s) that initiate disease onset have not been clearly elucidated, several lines of evidence, derived from the EAE (experimental autoimmune encephalomyelitis) model, support that the disease is autoimmune in nature, regulated by APCs (antigen-presenting cells) and auto-reactive Th1 and Th17 cells (Steinman, 2001; Sospedra and Martin, 2005; Korn et al., 2009). These cell types co-ordinate the production of inflammatory cytokines and chemokines within the CNS parenchyma, leading to the recruitment of macrophages, mast cells, neutrophils, CD8+ T-cells and B-cells, which subsequently attack components of the CNS, including the myelin sheath and axons (Siatskas and Bernard, 2009). The accumulation of axonal damage leads to a permanent loss of neurological function (Lassmann et al., 2007).
Given that a pathological hallmark of MS is an overactive immune response, strategies for the treatment of MS have focused on suppressing inflammation, resulting in an emergence of DMDs (disease-modifying drugs). As reviewed in Barten et al. (2010) and Farrell and Giovannoni (2010), four approved DMDs are currently available which target the immune system. They include recombinant IFNβ1a (interferon β1a), IFNβ1b (The IFNB Multiple Sclerosis Study Group, 1993; Jacobs et al., 1996; PRISMS Study Group, 1998) and glatiramer acetate (Ge et al., 2000; Comi et al., 2001; Filippi et al., 2001) as first-line treatments, with mitoxantrone (Edan et al., 1997; Hartung et al., 2002; Le Page et al., 2008) reserved as a second-line therapy due to its association with cardiomyopathy, leukaemia, leucopenia, and infection (Barten et al. 2010; Farrell and Giovannoni 2010). Unfortunately, the efficacy of the DMDs appears to be modest. Indeed, they are able to delay MS relapse and slow disease progression for RRMS (relapsing-remitting multiple sclerosis), but fail to show significant benefit in progressive forms of MS such as SPMS (secondary progressive form of MS) and PPMS (primary progressive form of MS) (Goodin et al., 2002; Sorensen et al., 2005; Miller and Leary, 2007).
Currently, only injectable drugs are available for the treatment of MS. Therefore emerging oral therapies for MS are an especially attractive alternative to current DMDs. As they do not rely on injections, they are convenient in use, hence the hope for a much better compliance. Thus, much attention has centred around four emerging oral formulations, cladribine, laquinimod, fingolimod and dalfampridine (Fox, 2010; Losy and Kalinowska-Lyszczarz, 2011). These have demonstrated impressive efficacy in terms of clinical outcomes (Polman et al., 2005; Kappos et al., 2006, 2010; Fox, 2010; Giovannoni et al. 2010; Losy and Kalinowska-Lyszczarz, 2011). Interestingly, unlike the other agents, the mechanism of action of dalfampridine which is a selective potassium channel blocker, does not rely on immunomodulation but rather on restoring the conduction of injured axons (Judge and Bever, 2006). Thus, dalfampridine is indicated as a symptomatic therapy to improve walking in MS patients (Judge and Bever, 2006; Goodman et al., 2009), but is not necessarily efficacious in other domains of MS. However, as reviewed in Losy and Kalinowska-Lyszczarz (2011) and in Fox (2010), there are a multitude of tolerable and/or serious adverse effects associated with each of these drugs. At present, laquinimod and fingolimod have received fast-track review status from the FDA (Food and Drug Administration); they may reach the market in late 2011. In contrast, cladribine and dalfampridine are still in the FDA's approval review for MS. Based on their side effect profiles and the unknown long-term safety outcomes, it remains to be seen whether these emerging DMDs will allow them to displace existing first-line therapies.
Although these current and emerging drugs provide benefit for MS, especially in the early phases of the disease, existing drugs have little effect on the neurodegenerative component that occurs at a secondary stage of the disease. Thus, the development of effective therapy for slowing down the course of SPMS and PPMS has been and remains challenging. Stem cell therapy has shown some benefit, but this therapy is still investigational and controversial. For example, due to their potent immune regulatory, neuroprotective properties, and capability to home to inflammatory or injured sites (Fox et al., 2007; Karp et al., 2009), MSCs (mesenchymal stem cells) have recently emerged as a promising alternative for the treatment of MS (reviewed in Siatskas et al., 2010). The therapeutic efficacy of MSCs has also been established in EAE. Systemic transplantation of autologous or allogeneic MSCs in relapsing remitting (Gerdoni et al., 2007) or chronic progressive models of EAE (Zappia et al., 2005) has shown that MSCs are efficacious in suppressing clinical EAE disease in the early stages of disease onset, limiting the number of CNS inflammatory lesions, reducing axonal loss and preserving myelin structure (Zappia et al., 2005; Gerdoni et al., 2007). Despite these positive outcomes, solid data regarding the safety of MSCs in MS are still lacking. In addition, because of their ability to suppress immune responses, adverse effects such as immune deficiency leading to infection, activation of tumour growth and metastasis must be ruled out (Djouad et al., 2003; Karnoub et al., 2007; Wang et al., 2009).
While having moderate success in slowing the progression of disability as well as reducing the severity and frequency of exacerbations of MS, the fact remains that these treatment modalities do not cure the disease. As such, the need for innovative approaches becomes of paramount importance. In this respect, VIP (vasoactive intestinal peptide) and PACAP (pituitary adenylate cyclase-activating peptide), two highly homologous neuroprotective peptides initially characterized as neuropeptide neurotransmitters, have recently been found to show pronounced anti-inflammatory actions when administered systemically in animal models of inflammatory disease, including EAE (Abad et al., 2001, 2003; Delgado et al., 2001; Kato et al., 2004; Gonzalez-Rey et al., 2006b). The immunosuppressive effects of these peptides have, in the past, been attributed to their capacities to modulate macrophage and DC (dendritic cell) function and to inhibit antigen-specific Th1-driven responses (Delgado et al., 2004b). While these mechanisms may be relevant in EAE and other inflammatory diseases, other evidence as well as our data indicate that these peptides may modulate the production and/or activities of Treg (regulatory T) and Th17 cells (Chorny et al., 2005; Juarranz et al., 2005; Fernandez-Martin et al., 2006). Thus, because VIP and PACAP are potent anti-inflammatory factors and because their receptors are widely distributed in the immune system, VIP and PACAP have recently emerged as candidates for the treatment of MS as well as other inflammatory diseases. In this review, we will concentrate our discussion on VIP, PACAP and their receptors and provide an overview of what it is currently known and accepted concerning the structure of both peptides and their respective targets, the signalling pathways involved and their biological functions with a focus on the immunomodulatory and neuroprotective actions. Finally to guide investigators towards drug design and new therapeutic pathways that can be exploited in MS and other inflammatory diseases, we will describe structure–activity relationship data and biophysical studies that probe the interaction between VIP/PACAP and their cognate receptors at the molecular level.
VIP AND PACAP BACKGROUND
Isolated in porcine duodenum by Said and Mutt (1970), VIP is a neuropeptide of 28 amino acids. Almost 20 years later, PACAP was isolated from ovine hypothalamus extracts on the basis of its ability to stimulate the production of cAMP in cultured pituitary cells (Miyata et al., 1989). Furthermore, PACAP exists in two forms as PACAP38 (38 amino acid residues) and PACAP27 that corresponds to the N-terminal 27 residues of PACAP38 (Miyata et al., 1989, 1990). Both forms were found to be generally equipotent in receptor binding and AC (adenylate cyclase) assays in some cells (Buscail et al., 1990; Miyata et al., 1990). VIP and PACAP exhibit very high similarity with 68% sequence identity and belong to the secretin/VIP peptide family as discussed below in more detail in the section titled ‘Structural and functional properties’.
VIP and PACAP actions are mediated through heterotrimeric GPCRs (G-protein-coupled receptors). Three VIP/PACAP receptor genes have been cloned (Laburthe et al., 2007; Dickson and Finlayson, 2009; Vaudry et al., 2009). The VIPR1 and VIPR2 genes encode receptors that respond equally to VIP and PACAP named VPAC1 and VPAC2 respectively (Ishihara et al., 1992; Lutz et al., 1993; Sreedharan et al., 1993; Couvineau et al., 1994), whereas the ADCYAP1R gene encodes the PACAP-preferring receptor PAC1 (Hashimoto et al., 1993; Hosoya et al., 1993; Pisegna and Wank, 1993). In mammals, both peptides are widely expressed in the central and peripheral nervous systems (Pozo and Delgado, 2004; Laburthe et al., 2007; Dickson and Finlayson, 2009; Vaudry et al., 2009), are also produced within immune cells where they play the role of a ‘cytokine-like peptide’ (Gomariz et al., 2001; Delgado et al., 2004b), and are induced in both neurons and immune cells during inflammation (Gomariz et al., 1993; Gaytan et al., 1994; Leceta et al., 1996; Zhang et al., 1998; Vassiliou et al., 2001; Abad et al., 2002; Armstrong et al., 2004; Delgado et al., 2004b; Laburthe et al., 2007; Vaudry et al., 2009). Likewise, their receptors are mainly distributed in the nervous, endocrine and immune systems (Delgado et al., 2004b; Laburthe et al., 2007; Vaudry et al., 2009). In consonance with this large distribution, they are pleiotropic neuropeptides involved in many physiological and pathophysiological processes (Vaudry et al., 2009) and will be discussed in particular with respect to MS-relevant actions in the ‘Potent immunomodulatory actions of VIP and PACAP’ section.
STRUCTURAL AND FUNCTIONAL PROPERTIES
VIP and PACAP
VIP and PACAP belong to the amidated VIP/secretin family that adopts common properties: (i) a length of 27–44 amino acid residues, (ii) an α-helical configuration along the sequence from residue 6 to the C-terminal end of the peptide, and a non-structured N-terminal end (Gronenborn et al., 1987; Romier et al., 1993; Thornton and Gorenstein, 1994; Pellegrini et al., 1998; Inooka et al., 2001; Tan et al., 2006) and (iii) the presence of a common N-terminal structural motif, named N-cap (Neumann et al., 2008). Utilizing CD spectroscopy and/or NMR spectroscopy, it has been reported that most of the VIP-28 amino acid sequences have an α-helical structure (sequence 7–28) with the exception of the N-terminal 1–5 sequence that has no defined structure in solution when unbound to the receptor (Tan et al., 2006) (Figure 1), whereas PACAP27 peptide is characterized by a disordered N-terminal domain consisting of eight amino acids, followed by an α-helical structure (Inooka et al., 2001; Bourgault et al., 2009b). In addition, the conformation of PACAP38 mirrors that of PACAP27 with the C-terminal 28–38 short helix connected by a flexible hinge to the 1–27 region (Wray et al., 1993). Furthermore, it is also widely agreed that the disorganized N-terminal 1–5 segment plays a crucial role in activation of AC (Laburthe et al., 2007; Vaudry et al., 2009). Particularly, the N-cap motif was suggested to be involved in receptor activation and may possibly be used for the design of drugs targeting VPAC receptors and other members of the class B GPCRs (Neumann et al., 2008), while the α-helical conformation is mainly involved in the peptide binding and receptor specificity (Laburthe et al., 2007; Vaudry et al., 2009).
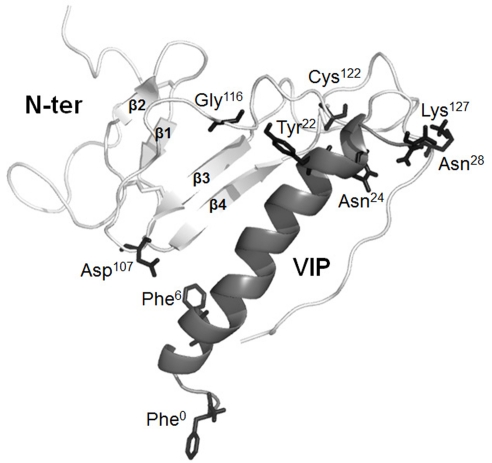
Figure 1. A 3-D ribbon representation of VIP interaction with the VPAC1 N-terminal domain.
The VPAC1 receptor N-terminal domain encompassing sequence 44–137 is shown in light grey. The structure reveals a Sushi domain characterized by two anti-parallel β-sheets named β1, β2, β3 and β4. Most of the VIP-28 sequence, which is shown in middle grey, has an α-helical structure (sequence 7–28). Photoaffinity labelling experiments showed that Asp107, Gly116, Cys122, Lys127 and sequence 129–137 that connects the Nter domain and the first helical TM (shown in dark grey) in the N-terminal domain are physically in contact with the side chains of Phe6, Tyr22, Asn24, Asn28 and Phe0 (shown in dark grey) of VIP respectively. Reprinted from Neuropeptides, 44, A. Couvineau, E. Ceraudo, Y.-V. Tan and M. Laburthe, VPAC1 receptor binding site: contribution of photoaffinity labeling approach, 127–132, © 2010, with permission from Elsevier.
VIP/PACAP receptors, class B GPCRs
Approx. 20 years ago, VIP and PACAP receptors were designated as VPAC1, VPAC2 and PAC1 receptors by the International Union of Pharmacology according to their relative affinity for their ligands, PACAP and VIP (Harmar et al., 1998). With the cloning of many other receptors for peptides of the secretin family, a new GPCR subfamily was born and named class B GPCR or class II GPCR or also ‘secretin-like’ receptor family (Laburthe et al., 2007; Vaudry et al., 2009). Subfamily B GPCRs exhibit 25–50% amino acid identity among themselves and low primary sequence homology with other GPCR classes, but display a common seven TM (transmembrane) helical configuration denoted as TM I to TM VII, which are interconnected by extracellular and intracellular loops (Hosoya et al., 1993; Pisegna and Wank, 1993; Sreedharan et al., 1993; Usdin et al., 1994; Laburthe et al., 2007; Dickson and Finlayson, 2009; Vaudry et al., 2009). In addition, they exhibit specific features: (i) a relatively large (>120 residues) N-terminal domain (Nter domain), which is usually smaller in the class A GPCRs or Rhodopsin-like receptors, (ii) six highly conserved cysteine residues forming three disulfide bridges that structure the Nter domain, (iii) the presence of a signal peptide probably involved in addressing the receptor towards the plasma membrane, (iv) several conserved glycosylation sites within the Nter domain involved in receptor trafficking, (v) the absence of archetypical A subfamily GPCR motifs such as E/D-R-Y or NP-xx(x)-Y and (vi) a common but complex gene organization (Laburthe et al., 1996, 2007).
Molecular anatomy of VIP–PACAP interaction with their receptors
To dissect the binding mechanism between VIP/PACAP and their receptors and particularly to define the residues and structural elements involved, numerous structure–activity relationship experiments have been conducted. These have included chemical modifications, amino acid substitutions, deletion mutagenesis, molecular chimaerisms and photoaffinity labelling analyses as reviewed in Laburthe et al. (2007), Vaudry et al. (2009) and Couvineau et al. (2010). Interestingly, the data indicate that the Nter domain of the VIP and PACAP receptors is a major binding site, essential for the recognition of their respective ligands (Cao et al., 1998; Laburthe et al., 2007; Vaudry et al., 2009; Couvineau et al., 2010; Dejda et al., 2011). Due to its quite recent recognition, no 3-D structure of a B family receptor is yet available unlike some class A GPCRs. However, the N-terminal domain structures of five type B receptors including human PAC1 receptor (Sun et al., 2007a) have been determined by NMR spectroscopy or X-ray crystallography. The structures revealed a Sushi domain characterized by two anti-parallel β-sheets (named β1, β2, β3 and β4), and stabilized by three disulfide bonds between six highly conserved cysteine residues and by a putative salt bridge involving acidic and basic residues (Grace et al., 2004). This Sushi domain has been shown to play a crucial role in peptide ligand binding (Grace et al., 2004). Recently, the development of a structural model of VPAC1 N-terminal domain indicated a structure similar to that of a Sushi domain (Tan et al., 2006) (Figure 1).
A detailed molecular description of the interaction of VIP with one of its receptors, VPAC1 receptor, has recently been provided by performing extensive photoaffinity studies (Tan et al., 2003, 2004, 2006; Ceraudo et al., 2008a) in which the full-length ligand is covalently bound to its native receptor. This has allowed: (i) analyses of point-to-point interactions between the peptide and its target protein and (ii) the accurate determination of a spatial view of the ligand within the binding site of the receptor. For example, the residues in positions 6, 22, 24 and 28 within the VIP sequence were replaced by a photoreactive Bz-Phe (benzophenone-Phe) amino acid. As illustrated in Figure 1, these studies hence demonstrated that side chains in positions 6, 22, 24 and 28 of VIP are in direct contact with different amino acids in the Nter domain of the receptor e.g. Asp107, Gly116, Cys122 and Lys127 respectively (Tan et al., 2003, 2004, 2006; Ceraudo et al., 2008a). Based on the four physical interaction sites, identified by the photoaffinity strategy, to the NMR structure of VIP, and the structural model of the VPAC1 Nter domain, the structure of VIP has been docked in the VPAC1 Nter domain. This docking created an accurate 3-D structural model in which the C-terminal segment of the Nter domain of the receptor accommodates the VIP molecule, at least for the 6–28 sequence (Tan et al., 2003, 2004, 2006; Ceraudo et al., 2008a) (Figure 1). In parallel, under molecular dynamic simulations in water for over 14 ns, this model exhibited a remarkable stability (Ceraudo et al., 2008a).
A ‘two-site’ binding model
The model described above may realistically illustrate the binding between VIP and VPAC1, and potentially explains how the receptor is activated. It is in agreement with the currently accepted concept of a ‘two-site’ binding model for the interaction of natural ligands to family B GPCRs (Hoare, 2005). In this model, the central and C-terminal α-helical segments of the ligand are trapped by the Nter domain of the receptor. This step positions the disordered N-terminal region of the peptide ligand for its interaction with the TM domain of the receptor to initiate intracellular signalling (Hoare, 2005). In this respect, the integrity of the α-helical conformation seems crucial for the binding of VIP and PACAP to their receptors. Accordingly, deletion in the C-terminal domain of PACAP reduces drastically the binding affinity without altering the propensity of the analogues to activate the receptor at high concentration (Bourgault et al., 2008b). Conversely, modifications of the disordered N-terminal portion of PACAP molecule decrease the biological activity of the peptide (Onoue et al., 2001; Bourgault et al., 2009b). In fact, deletion of the entire N-terminal segment, i.e. PACAP(6–38) leads to a potent PAC1/VPAC2 antagonist (Vandermeers et al., 1992). It seems clear that the presence of an N-capping signature in the N-terminal end of VIP, PACAP and other members of the VIP/secretin family may contribute to position His1 of these peptides into their cognate receptors, triggering the release of the Gαs subunit from the heterotrimeric Gs-protein and activation of AC (Neumann et al., 2008). Recently, in order to attempt to identify the molecular determinants of VPAC1 involved in the recognition of VIP's N-terminal part, photoaffinity labelling experiments have been carried out with a new VIP photoreactive probe containing a Bz-Phe in position 0 (Ceraudo et al., 2008b). The results revealed that the VIP N-terminal part physically interacts with the receptor domain connecting the Nter domain and the first TM helix (Ceraudo et al., 2008b) (Figure 1).
Although numerous structure–activity studies and photoaffinity labelling experiments have provided interesting insights to understand the molecular interactions between VIP/PACAP peptides and their receptors, the determination of the structure of the full-length VIP/PACAP receptors clearly remains a major challenge for all investigators in the field, and will facilitate the emergence of new potent drugs.
SIGNALLING PATHWAYS
As discussed earlier, two of the receptors, VPAC1 and VPAC2, recognize PACAP and the related peptide VIP with equally high affinity. The third, PAC1, is a PACAP-preferring receptor. Due to alternative splicing, different PAC1 variants exist and mediate differential coupling to second messengers. Indeed, five variants resulting from alternative splicing in the third intracellular loop region have been identified in rat (Spengler et al., 1993). These variants are characterized by the absence (short variant, S) or presence of either one or two cassettes of 28 amino acids (hip or hop1 variant) or 27 amino acids (hop2 variant) (Spengler et al., 1993). Furthermore, the presence of the hip cassette impairs AC stimulation and abolishes PLC (phospholipase C) activation. In addition, a very short splice variant of PAC1, characterized by a 21-amino acid deletion in the N-terminal extracellular domain showed altered selectivities for PACAP38 and PACAP27 isoforms (Dautzenberg et al., 1999). Another PACAP receptor variant termed as PAC1 TM4 (transmembrane domain 4) has been cloned in the rat cerebellum (Chatterjee et al., 1996).
Each of these GPCRs is typically coupled via the heterotrimeric Gs-protein (Gαs) to AC, cAMP production and the PKA (protein kinase A) pathway activation (Laburthe et al., 2007; Dickson and Finlayson, 2009; Vaudry et al., 2009). However, they can couple to other intracellular messenger systems in some cells in parallel or downstream of cAMP including pathways such as nitric oxide (Murthy et al., 1993), Gαq or Gαi/PLC leading to an increase in the intracellular calcium concentration ([Ca2+]i) and the PKC (protein kinase C) signalling stimulation (Spengler et al., 1993; Dickson and Finlayson, 2009), PI3K (phosphatidylinositol 3-kinase; Straub and Sharp, 1996), src (Koh, 1991), MAPKs (mitogen-activated protein kinases; Barrie et al., 1997; Villalba et al., 1997; Lelievre et al., 1998), Jak/STAT (signal transducer and activator of transcription) and NF-κB (nuclear factor κB; Delgado and Ganea 1999, 2000). Consistent with their diverse biological actions, VIP and PACAP receptor signalling cascades include a constellation of second messengers that are likely dependent not only on the receptor and G-proteins but also on the repertoire of accessory molecules. Thus, besides their interaction with G-proteins, recent results showed that VIP and PACAP receptors were also able to interact with non-G proteins or ‘accessory proteins’ and to transduce signals independently of the G proteins (Sun et al., 2007b). The ‘accessory protein’ group is known to modulate the overall function of the receptors, as extensively demonstrated for the A GPCR subfamily (Bockaert et al., 2004), but poorly documented for the subfamily B GPCRs. For example, a recent report clearly indicated that VPAC1 but not VPAC2 hetero-dimerizes with RAMPs (receptor-activity-modifying proteins) (Christopoulos et al., 2003; Sexton et al., 2009). Particularly, by interacting with RAMP2, VPAC1 induces inositol triphosphate production and increases intracellular calcium without affecting the coupling to AC (Christopoulos et al., 2003). Recently, the PDZ domain of S-SCAM (synaptic scaffolding molecule) was found in the VPAC1 receptor C-tail, implying its potential recruitment by S-SCAM to synaptic buds (Gee et al., 2009). The latter was reported to result in inhibition of VIP-induced cAMP production and inhibition of agonist-induced internalization of VPAC1 (Gee et al., 2009). Besides the role of RAMPs and/or S-SCAM interaction with VPAC1 receptors, one report describes the possible interaction between CaM (calmodulin) and this receptor although the functional role of this interaction remains unclear (Mahon and Shimada, 2005). Finally, it should be noted that VPAC1 receptor is able to homo-dimerize and hetero-dimerize with VPAC2 or secretin receptors (Harikumar et al., 2006), although again the functional signification remains unclear.
POTENT IMMUNOMODULATORY ACTIONS OF VIP AND PACAP
VIP, PACAP and their receptors are broadly expressed in the CNS and in most peripheral organs. Given this fact, it is not surprising that these peptides elicit pleiotropic physiological effects (Delgado et al., 2004b; Laburthe et al., 2007; Vaudry et al., 2009). Early reports suggested that VIP and PACAP exerted a multitude of effects on CNS, cardiovascular, circulatory and respiratory systems and on metabolic function (Said et al., 1970; McCulloch and Edvinsson, 1980; Miyata et al., 1989, 1990). Moreover, both peptides have been proposed for the treatment of asthma (Groneberg et al., 2001), impotence (Kalsi et al., 2002), treatment of septic shock (Delgado et al., 1999b, 2004b; Martinez et al., 2002, 2005) and diabetes (Nakata and Yada, 2007). More recent studies also point to a plethora of putative growth factor-like actions impacting on development and regeneration (Gozes and Brenneman, 2000; Dejda et al., 2005; Shioda et al., 2006) and cancer pathogenesis (Moody, 1996; Moody et al., 2003). In the following section, we focus on the immunomodulatory actions of VIP and PACAP (Gomariz et al., 2001; Delgado et al., 2004b; Pozo and Delgado, 2004; Gonzalez-Rey et al., 2007). Growth factor-like actions of these peptides are discussed in a subsequent section.
Macrophage function (reviewed in Delgado et al., 2004b)
Macrophages constitute a major source of chemokines and pro-inflammatory molecules implicated in inflammatory responses and constitute a high percentage of the migrating cells to inflamed tissues. They constitutively express VPAC1 and PAC1, and when exposed to inflammatory stimulus express VPAC2. The effect of VIP and PACAP on macrophage function has been repeatedly demonstrated. In unstimulated macrophages, VIP and PACAP increase IL-6 (interleukin 6) production through activation of the PKA and PKC pathways (Martinez et al., 1998a, 1998b). In contrast, it was reported that they inhibited the production of pro-inflammatory TNFα (tumour necrosis factor α), IL-6 and IL-12 in macrophage cultures in response to LPS (lipopolysaccharide) (Martinez et al., 1998a; Delgado et al., 1999d, 1999e). The negative effect on TNFα and other macrophage derived pro-inflammatory mediators (IL-12, IFNγ and NO) appeared to be mediated through the constitutively expressed VPAC1, although the inducible expressed VPAC2 could also participate (Delgado et al., 2000). More recently, both peptides were shown to increase the synthesis and release of anti-inflammatory molecules like IL-10 and the IL-1Rα (IL-1 receptor antagonist), leading to a suppression of inflammatory responses (Delgado et al., 1999c). Finally, it has been reported that VIP and PACAP inhibit the production of several macrophage-derived chemokines such as MIP-2 (macrophage inflammatory protein 2), KC (keratinocyte chemoattractant) (IL-8), MIP-1α, MIP-1β, MCP-1 (monocyte chemoattractant protein 1) and RANTES (regulated upon activation, normal T-cell expressed and secreted), produced by LPS-activated macrophages (Delgado and Ganea, 2001).
APC/T-cell function
T-lymphocyte differentiation is finely orchestrated by APCs through co-stimulatory molecules and the production of specific APC cytokines. Thus, Th cells differentiate upon antigen presentation into four main cell subsets named Th1, Th2, Th17 and adaptive (also called inducible) regulatory T-cells (Zhu and Paul, 2008). Interestingly, in vitro studies showed that both neuropeptides were able to modify via VPAC1 receptors the expression of the APC co-stimulatory molecules B7.1 and B7.2 (or also called CD80/CD86) (Delgado et al., 1999a, 1999f, 2000). For example, in resting macrophage cultures, VIP and PACAP promoted B7.2, but not B7.1, expression. In contrast, both peptides were shown to inhibit in vivo and in vitro the expression of B7.1 and B7.2 of LPS/IFNγ-activated macrophages (Delgado et al., 1999a, 1999f, 2000). VIP and PACAP also appear to regulate the ability of DCs to activate T-cells (Delgado et al., 2004c). Indeed, in BM (bone marrow)-derived DCs, they up-regulated, via VPAC1, CD86 (B7.2) expression, and enabled them to stimulate T-cell proliferation and differentiation into Th2 effectors in vivo and in vitro (Delgado et al., 2000, 2004c). In contrast, VIP/PACAP down-regulated CD80/CD86 (B7.1/B7.2) expression in LPS-stimulated DC cells and strongly reduced their capacity to stimulate T-cell proliferation and to secrete Th1 and Th2 cytokines. More importantly, related to these results, it has been shown that both human and murine DCs generated in vitro in the presence of VIP exhibited a ‘tolerogenic’ phenotype characterized as CD11clowCD45RBhigh with the absence of CD80, CD86 and CD40 expression and ability to secrete high amount of the anti-inflammatory IL-10 cytokine after LPS stimulation. Remarkably, these VIP-induced tolerogenic DCs promoted the generation of Treg cells in vivo and in vitro (Delgado et al., 2005; Gonzalez-Rey et al., 2006a).
T-cell function (reviewed in Delgado et al., 2004b)
VIP/PACAP and their GPCRs also appear to play an important role in many aspects of T-cell-dependent immunity, imperative to control infectious, autoimmune diseases and organ transplant rejection. On resting CD4+ T-cells, VPAC1 is expressed constitutively, whereas upon activation by anti-CD3 and anti-CD28 antibodies, VPAC1 is down-regulated while VPAC2 is induced (Lara-Marquez et al., 2001). Interestingly, although most of the Th differentiation occurs via APC interaction as discussed above, a few in vivo and in vitro studies suggested that VIP and PACAP may in some cases, promote Th2 responses by regulating Th2-specific transcription factors, cytokine responses, proliferation, survival and chemotaxis (Delgado et al., 2002; Voice et al., 2004). VPAC 1 and VPAC2 receptors may be involved in these actions. For example, it was shown that activated CD4+ T-cells expressed VPAC2 during differentiation to the Th2 phenotype, suggesting VPAC2 as a modulator for Th2 phenotype. On the other hand, VIP and PACAP via VPAC1 down-regulated in vivo and in vitro the production of CXCL10 (CXC chemokine ligand 10; IP-10) and up-regulated CCL22 (CC chemokine ligand 22) [MDC (macrophage-derived chemokine)], purported to be Th1- and Th2-specific chemokines respectively. This might lead to the preferential recruitment of the anti-inflammatory Th2 cell population (Jiang et al., 2002; Delgado et al., 2004a).
VIP/PACAP effects on human T-cell function in MS
The dynamics of VPAC receptor expression in peripheral blood T-lymphocytes from MS patients compared with healthy controls has been investigated (Sun et al., 2006). Similar to what had been shown previously for mouse lymphocytes, resting human T-cells from both MS patients and controls expressed high levels of VPAC1, but very low levels of VPAC2. Like in mice (Vomhof-DeKrey and Dorsam, 2008; Vomhof-DeKrey et al., 2008), when lymphocytes from either group were activated in vitro with anti-CD3 and anti-CD28 antibodies, VPAC1 expression was significantly down-regulated. This decrease in VPAC1 expression was compensated by a strong up-regulation of the VPAC2 receptor in healthy control lymphocytes, whereas the induction was severely impaired in MS patients (Sun et al., 2006). Interestingly, while CD4+ lymphocytes from healthy controls were able in the presence of VIP, to polarize into a Th2 phenotype with increased expression of the Th2-associated transcription factor Stat-6, this VIP effect was significantly dampened in MS patients, which could be due to the aberrant expression of its receptors (Sun et al., 2006).
VIP and PACAP as therapeutic agents in EAE (Figure 2)
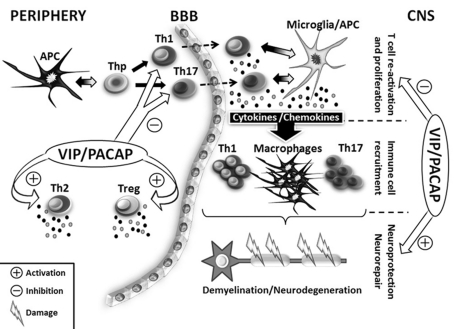
Figure 2. Potential effects of VIP and PACAP in EAE, a mouse MS model.
This is a schematic representation of the EAE-mediated immune response with a focus on APCs and T-cells. Briefly, after EAE induction, APCs present in the periphery of the MOG antigen to Th progenitors (Thp) which subsequently differentiate into Th1 or Th17 cell subsets. T effector Th1 and Th17 cells, so-called encephalitogenic Th cells, migrate towards the CNS crossing the blood−brain barrier (BBB). In the CNS, once re-activated by microglia/APC cells, Th1 and Th17 cells proliferate and secrete IFN-γ and IL-17 respectively, mounting a robust pro-inflammatory immune response against endogenous myelin that involves the secretion of multiple pro-inflammatory cytokines and chemokines. This leads to the recruitment of increasing numbers of immune cells, which amplifies the pro-inflammatory response. Ultimately, demyelination and neurodegeneration events occur, leading to the development of EAE symptoms. Due to their potent immunomodulatory and neuroprotective functions, VIP and PACAP can potentially act both in the periphery and the CNS to reduce and block the EAE pathogenesis. In the periphery, both peptides have been shown to inhibit Th1 and Th17 activities, while stimulating anti-inflammatory response by favouring Th2 and Treg generation. In the CNS, VIP and PACAP down-regulate T-cell re-activation and promote neuroprotection and neurorepair. These two phenomena lead to the suppression of EAE symptoms.
Based on the substantial success of systemic administrations of VIP and PACAP in other animal models of chronic inflammatory disease such as rheumatoid arthritis (Abad et al., 2001; Delgado et al., 2001) and Crohn's disease (Abad et al., 2003), investigators have begun to examine whether or not these peptides might have efficacy in the EAE model of MS. For instance, in the earliest report, the effects of PACAP on MOG35–55 (myelin oligodendrocyte glycoprotein 35–55)-induced EAE were examined in C57BL/6 mice (Kato et al., 2004). Intraperitoneal administration of PACAP every other day after immunization with MOG ameliorated both the clinical and pathological manifestations of EAE. Splenocyte cultures from mice treated with PACAP exhibited significantly reduced MOG-specific Th1 IFNγ production. In vitro analysis revealed that PACAP suppressed the production of inflammatory cytokines, including TNFα, IL-1β and IL-12, and expression of the co-stimulatory factor B7.2 on macrophages and microglia. The authors suggested that PACAP suppressed induction of EAE primarily via suppression of APC function and inflammatory cytokine production. Other work focused on the therapeutic action of VIP in EAE. In these studies, the clinical and pathological scores in chronic MOG35-55-induced EAE in C57BL/6 mice were dramatically reduced by a 3-day treatment with VIP begun before or after the onset of EAE (Gonzalez-Rey et al., 2006b). Three-day treatment with either VIP or PACAP was also found to ameliorate EAE in a relapsing-remitting model (PLP139-151-induced SJL/J mice), with a blockade of symptoms lasting 60 days. The reduction of EAE and decreased inflammation after VIP administration was associated with decreased spinal cord levels of pro-inflammatory cytokines (TNFα, IL-6, IL-1β, IL-18 and IL-12), enzymes [iNOS (inducible nitric oxide synthase)], chemokines (RANTES, MIP-1, MIP-2, MCP-1 and IP-10) and CCRs (CC chemokine receptors; CCR-1, CCR-2 and CCR-5) and increased levels of the anti-inflammatory cytokines IL-10, IL-1Ra and TGFβ (transforming growth factor β). The latter inductions occurred despite a 4-fold reduction in the number of inflammatory cells in the spinal cord as detected by FACS. Lymph nodes from VIP-treated mice showed reduced antigen-induced proliferation, and lower and higher production of Th1 and Th2 cytokine respectively.
VIP and PACAP as potential modulators of Treg and Th17 production and activity: significance in EAE (Figure 2)
As previously discussed, these peptides have formally been thought to act primarily via VPAC receptors by inhibiting macrophage activity and skewing Th1/Th2 responses in models of autoimmune diseases. However, with the increased interest in Treg cells in autoimmunity, and the discovery that many autoimmune diseases seem to be driven primarily by Th17 responses, emerging data indicated that VIP and PACAP might regulate the production or expansion of regulatory T-cells and Th17 responses. In the context of the EAE model, the administration of VIP to mice resulted in the expansion of the CD4+CD25+Foxp3+ expressing T-cells (Tregs) in the periphery and the nervous system, which were shown to inhibit encephalitogenic T-cell activation (Fernandez-Martin et al., 2006). VIP was also reported to enhance the suppressive efficiency of Tregs on a per cell basis. In adoptive transfer experiments, VIP-generated CD4+CD25+ Tregs were found to transfer suppression and significantly ameliorate the progression of the disease. Conversely, CD4+ T-cells from VIP-treated EAE mice did not transfer disease, whereas CD4+CD25+-depleted T-cells from VIP-treated EAE mice did. In another study, the same investigators studied potential mechanisms by which VIP induced expansion of Tregs (Chorny et al., 2005). In the ex vivo experiments, mice with evolving EAE were injected with DCs generated from cultures of syngenic BM cells in the presence or absence of VIP (cells were termed DCVIP and DCcon respectively). Injection of DCVIP but not DCcon was found to reduce EAE symptoms in association with a decreased ability of draining lymph node cultures to proliferate and produce IFNγ cytokine in response to autoantigen. Moreover, injection of DCVIP-induced MOG-specific regulatory T-cells, but not DCcon-induced cells, significantly attenuated EAE. These results suggest that the induction and/or activation of regulatory T-cells via the production of tolerogenic DCs may be a mechanism by which exogenous VIP (or perhaps PACAP) alleviates EAE. No studies have specifically examined the role of VIP or PACAP in Th17 cells in EAE or other inflammatory disease models, although IL-17 gene expression was found to be reduced in VIP-treated against untreated mice in CIA (collagen-induced arthritis; Juarranz et al., 2005) and in colonic extracts in a chemical-induced colitis model (Abad et al., 2005).
One of the problems that could limit the use of VIP as a therapeutic agent in MS or other diseases is the short biological half-life (t½) of the peptide. This latter topic will be discussed later in the ‘Pharmacology and drug design’ section. In order to sustain beneficial VIP levels in the organism, the group of Toscano et al. (2010) tested in mice a strategy based on the transfer of DCs transduced with vectors expressing VIP (lenti-VIP DCs). The intravenous transfer of these cells to mice with EAE reduced the clinical symptoms of the disease as well as the mRNA expression of the key pro-inflammatory cytokines IL-1β, TNFα and IL-6. After inoculation, these cells were found in several organs such as the liver, lung, lymph nodes and spleen. The mechanisms for the therapeutic actions of these cells could be double. On the one hand, before transfer, the lenti-VIP DCs were functionally ‘tolerogenic’. On the other hand, the serum levels of VIP in these mice assessed 48 h after inoculation were 3-fold higher than those in non-injected mice, demonstrating that transfer of these cells resulted in systemic levels of VIP, leading to a more robust inhibition of the inflammatory response.
GENETIC MANIPULATION OF VIP, PACAP AND THEIR RECEPTORS IN MICE AND THEIR APPLICATION TO INFLAMMATORY DISEASE MODELS
VIP KO (knockout) and PACAP KO mice in EAE
As for many peptides and their cognate receptors, clear insights into their physiological roles have recently been gained through the use of genetically engineered mice. These have been used to investigate immunomodulatory actions of endogenous VIP and PACAP. For example, although the mechanism is still uncertain, we showed that the severity of MOG35–55-induced EAE is increased in PACAP-deficient mice in association with enhanced Th1/Th17 and diminished Th2 responses in the spinal cord, indicating that endogenous PACAP is protective in this model of autoimmune disease (Tan et al., 2009). Moreover, we found a significant reduction in the abundance of Tregs in the lymph nodes of PACAP KO mice during the recovery phase of the disease. It has been experimentally demonstrated in this model of EAE that natural, but not inducible, Tregs are involved in the recovery phase (Korn et al., 2007). Thus, the results from PACAP KO mice suggest a new role for PACAP as an endogenous modulator of natural Treg expansion. We also recently studied the response of VIP KO mice to EAE induction (Abad et al., 2010). In contrast to PACAP KO mice, VIP KO mice were highly resistant to EAE at the clinical and histopathological levels. Supporting this phenotype, the levels of multiple pro-inflammatory cytokines in the spinal cord were strikingly reduced in the KO mice. The lack of inflammatory disease in these mice precluded an investigation of how VIP loss affected Treg expansion. Interestingly, ex vivo antigen-rechallenge studies suggested that the immunization phase of EAE was unimpaired in VIP KO mice. Moreover, immune cells isolated from MOG-immunized VIP KO mice had encephalitogenic properties as exhibited by the fact that they were capable of inducing EAE when adoptively transferred to WT (wild-type) hosts. Although the mechanism for the EAE resistance of VIP KO mice remains to be elucidated, immune cells were found to be trapped in the meninges of the brain and spinal cords, and failed to invade the CNS parenchyma of VIP KO mice, suggesting a defect in immune cell migration.
VIP/PACAP receptor KO mice in inflammatory disease models
In addition to mice deficient in VIP or PACAP, the development of receptor KO mice has provided valuable tools to elucidate the mechanisms responsible for the actions of these peptides in the immune system. Although not yet tested for EAE susceptibility, they have been subjected to different models of inflammatory diseases. For example, PAC1 KO mice showed increased mortality after LPS injection in comparison with their WT counterparts, a finding linked to an overstimulation of the IL-6-dependent coagulation and chemotaxis cascades (Martinez et al., 2002, 2005). Inflammatory phenotypes of VPAC2 receptor KO mice have also been examined. In these mice, anti-CD3/CD28 stimulation of purified splenic CD4+ T resulted in 3-fold higher levels of IL-2 and IFNγ than in WT mice and 90% lower levels of IL-4 (Goetzl et al., 2001). These results, along with a finding of relative resistance to cutaneous anaphylaxis and an enhanced hapten-evoked cutaneous DTH (delayed-type hypersensitivity) response in KO mice, suggested that loss of VPAC2 leads to a state of enhanced delayed-type hypersensitivity and depressed immediate-type hypersensitivity (Goetzl et al., 2001). Conversely, transgenic mice overexpressing VPAC2 receptors specifically in CD4+ T-cells exhibited increased Th2/Th1 cytokine profiles and alterations in delayed against immediate hypersensitivity (Voice et al., 2001). The studies reinforce the in vivo and in vitro data suggesting that PACAP and VIP serve to promote and/or reinforce Th2-type responses at the expense of Th1.
In vitro studies by Yadav et al. (2008) addressed the complexity of VPAC1 and VPAC2 signalling effects in lymphocyte differentiation. Interestingly, in vitro treatment of isolated CD4+ cells with VIP in the presence of TGFβ promoted the generation of T-cells with a Th17-like phenotype, producing IL-17 and IL-22 but not IL-21. It was inferred that this effect was mediated by VPAC1 because this is the main receptor expressed in resting lymphocytes. Moreover, in CD4+ cells from VPAC2 KO mice, where the expression of VPAC1 is enhanced, Th17-like polarization was even stronger. This suggests that VIP signalling through VPAC1 receptor can, at least in some contexts, promote a potential pro-inflammatory T-cell phenotype. Studies using the model of DSS (dextran sodium sulfate) colitis supported this possibility (Yadav et al., 2011), because VPAC1 KO mice exhibited a milder disease than WT controls, whereas VPAC2 KO mice exhibited enhanced colitis after DSS treatment.
Therapeutic actions of VIP/PACAP in other mouse models of inflammatory disease
VIP and PACAP have also been shown to have beneficial therapeutic effects in mouse models of rheumatoid arthritis, septic shock and Crohn's Disease. CIA exhibits most of the clinical and histological features of rheumatoid arthritis in humans. Either VIP or PACAP, injected intraperitoneally every day or every other day, reduced dramatically the incidence and the severity of arthritis, even when they were administered in the late stages of the disease (Abad et al., 2001; Delgado et al., 2001). After treatment, paw joints of the VIP- and PACAP-treated animals, unlike controls, showed no evident signs of inflammation (redness and swelling), and in the histological study of the joint tissue there was no pannus formation in the treated animals, and the joint structure was preserved. Administration of VIP or PACAP was found to inhibit both the inflammatory and the autoimmune (Th1) components of the disease and to promote a Th2 cytokine profile. In another model, VIP and PACAP were also found to protect against endotoxin-induced lethality and prevent septic shock-associated histopathological alterations (Delgado et al., 1999b). Finally, in a chemical-induced colitis model, VIP was found reduce the clinical and histopathological severity, abrogating body weight loss, diarrhoea and macroscopic and microscopic intestinal inflammation (Abad et al., 2003). The therapeutic effects were associated with down-regulation of both inflammatory and Th1-driven autoimmune responses, and an up-regulation of Th2 anti-inflammatory cytokines (Abad et al., 2003). Thus, PACAP and VIP have emerged as candidates for the treatment of autoimmune and other inflammatory diseases, especially those associated with an overproduction of Th1 cytokines.
Taken together, through their actions on cytokines, chemokines, cell adhesion molecules, and co-stimulatory molecules produced or expressed by activated APCs, and through its direct and/or indirect effects on Th cell responses, VIP and PACAP appear as important endogenous immunomodulatory molecules that exert mainly protective anti-inflammatory actions in many different models of autoimmune diseases.
OTHER VIP/PACAP ACTIONS RELEVANT TO MULTIPLE SCLEROSIS
Potent neuroprotective properties
Evidence from both in vitro and in vivo experiments described below suggest that VIP and PACAP may promote neural cell proliferation, survival and axon regeneration as well as oligodendrocyte progenitor proliferation and maturation. However, despite their homologies, it is apparent that in most cases, PACAP was shown to be significantly more potent than VIP. The latter may be due to the fact that many of these growth factor-like actions are mediated by PAC1, the PACAP-preferring receptor.
Growth factor-like actions of PACAP
PACAP has been shown to exhibit a variety of trophic or growth factor-like actions on cultured neurons and glia subtypes. Early studies showed that PACAP stimulated the outgrowth of neurites from PC12 cells (Deutsch and Sun, 1992). It was later shown in cultured cortical precursor cells from E13 rats (E is embryonic day) that PACAP induced cell cycle withdrawal and promoted the transition from proliferation to differentiation (Lu and DiCicco-Bloom, 1997). PACAP also potently increased cAMP levels in cultured hindbrain neuroepithelial cells from E10.5 mice and down-regulated the expression of the sonic hedgehog-dependent target gene gli-1 (Waschek et al., 1998). In other work, it was reported that PACAP increased mitosis in cultured embryonic SCG (superior cervical ganglion) precursors and potently enhanced precursor survival via the PACAP-preferring PAC1 receptor (DiCicco-Bloom et al., 2000). Furthermore, PACAP promoted SCG neuronal differentiation, increased neurite outgrowth and enhanced expression of the neurotrophin receptors trkC and trkA (DiCicco-Bloom et al., 2000). PACAP effects on SCG precursors were shown to be mediated by PAC1, via increased intracellular second messengers including cAMP and phosphatidylinositol as well as Ca2+. This findings in SCG cultures explained early studies demonstrating that high concentrations of VIP stimulate mitosis, promote neurite outgrowth and enhance survival of sympathetic neuroblasts in culture (Pincus et al., 1990).
PACAP has been reported to prevent PCD (programmed cell death) in cultured CGCs (cerebellar granule cells; Villalba et al., 1997; Vaudry et al., 2000), as well as apoptosis induced by different neurotoxins such as ethanol (Vaudry et al., 2002b), H2O2 and H2O2 during oxidative stress (Vaudry et al., 2002a), and ceramides (Vaudry et al., 2003). Using PC12 cells, it was shown that PACAP provided protection against cytotoxicity of β-amyloid, a key factor in AD (Alzheimer's disease; Onoue et al., 2002a) and of human prion protein fragment 106–126 [PrP(106–126)] (Onoue et al., 2002b). In purified embryonic rat cortical cultures, PACAP suppressed glutamate-induced neuronal cell death in up to 50% of the cells compared with control (Morio et al., 1996). Moreover, PACAP was shown to protect cerebral cortical neurons from neuronal loss triggered by gp120, the envelope glycoprotein from the HIV (Brenneman et al., 2002). These in vitro data supported those performed in vivo. For example, in the ischaemia-induced PMCAO (permanent middle cerebral artery occlusion) model, PACAP, administered to rats either before (Reglodi et al., 2002) or after the occlusion (Reglodi et al., 2000), significantly reduced the infarct size. Likewise, in a model of Parkinson's disease, PACAP treatment effectively protected the dopaminergic nigrostriatal neurons from apoptotic death, and resulted in reduced neurological deficits and more rapid amelioration of behavioural abnormalities (Reglodi et al., 2004). Moreover, PACAP increased neuronal survival after spinal cord compression (Chen and Tzeng, 2005), suggesting that PACAP could have beneficial effects in tissue restoration after nerve injury. Consistent with this hypothesis, PACAP mRNA was up-regulated for as long as 30 days after facial motor neuron axotomy (Zhou et al., 1999) and nerve regeneration was impaired in PACAP KO animals (Armstrong et al., 2008).
In most of the aforementioned studies, the effects of PACAP were mediated through PAC1 receptor and linked to the PKA pathway (Morio et al., 1996; Onoue et al., 2002b; Vaudry et al., 2002a, 2003). However, it is clear that neuroprotective actions include, but are not limited to, cAMP-mediated processes. Thus, MAPK pathways also contribute to the neuroprotective action of PACAP (Onoue et al., 2002b; Vaudry et al., 2002a, 2003). For example, it was indicated that under brain ischaemic stress, PACAP acted in neurons through the inhibition of JNK (c-Jun N-terminal kinase)/SAPK (stress-activated protein kinase) and p38 signalling pathways (Dohi et al., 2002). Furthermore, this latter action was also accompanied by blockade of DNA fragmentation and inhibition of caspase-3 activation, the key enzyme of the apoptosis process (Vaudry et al., 2000, 2002a, 2003; Onoue et al., 2002a). The effect of PACAP was mimicked by cAMP analogues, and abrogated by inhibitors of PKA and PKC, which implies that the process of caspase-3 de-activation may be mediated via both the AC/cAMP/PKA and PLC/IP3/DAG (diacylglycerol)/PKC signalling pathways (Vaudry et al., 2000; Onoue et al., 2002a). It was observed that PACAP, in addition to direct stimulation of neurons, can also affect them indirectly. For example, in the ischaemic region, the peptide stimulated astrocytes that secrete IL-6 into the cerebrospinal fluid. IL-6, similar to PACAP, inhibited the activation of JNK/SAPK in neuronal cells (Shioda et al., 1998). Finally, it has been suggested that PACAP protects neurons from death after ischaemia through induction of IL-6 and consequent activation of the STAT3 and ERK (extracellular-signal-regulated kinase) signalling pathways (Ohtaki et al., 2006).
Growth factor-like actions of PACAP are reviewed in Dejda et al. (2005) and Shioda et al. (2006).
Growth factor-like actions of VIP
Well before the discovery of PACAP in 1989, the first report of VIP neuroprotection appeared, showing that subnanomolar concentrations of VIP rescued neurons in primary embryonic spinal cord cultures deprived of electrical activity (Brenneman et al., 1985; Brenneman and Eiden, 1986). Later, it was reported that VIP provides neuronal defence by inducing the synthesis and secretion of diverse neuroprotective glia-derived trophic substances such as nerve growth factor (Hill et al., 2002), cytokines (Brenneman et al., 2003), chemokines (Brenneman et al., 2000a), protease nexin-1 (Festoff et al., 1996) and particularly ADNF (activity-dependent neurotrophic factor; Brenneman and Gozes, 1996) and ADNP (activity-dependent neuroprotective protein; Sigalov et al., 2000). ADNF is reported to be a potent neuroprotective astroglia-derived factor (Brenneman et al., 1996). Two fragments of ADNF, ADNF-14 and ADNF-9, prevented cell death induced by gp120 (HIV envelope protein), β-amyloid (fragment 25–35) and activation of glutamate NMDA (N-methyl-d-aspartate) type receptors, being effective at femtomolar concentrations (Brenneman et al., 1996, 1998, 1999, 2000b). Moreover, ADNF-9 was reported to exhibit greater potency and efficacy in preventing cell death than ADNF-14 or the parent protein ADNF (Brenneman et al., 1996, 2000b). In addition, experimental data showed that ADNP mRNA concentration in the rat astrocytes was increased 2–3-fold after a short exposure to VIP (Bassan et al., 1999; Gozes et al., 2003). In cerebral cortical cultures exposed to TTX (tetrodotoxin), gp120, β-amyloid or NMDA, NAP (neutrophil-activating protein), an 8-amino acid fragment of ADNP, exhibited stronger neuroprotective efficacy than ADNF-9 itself (Gozes et al., 2003).
In vivo studies have corroborated the in vitro actions of ADNF-9 and NAP described as follows: (i) ADNF-9 has been shown to protect against gp120 in a model mimicking intrauterine growth retardation which is common in infants born to HIV-positive mothers (Dibbern et al., 1997) and (ii) NAP improved the developmental reflexes and prevented short-term memory deficits in a model of ApoE (apolipoprotein E)-deficient mice for AD (Bassan et al., 1999). Other investigations have demonstrated that ADNF-9 may act as an endogenous growth-regulating factor during embryonic development (Hill et al., 1999). NAP and ADNF-9 prevented cellular oxidative stress in a mouse model of fetal alcohol syndrome where pregnant mice were exposed to ethanol during mid-gestation (E8) (Spong et al., 2001). Finally, in the PMCAO model, NAP provided a significant protection against neuronal damage associated with stroke by inhibiting key factors inducing apoptosis of neuronal cells (Leker et al., 2002).
In other studies, VIP was shown to inhibit pro-inflammatory mediators released by activated microglia, which purportedly contribute to the neuronal cell death in neurodegenerative disorders. VIP also significantly blocked the microglia activation and production of neurotoxic factors including TNFα, IL-1β and NO (nitric oxide) in a model of Parkinson's disease and brain trauma (Delgado and Ganea, 2003a, 2003b). Recently, it has been demonstrated that VIP has a unique and potent neuroprotective action of the perinatal white matter against excitotoxic insults that is not recapitulated by PACAP (Gressens et al., 1998). Moreover, when given systemically, VIP and stable VIP agonists appeared to promote axonal regrowth in this model through VPAC2 receptors and PKC and MAPK pathways (Passemard et al., 2011). On the other hand, VIP cytoprotective effects in some contexts have been attributed to action on the PAC1, specifically on the Hop2 splice variant of PAC1 (Pilzer and Gozes, 2006). For example, the Hop2 splice variant has been shown to be expressed and prevent toxicity during oxidative stress (Pilzer and Gozes, 2006) and ischaemic injury (Shioda et al., 2006).
Growth factor-like actions of VIP are reviewed in Waschek (1995) and Dejda et al. (2005).
Taken together, interestingly, unlike the most current and emerging therapeutic agents designed for the treatment of MS, the therapeutic mechanisms of VIP and PACAP peptides are not only delimited to their remarkable anti-inflammatory function but also to their potent growth factor-like and specifically neuroprotective effects.
PHARMACOLOGY AND DRUG DESIGN
Based on the above observations, VIP, PACAP, and their receptors are currently being considered as therapeutic targets for MS and other chronic inflammatory disorders. However, a major drawback with the use of native peptides in therapy is their high sensitivity to protease degradation and ultimately their bioavailability. Indeed, VIP is a 28-amino acid peptide that undergoes rapid degradation in vivo with a t½ of less than 1 min in the blood (Domschke et al., 1978). Moreover, the 38-residue isoform of PACAP displays a t½ of less than 5 min in isolated human plasma, whereas PACAP27 is relatively resistant to degradation in vitro (Bourgault et al., 2008a). This observation suggests that the 28–38 region is essential for the degradation of PACAP (Bourgault et al., 2008a). To circumvent this problem, recent data indicated that acetylation of the N-terminal end of PACAP confers resistance to DPPIV (dipeptidyl peptidase IV) (Bourgault et al., 2008a). Other strategies have been developed to protect the peptide against degradation by insertion of VIP into micelles or nanoparticles (Fernandez-Montesinos et al., 2009; Onyuksel et al., 2009). A second major obstacle that reduces the therapeutic use of VIP and PACAP in humans is their relative ability to act on multiple VIP and PACAP receptor subtypes, or when given at high doses even on other class B GPCRs (Laburthe et al., 2007; Vaudry et al., 2009). These cross-interactions may result in peripheral side effects induced by VIP and PACAP (Laburthe et al., 2007; Vaudry et al., 2009). Strategies to overcome these compromising conditions include the development of specific and metabolically stable ligands of VPAC1, VPAC2, and PAC1 receptors.
Regarding VPAC receptors, most of the structure–activity relationship analyses so far have been carried out with VIP and PACAP derivatives and have contributed to the development of valuable pharmacological tools that discriminate between VPAC1 and VPAC2 (Laburthe and Couvineau, 2002; Laburthe et al., 2007; Vaudry et al., 2009). For instance, the PACAP (6–38) fragment exhibits a 15-fold higher affinity for VPAC2 than for VPAC1 (Gourlet et al., 1995), whereas PACAP (1–25) possesses a 66-fold higher affinity for VPAC1 than for VPAC2 (Gourlet et al., 1998). Thus, a complete alanine scanning (Nicole et al., 2000; Igarashi et al., 2002a, 2002b) as well as D-amino acid scanning of VIP (Igarashi et al., 2002a, 2002b) allowed the rationally design of selective, high affinity and metabolically stable analogues of VPAC receptors. The most potent and specific peptide agonist for VPAC1 receptor currently available is [Ala11,22,28]-VIP (Nicole et al., 2000). Moreover, two analogues ([Ala2,8,9,11,19,22,24,25,27,28])VIP and [Ala2,8,9,11,19,24−28]VIP) were found to have high selectivity for hVPAC1 (Onoue et al., 2011). [Ala2,8,9,16,19,24,25]VIP agonist for VPAC2 was much more metabolically stable than VIP (Onoue et al., 2011). With regard to antagonists, [Ac-His1D-Phe2,K15R16L27]VIP(3–7)/GRF(8–27) (also called PG 97–269) is a selective high-affinity antagonist of the VPAC1 receptor (Gourlet et al., 1997a). Regarding the VPAC2 receptor, the cyclic peptide analogue of VIP [Ac-Glu8OCH3-Tyr10Lys12Nle17Ala19Asp25Leu26-Lys27,28-VIP(cyclo 21–25)] or Ro 25–1392 is a potent and selective agonist (Xia et al., 1997). Likewise, the VIP analogue RO 25–1553, that possesses a C-terminally extended tail and an α-helix-stabilizing lactam bridge between residues 21 and 25, behaves as a selective VPAC2 agonist (Bolin et al., 1995; Gourlet et al., 1997b). In our opinion, there is still no highly selective VPAC2 receptor antagonist because PG 99-465, a VIP analogue that antagonizes VIP action on the VPAC2 receptor, has significant agonist activity on human VPAC1 receptor (Moreno et al., 2000). Together, these data suggest that the C-terminal helical domains of PACAP and VIP are important for the binding affinity towards VPAC2, whereas, conversely, VPAC1 seems tolerant to deletion at the C terminus.
Regarding PAC1 receptors, as for VPAC receptors, an extensive set of structure–activity relationship studies was performed combining alanine scanning, directed mutagenesis, deletion and/or chimaerism analyses. Thus, numerous PACAP analogues have been synthesized to identify the molecular determinants responsible for the recognition and activation of the receptors (Bourgault et al., 2009a). In 1991, a natural 61-amino acid vasodilator polypeptide called maxadilan was isolated from the salivary gland extracts of the blood-feeding sand fly (Lerner et al., 1991). Later, although there is a lack of primary sequence homology with PACAP, maxadilan was definitively characterized as a potent and highly selective agonist of PAC1 (Moro and Lerner, 1997; Lerner et al., 2007). A shortened maxadilan synthetic analogue, termed as M65, in which the amino acid sequence 25–41 was deleted, acts as a specific PAC1 antagonist (Uchida et al., 1998; Moro et al., 1999; Lerner et al., 2007). As previously reported, the N-terminal region of PACAP seems to play a crucial role for the biological activity of the peptide (Gourlet et al., 1991; Robberecht et al., 1992a; Bitar and Coy, 1993). Indeed, gradual deletion of the N-terminal residues of PACAP38 showed that PACAP(6–38) is a potent antagonist (Robberecht et al., 1992b). Moreover, the chemical modification of PACAP38 led to the development of [R15, 20, 21,L17]-PACAP38 which exhibited enhanced stability with a 42% reduction in degradation kinetics compared with that of PACAP38 and biological activity (Onoue et al., 2011). As discussed, PACAP27 and VIP possess a high degree of sequence homology (68%). However, VIP is not able to bind to PAC1 efficiently. Because sequence differences between VIP and PACAP are restricted to regions 4–13 and 24–28, the PAC1 selectivity should reside within these two regions. Synthesis and pharmacological characterization of VIP/PACAP chimaeras showed that the selectivity of PAC1 towards PACAP implicates not the C-terminal domain but rather the chemical motifs of the 4–13 region (Schafer et al., 1999; Onoue et al., 2001).
Although a multitude selective peptide agonists and, to a lesser extent, antagonists have already been described, the development of potent small non-peptide molecules acting on VIP/PACAP receptors is a challenging goal. Indeed, the hydrazides have been discovered recently as specific antagonist for PAC1 receptor, and are the first potent small molecule antagonists ever reported for VIP/PACAP receptors (Beebe et al., 2008). In parallel, the recent finding of N-cap motifs which orientate the N-terminal part of members of VIP/secretin family, and are presumably involved in receptor activation, may serve as a template for a new rational drug design. Thus, the further structural analyses and structure–activity relationship studies of the receptors should be very helpful to understand the mechanism of activation of the VIP/PACAP receptors, and ultimately for the design of new non-peptide drugs targeting VIP and PACAP receptors.
CONCLUSION
Current and emerging therapeutic agents have so far been primarily designed to suppress the immune response in MS patients. They are capable of reducing the severity of early stage MS and slowing its progression, but without having significant action during the secondary phase of the disorder. Thus, it is important to find new promising therapeutic tools acting also on the neurodegenerative aspect of MS physiopathology. VIP and PACAP exhibit not only a potent anti-inflammatory action, but also remarkable neurotrophic and neuroprotective actions. These peptides and their receptors may modulate both the excess of inflammation and the neurodegenerative processes associated with MS. Recently, the development of VIP and PACAP-related drugs in the treatment of multiple sclerosis has become a new challenge.
Footnotes
This work was supported by National Multiple Sclerosis Society Grants [grant numbers RG3928 and TA 3048-A-1].
REFERENCES
- Abad C, Juarranz Y, Martinez C, Arranz A, Rosignoli F, Garcia-Gomez M, Leceta J, Gomariz RP. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflamm Bowel Dis. 2005;11:674–684. doi: 10.1097/01.mib.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M. Pituitary adenylate cyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol. 2001;167:3182–3189. doi: 10.4049/jimmunol.167.6.3182. [DOI] [PubMed] [Google Scholar]
- Abad C, Martinez C, Leceta J, Juarranz MG, Delgado M, Gomariz RP. Pituitary adenylate-cyclase-activating polypeptide expression in the immune system. Neuroimmunomodulation. 2002;10:177–186. doi: 10.1159/000067180. [DOI] [PubMed] [Google Scholar]
- Abad C, Tan YV, Lopez R, Nobuta H, Dong H, Phan P, Feng JM, Campagnoni AT, Waschek JA. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:19555–19560. doi: 10.1073/pnas.1007622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Nobuta H, Waschek JA. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Lee M, Chhith S, Gomariz RP, Waschek JA. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience. 2004;129:93–99. doi: 10.1016/j.neuroscience.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Barrie AP, Clohessy AM, Buensuceso CS, Rogers MV, Allen JM. Pituitary adenylyl cyclase-activating peptide stimulates extracellular signal-regulated kinase 1 or 2 (ERK1/2) activity in a Ras-independent, mitogen-activated protein Kinase/ERK kinase 1 or 2-dependent manner in PC12 cells. J Biol Chem. 1997;272:19666–19671. doi: 10.1074/jbc.272.32.19666. [DOI] [PubMed] [Google Scholar]
- Barten LJ, Allington DR, Procacci KA, Rivey MP. New approaches in the management of multiple sclerosis. Drug Des Dev Ther. 2010;4:343–366. doi: 10.2147/DDDT.S9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, Bassan H, Blat C, Gibney G, Glazner G, Brenneman DE, Gozes I. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- Beebe X, Darczak D, Davis-Taber RA, Uchic ME, Scott VE, Jarvis MF, Stewart AO. Discovery and SAR of hydrazide antagonists of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor type 1 (PAC1-R). Bioorg Med Chem Lett. 2008;18:2162–2166. doi: 10.1016/j.bmcl.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Bitar KG, Coy DH. Interaction of ovine pituitary adenylate cyclase-activating peptide (PACAP-38) with rat lung membranes. Peptides. 1993;14:621–627. doi: 10.1016/0196-9781(93)90154-9. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP). Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bolin DR, Michalewsky J, Wasserman MA, O'Donnell M. Design and development of a vasoactive intestinal peptide analog as a novel therapeutic for bronchial asthma. Biopolymers. 1995;37:57–66. doi: 10.1002/bip.360370203. [DOI] [PubMed] [Google Scholar]
- Bourgault S, Vaudry D, Botia B, Couvineau A, Laburthe M, Vaudry H, Fournier A. Novel stable PACAP analogs with potent activity towards the PAC1 receptor. Peptides. 2008a;29:919–932. doi: 10.1016/j.peptides.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Bourgault S, Vaudry D, Dejda A, Doan ND, Vaudry H, Fournier A. Pituitary adenylate cyclase-activating polypeptide: focus on structure-activity relationships of a neuroprotective Peptide. Curr Med Chem. 2009a;16:4462–4480. doi: 10.2174/092986709789712899. [DOI] [PubMed] [Google Scholar]
- Bourgault S, Vaudry D, Guilhaudis L, Raoult E, Couvineau A, Laburthe M, Segalas-Milazzo I, Vaudry H, Fournier A. Biological and structural analysis of truncated analogs of PACAP27. J Mol Neurosci. 2008b;36:260–269. doi: 10.1007/s12031-008-9081-7. [DOI] [PubMed] [Google Scholar]
- Bourgault S, Vaudry D, Segalas-Milazzo I, Guilhaudis L, Couvineau A, Laburthe M, Vaudry H, Fournier A. Molecular and conformational determinants of pituitary adenylate cyclase-activating polypeptide (PACAP) for activation of the PAC1 receptor. J Med Chem. 2009b;52:3308–3316. doi: 10.1021/jm900291j. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Eiden LE. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc Natl Acad Sci USA. 1986;83:1159–1162. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Eiden LE, Siegel RE. Neurotrophic action of VIP on spinal cord cultures. Peptides. 1985;6(Suppl. 2):35–39. doi: 10.1016/0196-9781(85)90132-9. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Glazner G, Hill JM, Hauser J, Davidson A, Gozes I. VIP neurotrophism in the central nervous system: multiple effectors and identification of a femtomolar-acting neuroprotective peptide. Ann NY Acad Sci. 1998;865:207–212. doi: 10.1111/j.1749-6632.1998.tb11180.x. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Gozes I. A femtomolar-acting neuroprotective peptide. J Clin Invest. 1996;97:2299–2307. doi: 10.1172/JCI118672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Hauser J, Phillips TM, Davidson A, Bassan M, Gozes I. Vasoactive intestinal peptide.Link between electrical activity and glia-mediated neurotrophism. Ann NY Acad Sci. 1999;897:17–26. doi: 10.1111/j.1749-6632.1999.tb07875.x. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Hauser J, Spong CY, Phillips TM. Chemokines released from astroglia by vasoactive intestinal peptide.Mechanism of neuroprotection from HIV envelope protein toxicity. Ann NY Acad Sci. 2000a;921:109–114. doi: 10.1111/j.1749-6632.2000.tb06956.x. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Hauser JM, Spong C, Phillips TM. Chemokine release is associated with the protective action of PACAP-38 against HIV envelope protein neurotoxicity. Neuropeptides. 2002;36:271–280. doi: 10.1016/s0143-4179(02)00045-8. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Phillips TM, Hauser J, Hill JM, Spong CY, Gozes I. Complex array of cytokines released by vasoactive intestinal peptide. Neuropeptides. 2003;37:111–119. doi: 10.1016/s0143-4179(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Spong CY, Gozes I. Protective peptides derived from novel glial proteins. Biochem Soc Trans. 2000b;28:452–455. [PubMed] [Google Scholar]
- Buscail L, Gourlet P, Cauvin A, De Neef P, Gossen D, Arimura A, Miyata A, Coy DH, Robberecht P, Christophe J. Presence of highly selective receptors for PACAP (pituitary adenylate cyclase activating peptide) in membranes from the rat pancreatic acinar cell line AR 4–2J. FEBS Lett. 1990;262:77–81. doi: 10.1016/0014-5793(90)80158-f. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Kojro E, Jasionowski M, Lankiewicz L, Grzonka Z, Fahrenholz F. Identification of binding domains of pituitary adenylate cyclase activating polypeptide (PACAP) for its type 1 receptor by photoaffinity labeling. Ann NY Acad Sci. 1998;865:82–91. doi: 10.1111/j.1749-6632.1998.tb11166.x. [DOI] [PubMed] [Google Scholar]
- Ceraudo E, Murail S, Tan YV, Lacapere JJ, Neumann JM, Couvineau A, Laburthe M. The vasoactive intestinal peptide (VIP) alpha-Helix up to C terminus interacts with the N-terminal ectodomain of the human VIP/Pituitary adenylate cyclase-activating peptide 1 receptor: photoaffinity, molecular modeling, and dynamics. Mol Endocrinol. 2008a;22:147–155. doi: 10.1210/me.2007-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceraudo E, Tan YV, Nicole P, Couvineau A, Laburthe M. The N-terminal parts of VIP and antagonist PG97–269 physically interact with different regions of the human VPAC1 receptor. J Mol Neurosci. 2008b;36:245–248. doi: 10.1007/s12031-008-9073-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Sharma RV, Fisher RA. Molecular cloning of a novel variant of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor that stimulates calcium influx by activation of L-type calcium channels. J Biol Chem. 1996;271:32226–32232. doi: 10.1074/jbc.271.50.32226. [DOI] [PubMed] [Google Scholar]
- Chen WH, Tzeng SF. Pituitary adenylate cyclase-activating polypeptide prevents cell death in the spinal cord with traumatic injury. Neurosci Lett. 2005;384:117–121. doi: 10.1016/j.neulet.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci USA. 2005;102:13562–13567. doi: 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, Kuwasako K, Tilakaratne N, Sexton PM. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging – measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol. 2001;49:290–297. [PubMed] [Google Scholar]
- Couvineau A, Ceraudo E, Tan YV, Laburthe M. VPAC1 receptor binding site: contribution of photoaffinity labeling approach. Neuropeptides. 2010;44:127–132. doi: 10.1016/j.npep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Rouyer-Fessard C, Darmoul D, Maoret JJ, Carrero I, Ogier-Denis E, Laburthe M. Human intestinal VIP receptor: cloning and functional expression of two cDNA encoding proteins with different N-terminal domains. Biochem Biophys Res Commun. 1994;200:769–776. doi: 10.1006/bbrc.1994.1517. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Mevenkamp G, Wille S, Hauger RL. N-terminal splice variants of the type I PACAP receptor: isolation, characterization and ligand binding/selectivity determinants. J Neuroendocrinol. 1999;11:941–949. doi: 10.1046/j.1365-2826.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- Dejda A, Bourgault S, Doan ND, Letourneau M, Couvineau A, Vaudry H, Vaudry D, Fournier A. Identification by photoaffinity labeling of the extracellular N-terminal domain of PAC1 receptor as the major binding site for PACAP. Biochimie. 2011;93:669–677. doi: 10.1016/j.biochi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Dejda A, Sokolowska P, Nowak JZ. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol Rep. 2005;57:307–320. [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit interleukin-12 transcription by regulating nuclear factor kappaB and Ets activation. J Biol Chem. 1999;274:31930–31940. doi: 10.1074/jbc.274.45.31930. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of IFN-gamma-induced janus kinase-1-STAT1 activation in macrophages by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. J Immunol. 2000;165:3051–3057. doi: 10.4049/jimmunol.165.6.3051. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J Immunol. 2001;167:966–975. doi: 10.4049/jimmunol.167.2.966. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson's disease by blocking microglial activation. FASEB J. 2003a;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. FASEB J. 2003b;17:1922–1924. doi: 10.1096/fj.02-1029fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide stimulate the induction of Th2 responses by up-regulating B7.2 expression. J Immunol. 1999a;163:3629–3635. [PubMed] [Google Scholar]
- Delgado M, Martinez C, Pozo D, Calvo JR, Leceta J, Ganea D, Gomariz RP. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-alpha and IL-6. J Immunol. 1999b;162:1200–1205. [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J Immunol. 1999c;162:1707–1716. [PubMed] [Google Scholar]
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFNgamma synthesis by T cells. J Neuroimmunol. 1999d;96:167–181. doi: 10.1016/s0165-5728(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Martinez C, Leceta J, Calvo JR, Ganea D, Gomariz RP. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit endotoxin-induced TNF-alpha production by macrophages: in vitro and in vivo studies. J Immunol. 1999e;162:2358–2367. [PubMed] [Google Scholar]
- Delgado M, Sun W, Leceta J, Ganea D. VIP and PACAP differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7.1 and B7.2 expression. J Immunol. 1999f;163:4213–4223. [PubMed] [Google Scholar]
- Delgado M, Gomariz RP, Martinez C, Abad C, Leceta J. Anti-inflammatory properties of the type 1 and type 2 vasoactive intestinal peptide receptors: role in lethal endotoxic shock. Eur J Immunol. 2000;30:3236–3246. doi: 10.1002/1521-4141(200011)30:11<3236::AID-IMMU3236>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide promote in vivo generation of memory Th2 cells. FASEB J. 2002;16:1844–1846. doi: 10.1096/fj.02-0248fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E, Ganea D. VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. FASEB J. 2004a;18:1453–1455. doi: 10.1096/fj.04-1548fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004b;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Delgado M, Reduta A, Sharma V, Ganea D. VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J Leukoc Biol. 2004c;75:1122–1130. doi: 10.1189/jlb.1203626. [DOI] [PubMed] [Google Scholar]
- Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. J Immunol. 2005;175:7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- Dibbern DA, Jr., Glazner GW, Gozes I, Brenneman DE, Hill JM. Inhibition of murine embryonic growth by human immunodeficiency virus envelope protein and its prevention by vasoactive intestinal peptide and activity-dependent neurotrophic factor. J Clin Invest. 1997;99:2837–2841. doi: 10.1172/JCI119476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Deutsch PJ, Maltzman J, Zhang J, Pintar JE, Zheng J, Friedman WF, Zhou X, Zaremba T. Autocrine expression and ontogenetic functions of the PACAP ligand/receptor system during sympathetic development. Dev Biol. 2000;219:197–213. doi: 10.1006/dbio.2000.9604. [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: From ligands to function. Pharmacol Ther. 2009;121:294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- Dohi K, Mizushima H, Nakajo S, Ohtaki H, Matsunaga S, Aruga T, Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) prevents hippocampal neurons from apoptosis by inhibiting JNK/SAPK and p38 signal transduction pathways. Regul Pept. 2002;109:83–88. doi: 10.1016/s0167-0115(02)00190-8. [DOI] [PubMed] [Google Scholar]
- Domschke S, Domschke W, Bloom SR, Mitznegg P, Mitchell SJ, Lux G, Strunz U. Vasoactive intestinal peptide in man: pharmacokinetics, metabolic and circulatory effects. Gut. 1978;19:1049–1053. doi: 10.1136/gut.19.11.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, Brochet B, Berry I, Rolland Y, Froment JC, Cabanis E, Iba-Zizen MT, Gandon JM, Lai HM, Moseley I, Sabouraud O. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62:112–118. doi: 10.1136/jnnp.62.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell RA, Giovannoni G. Current and future role of interferon beta in the therapy of multiple sclerosis. J Interferon Cytokine Res. 2010;30:715–726. doi: 10.1089/jir.2010.0089. [DOI] [PubMed] [Google Scholar]
- Fernandez-Martin A, Gonzalez-Rey E, Chorny A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory T cells during experimental autoimmune encephalomyelitis. Eur J Immunol. 2006;36:318–326. doi: 10.1002/eji.200535430. [DOI] [PubMed] [Google Scholar]
- Fernandez-Montesinos R, Castillo PM, Klippstein R, Gonzalez-Rey E, Mejias JA, Zaderenko AP, Pozo D. Chemical synthesis and characterization of silver-protected vasoactive intestinal peptide nanoparticles. Nanomedicine (Lond) 2009;4:919–930. doi: 10.2217/nnm.09.79. [DOI] [PubMed] [Google Scholar]
- Festoff BW, Nelson PG, Brenneman DE. Prevention of activity-dependent neuronal death: vasoactive intestinal polypeptide stimulates astrocytes to secrete the thrombin-inhibiting neurotrophic serpin, protease nexin I. J Neurobiol. 1996;30:255–266. doi: 10.1002/(SICI)1097-4695(199606)30:2<255::AID-NEU7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rovaris M, Rocca MA, Sormani MP, Wolinsky JS, Comi G. Glatiramer acetate reduces the proportion of new MS lesions evolving into ‘black holes’. Neurology. 2001;57:731–733. doi: 10.1212/wnl.57.4.731. [DOI] [PubMed] [Google Scholar]
- Fox EJ. Emerging oral agents for multiple sclerosis. Am J Manag Care. 2010;16:S219–S226. [PubMed] [Google Scholar]
- Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Martinez-Fuentes AJ, Garcia-Navarro F, Vaudry H, Aguilar E. Pituitary adenylate cyclase-activating peptide (PACAP) immunolocalization in lymphoid tissues of the rat. Cell Tissue Res. 1994;276:223–227. doi: 10.1007/BF00306107. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Udupa JK, Fulton J, Constantinescu CS, Gonzales-Scarano F, Babb JS, Mannon LJ, Kolson DL, Cohen JA. Glatiramer acetate (Copaxone) treatment in relapsing-remitting MS: quantitative MR assessment. Neurology. 2000;54:813–817. doi: 10.1212/wnl.54.4.813. [DOI] [PubMed] [Google Scholar]
- Gee HY, Kim YW, Jo MJ, Namkung W, Kim JY, Park HW, Kim KS, Kim H, Baba A, Yang J, Kim E, Kim KH, Lee MG. Synaptic scaffolding molecule binds to and regulates vasoactive intestinal polypeptide type-1 receptor in epithelial cells. Gastroenterology 137. 2009;607–617:e601–604. doi: 10.1053/j.gastro.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sorensen P, Vermersch P, Chang P, Hamlett A, Musch B, Greenberg SJ. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Voice JK, Shen S, Dorsam G, Kong Y, West KM, Morrison CF, Harmar AJ. Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC(2) receptor for vasoactive intestinal peptide. Proc Natl Acad Sci USA. 2001;98:13854–13859. doi: 10.1073/pnas.241503798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomariz RP, Delgado M, Naranjo JR, Mellstrom B, Tormo A, Mata F, Leceta J. VIP gene expression in rat thymus and spleen. Brain Behav Immun. 1993;7:271–278. doi: 10.1006/brbi.1993.1027. [DOI] [PubMed] [Google Scholar]
- Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr Pharm Des. 2001;7:89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006a;107:3632–3638. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006b;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Varela N, Chorny A, Delgado M. Therapeutical approaches of vasoactive intestinal peptide as a pleiotropic immunomodulator. Curr Pharm Des. 2007;13:1113–1139. doi: 10.2174/138161207780618966. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Frohman EM, Garmany GP, Jr., Halper J, Likosky WH, Lublin FD, Silberberg DH, Stuart WH, van den Noort S. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58:169–178. doi: 10.1212/wnl.58.2.169. [DOI] [PubMed] [Google Scholar]
- Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, Marinucci LN, Blight AR. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373:732–738. doi: 10.1016/S0140-6736(09)60442-6. [DOI] [PubMed] [Google Scholar]
- Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997a;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Van Rampelbergh J, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Analogues of VIP, helodermin, and PACAP discriminate between rat and human VIP1 and VIP2 receptors. Ann NY Acad Sci. 1998;865:247–252. doi: 10.1111/j.1749-6632.1998.tb11184.x. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Vandermeers-Piret MC, Rathe J, De Neef P, Robberecht P. Fragments of pituitary adenylate cyclase activating polypeptide discriminate between type I and II recombinant receptors. Eur J Pharmacol. 1995;287:7–11. doi: 10.1016/0014-2999(95)00467-5. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vertongen P, Vandermeers A, Vandermeers-Piret MC, Rathe J, De Neef P, Waelbroeck M, Robberecht P. The long-acting vasoactive intestinal polypeptide agonist RO 25–1553 is highly selective of the VIP2 receptor subclass. Peptides. 1997b;18:403–408. doi: 10.1016/s0196-9781(96)00322-1. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Woussen-Colle MC, Robberecht P, de Neef P, Cauvin A, Vandermeers-Piret MC, Vandermeers A, Christophe J. Structural requirements for the binding of the pituitary adenylate-cyclase-activating peptide to receptors and adenylate-cyclase activation in pancreatic and neuronal membranes. Eur J Biochem. 1991;195:535–541. doi: 10.1111/j.1432-1033.1991.tb15734.x. [DOI] [PubMed] [Google Scholar]
- Gozes I, Brenneman DE. A new concept in the pharmacology of neuroprotection. J Mol Neurosci. 2000;14:61–68. doi: 10.1385/JMN:14:1-2:061. [DOI] [PubMed] [Google Scholar]
- Gozes I, Divinsky I, Pilzer I, Fridkin M, Brenneman DE, Spier AD. From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: a view of neuroprotection and cell division. J Mol Neurosci. 2003;20:315–322. doi: 10.1385/JMN:20:3:315. [DOI] [PubMed] [Google Scholar]
- Grace CR, Perrin MH, DiGruccio MR, Miller CL, Rivier JE, Vale WW, Riek R. NMR structure and peptide hormone binding site of the first extracellular domain of a type B1 G protein-coupled receptor. Proc Natl Acad Sci USA. 2004;101:12836–12841. doi: 10.1073/pnas.0404702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressens P, Marret S, Martin JL, Laquerriere A, Lombet A, Evrard P. Regulation of neuroprotective action of vasoactive intestinal peptide in the murine developing brain by protein kinase C and mitogen-activated protein kinase cascades: in vivo and in vitro studies. J Neurochem. 1998;70:2574–2584. doi: 10.1046/j.1471-4159.1998.70062574.x. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Springer J, Fischer A. Vasoactive intestinal polypeptide as mediator of asthma. Pulm Pharmacol Ther. 2001;14:391–401. doi: 10.1006/pupt.2001.0306. [DOI] [PubMed] [Google Scholar]
- Gronenborn AM, Bovermann G, Clore GM. A 1H-NMR study of the solution conformation of secretin.Resonance assignment and secondary structure. FEBS Lett. 1987;215:88–94. doi: 10.1016/0014-5793(87)80119-9. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Morfis MM, Lisenbee CS, Sexton PM, Miller LJ. Constitutive formation of oligomeric complexes between family B G protein-coupled vasoactive intestinal polypeptide and secretin receptors. Mol Pharmacol. 2006;69:363–373. doi: 10.1124/mol.105.015776. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Hartung HP, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, Krapf H, Zwingers T. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–2025. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Ishihara T, Shigemoto R, Mori K, Nagata S. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron. 1993;11:333–342. doi: 10.1016/0896-6273(93)90188-w. [DOI] [PubMed] [Google Scholar]
- Hill JM, Glazner GW, Lee SJ, Gozes I, Gressens P, Brenneman DE. Vasoactive intestinal peptide regulates embryonic growth through the action of activity-dependent neurotrophic factor. Ann NY Acad Sci. 1999;897:92–100. doi: 10.1111/j.1749-6632.1999.tb07881.x. [DOI] [PubMed] [Google Scholar]
- Hill JM, Mehnert J, McCune SK, Brenneman DE. Vasoactive intestinal peptide regulation of nerve growth factor in the embryonic mouse. Peptides. 2002;23:1803–1808. doi: 10.1016/s0196-9781(02)00137-7. [DOI] [PubMed] [Google Scholar]
- Hoare SR. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov Today. 2005;10:417–427. doi: 10.1016/S1359-6446(05)03370-2. [DOI] [PubMed] [Google Scholar]
- Hosoya M, Onda H, Ogi K, Masuda Y, Miyamoto Y, Ohtaki T, Okazaki H, Arimura A, Fujino M. Molecular cloning and functional expression of rat cDNAs encoding the receptor for pituitary adenylate cyclase activating polypeptide (PACAP). Biochem Biophys Res Commun. 1993;194:133–143. doi: 10.1006/bbrc.1993.1795. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Ito T, Hou W, Mantey SA, Pradhan TK, Ulrich CD, 2nd, Hocart SJ, Coy DH, Jensen RT. Elucidation of vasoactive intestinal peptide pharmacophore for VPAC(1) receptors in human, rat, and guinea pig. J Pharmacol Exp Ther. 2002a;301:37–50. doi: 10.1124/jpet.301.1.37. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Ito T, Pradhan TK, Mantey SA, Hou W, Coy DH, Jensen RT. Elucidation of the vasoactive intestinal peptide pharmacophore for VPAC(2) receptors in human and rat and comparison to the pharmacophore for VPAC(1) receptors. J Pharmacol Exp Ther. 2002b;303:445–460. doi: 10.1124/jpet.102.038075. [DOI] [PubMed] [Google Scholar]
- Inooka H, Ohtaki T, Kitahara O, Ikegami T, Endo S, Kitada C, Ogi K, Onda H, Fujino M, Shirakawa M. Conformation of a peptide ligand bound to its G-protein coupled receptor. Nat Struct Biol. 2001;8:161–165. doi: 10.1038/84159. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, Fischer JS, Goodkin DE, Granger CV, Simon JH, Alam JJ, Bartoszak DM, Bourdette DN, Braiman J, Brownscheidle CM, Coats ME, Cohan SL, Dougherty DS, Kinkel RP, Mass MK, Munschauer III FE, Priore RL, Pullicino PM, Scherokman BJ, Whitham RH. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Jiang X, Jing H, Ganea D. VIP and PACAP down-regulate CXCL10 (IP-10) and up-regulate CCL22 (MDC) in spleen cells. J Neuroimmunol. 2002;133:81–94. doi: 10.1016/s0165-5728(02)00365-x. [DOI] [PubMed] [Google Scholar]
- Juarranz Y, Abad C, Martinez C, Arranz A, Gutierrez-Canas I, Rosignoli F, Gomariz RP, Leceta J. Protective effect of vasoactive intestinal peptide on bone destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1034–1045. doi: 10.1186/ar1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SI, Bever CT, Jr. Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther. 2006;111:224–259. doi: 10.1016/j.pharmthera.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kalsi JS, Cellek S, Muneer A, Kell PD, Ralph DJ, Minhas S. Current oral treatments for erectile dysfunction. Expert Opin Pharmacother. 2002;3:1613–1629. doi: 10.1517/14656566.3.11.1613. [DOI] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, Ueda R, Suzumura A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- Koh SW. Signal transduction through the vasoactive intestinal peptide receptor stimulates phosphorylation of the tyrosine kinase pp60c-src. Biochem Biophys Res Commun. 1991;174:452–458. doi: 10.1016/0006-291x(91)91437-h. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC Receptors for VIP and PACAP. Regul Pept. 2002;108:165–173. doi: 10.1016/s0167-0115(02)00099-x. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Gaudin P, Maoret JJ, Rouyer-Fessard C, Nicole P. Receptors for VIP, PACAP, secretin, GRF, glucagon, GLP-1, and other members of their new family of G protein-linked receptors: structure-function relationship with special reference to the human VIP-1 receptor. Ann NY Acad Sci. 1996;805:94–109. doi: 10.1111/j.1749-6632.1996.tb17476.x. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides. 2007;28:1631–1639. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Lara-Marquez M, O'Dorisio M, O'Dorisio T, Shah M, Karacay B. Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol. 2001;166:2522–2530. doi: 10.4049/jimmunol.166.4.2522. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page E, Leray E, Taurin G, Coustans M, Chaperon J, Morrissey SP, Edan G. Mitoxantrone as induction treatment in aggressive relapsing remitting multiple sclerosis: treatment response factors in a 5 year follow-up observational study of 100 consecutive patients. J Neurol Neurosurg Psychiatry. 2008;79:52–56. doi: 10.1136/jnnp.2007.124958. [DOI] [PubMed] [Google Scholar]
- Leceta J, Martinez C, Delgado M, Garrido E, Gomariz RP. Expression of vasoactive intestinal peptide in lymphocytes: a possible endogenous role in the regulation of the immune system. Adv Neuroimmunol. 1996;6:29–36. doi: 10.1016/s0960-5428(96)00001-0. [DOI] [PubMed] [Google Scholar]
- Leker RR, Teichner A, Grigoriadis N, Ovadia H, Brenneman DE, Fridkin M, Giladi E, Romano J, Gozes I. NAP, a femtomolar-acting peptide, protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2002;33:1085–1092. doi: 10.1161/01.str.0000014207.05597.d7. [DOI] [PubMed] [Google Scholar]
- Lelievre V, Pineau N, Du J, Wen CH, Nguyen T, Janet T, Muller JM, Waschek JA. Differential effects of peptide histidine isoleucine (PHI) and related peptides on stimulation and suppression of neuroblastoma cell proliferation. A novel VIP-independent action of PHI via MAP kinase. J Biol Chem. 1998;273:19685–19690. doi: 10.1074/jbc.273.31.19685. [DOI] [PubMed] [Google Scholar]
- Lerner EA, Iuga AO, Reddy VB. Maxadilan, a PAC1 receptor agonist from sand flies. Peptides. 2007;28:1651–1654. doi: 10.1016/j.peptides.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner EA, Ribeiro JM, Nelson RJ, Lerner MR. Isolation of maxadilan, a potent vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. J Biol Chem. 1991;266:11234–11236. [PubMed] [Google Scholar]
- Losy J, Kalinowska-Lyszczarz A. Emerging disease-modifying oral therapies for multiple sclerosis. J Neuroimmunol. 2011;231:15–22. doi: 10.1016/j.jneuroim.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Lu N, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide is an autocrine inhibitor of mitosis in cultured cortical precursor cells. Proc Natl Acad Sci USA. 1997;94:3357–3362. doi: 10.1073/pnas.94.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Mahon MJ, Shimada M. Calmodulin interacts with the cytoplasmic tails of the parathyroid hormone 1 receptor and a sub-set of class b G-protein coupled receptors. FEBS Lett. 2005;579:803–807. doi: 10.1016/j.febslet.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Martinez C, Abad C, Delgado M, Arranz A, Juarranz MG, Rodriguez-Henche N, Brabet P, Leceta J, Gomariz RP. Anti-inflammatory role in septic shock of pituitary adenylate cyclase-activating polypeptide receptor. Proc Natl Acad Sci USA. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez C, Delgado M, Pozo D, Leceta J, Calvo JR, Ganea D, Gomariz RP. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide modulate endotoxin-induced IL-6 production by murine peritoneal macrophages. J Leukoc Biol. 1998a;63:591–601. doi: 10.1002/jlb.63.5.591. [DOI] [PubMed] [Google Scholar]
- Martinez C, Delgado M, Pozo D, Leceta J, Calvo JR, Ganea D, Gomariz RP. VIP and PACAP enhance IL-6 release and mRNA levels in resting peritoneal macrophages: in vitro and in vivo studies. J Neuroimmunol. 1998b;85:155–167. doi: 10.1016/s0165-5728(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Martinez C, Juarranz Y, Abad C, Arranz A, Miguel BG, Rosignoli F, Leceta J, Gomariz RP. Analysis of the role of the PAC1 receptor in neutrophil recruitment, acute-phase response, and nitric oxide production in septic shock. J Leukoc Biol. 2005;77:729–738. doi: 10.1189/jlb.0704432. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Edvinsson L. Cerebral circulatory and metabolic effects of vasoactive intestinal polypeptide. Am J Physiol. 1980;238:H449–H456. doi: 10.1152/ajpheart.1980.238.4.H449. [DOI] [PubMed] [Google Scholar]
- Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Moody TW. Peptides and growth factors in non-small cell lung cancer. Peptides. 1996;17:545–555. doi: 10.1016/0196-9781(95)02148-5. [DOI] [PubMed] [Google Scholar]
- Moody TW, Hill JM, Jensen RT. VIP as a trophic factor in the CNS and cancer cells. Peptides. 2003;24:163–177. doi: 10.1016/s0196-9781(02)00290-5. [DOI] [PubMed] [Google Scholar]
- Moreno D, Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Development of selective agonists and antagonists for the human vasoactive intestinal polypeptide VPAC(2) receptor. Peptides. 2000;21:1543–1549. doi: 10.1016/s0196-9781(00)00309-0. [DOI] [PubMed] [Google Scholar]
- Morio H, Tatsuno I, Hirai A, Tamura Y, Saito Y. Pituitary adenylate cyclase-activating polypeptide protects rat-cultured cortical neurons from glutamate-induced cytotoxicity. Brain Res. 1996;741:82–88. doi: 10.1016/s0006-8993(96)00920-1. [DOI] [PubMed] [Google Scholar]
- Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem. 1997;272:966–970. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- Moro O, Wakita K, Ohnuma M, Denda S, Lerner EA, Tajima M. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem. 1999;274:23103–23110. doi: 10.1074/jbc.274.33.23103. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhang KM, Jin JG, Grider JR, Makhlouf GM. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol. 1993;265:G660–G671. doi: 10.1152/ajpgi.1993.265.4.G660. [DOI] [PubMed] [Google Scholar]
- Nakata M, Yada T. PACAP in the glucose and energy homeostasis: physiological role and therapeutic potential. Curr Pharm Des. 2007;13:1105–1112. doi: 10.2174/138161207780618948. [DOI] [PubMed] [Google Scholar]
- Neumann JM, Couvineau A, Murail S, Lacapere JJ, Jamin N, Laburthe M. Class-B GPCR activation: is ligand helix-capping the key? Trends Biochem Sci. 2008;33:314–319. doi: 10.1016/j.tibs.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Nicole P, Lins L, Rouyer-Fessard C, Drouot C, Fulcrand P, Thomas A, Couvineau A, Martinez J, Brasseur R, Laburthe M. Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a highly selective VPAC1 receptor agonist. Alanine scanning and molecular modeling of the peptide. J Biol Chem. 2000;275:24003–24012. doi: 10.1074/jbc.M002325200. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, Yofu S, Hashimoto H, Shintani N, Baba A, Kopf M, Iwakura Y, Matsuda K, Arimura A, Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue S, Endo K, Ohshima K, Yajima T, Kashimoto K. The neuropeptide PACAP attenuates beta-amyloid (1–42)-induced toxicity in PC12 cells. Peptides. 2002a;23:1471–1478. doi: 10.1016/s0196-9781(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Onoue S, Hanato J, Kuriyama K, Mizumoto T, Yamada S. Development of PACAP38 analogue with improved stability: physicochemical and in vitro/in vivo pharmacological characterization. J Mol Neurosci. 2011;43:85–93. doi: 10.1007/s12031-010-9415-0. [DOI] [PubMed] [Google Scholar]
- Onoue S, Ohshima K, Endo K, Yajima T, Kashimoto K. PACAP protects neuronal PC12 cells from the cytotoxicity of human prion protein fragment 106–126. FEBS Lett. 2002b;522:65–70. doi: 10.1016/s0014-5793(02)02886-7. [DOI] [PubMed] [Google Scholar]
- Onoue S, Waki Y, Nagano Y, Satoh S, Kashimoto K. The neuromodulatory effects of VIP/PACAP on PC-12 cells are associated with their N-terminal structures. Peptides. 2001;22:867–872. doi: 10.1016/s0196-9781(01)00411-9. [DOI] [PubMed] [Google Scholar]
- Onyuksel H, Mohanty PS, Rubinstein I. VIP-grafted sterically stabilized phospholipid nanomicellar 17-allylamino-17-demethoxy geldanamycin: a novel targeted nanomedicine for breast cancer. Int J Pharm. 2009;365:157–161. doi: 10.1016/j.ijpharm.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passemard S, Sokolowska P, Schwendimann L, Gress P. VIP-Induced Neuroprotection of the Developing Brain. Curr Pharm Des. 2011;17:1036–9. doi: 10.2174/138161211795589409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Royo M, Rosenblatt M, Chorev M, Mierke DF. Addressing the tertiary structure of human parathyroid hormone-(1–34). J Biol Chem. 1998;273:10420–10427. doi: 10.1074/jbc.273.17.10420. [DOI] [PubMed] [Google Scholar]
- Peterson LK, Fujinami RS. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. J Neuroimmunol. 2007;184:37–44. doi: 10.1016/j.jneuroim.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilzer I, Gozes I. VIP provides cellular protection through a specific splice variant of the PACAP receptor: a new neuroprotection target. Peptides. 2006;27:2867–2876. doi: 10.1016/j.peptides.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Pincus DW, DiCicco-Bloom EM, Black IB. Vasoactive intestinal peptide regulates mitosis, differentiation and survival of cultured sympathetic neuroblasts. Nature. 1990;343:564–567. doi: 10.1038/343564a0. [DOI] [PubMed] [Google Scholar]
- Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci USA. 1993;90:6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C, Barkhof F, Sandberg-Wollheim M, Linde A, Nordle O, Nederman T. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology. 2005;64:987–991. doi: 10.1212/01.WNL.0000154520.48391.69. [DOI] [PubMed] [Google Scholar]
- Pozo D, Delgado M. The many faces of VIP in neuroimmunology: a cytokine rather a neuropeptide? FASEB J. 2004;18:1325–1334. doi: 10.1096/fj.03-1440hyp. [DOI] [PubMed] [Google Scholar]
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- Reglodi D, Lubics A, Tamas A, Szalontay L, Lengvari I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson's disease. Behav Brain Res. 2004;151:303–312. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Vigh S, Kozicz T, Arimura A. Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke. 2000;31:1411–1417. doi: 10.1161/01.str.31.6.1411. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Somogyvari-Vigh A, Szanto Z, Kertes E, Lenard L, Arimura A, Lengvari I. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides. 2002;23:2227–2234. doi: 10.1016/s0196-9781(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Gourlet P, De Neef P, Woussen-Colle MC, Vandermeers-Piret MC, Vandermeers A, Christophe J. Receptor occupancy and adenylate cyclase activation in AR 4-2J rat pancreatic acinar cell membranes by analogs of pituitary adenylate cyclase-activating peptides amino-terminally shortened or modified at position 1, 2, 3, 20, or 21. Mol Pharmacol. 1992a;42:347–355. [PubMed] [Google Scholar]
- Robberecht P, Gourlet P, De Neef P, Woussen-Colle MC, Vandermeers-Piret MC, Vandermeers A, Christophe J. Structural requirements for the occupancy of pituitary adenylate-cyclase-activating-peptide (PACAP) receptors and adenylate cyclase activation in human neuroblastoma NB-OK-1 cell membranes. Discovery of PACAP(6–38) as a potent antagonist. Eur J Biochem. 1992b;207:239–246. doi: 10.1111/j.1432-1033.1992.tb17043.x. [DOI] [PubMed] [Google Scholar]
- Romier C, Bernassau JM, Cambillau C, Darbon H. Solution structure of human corticotropin releasing factor by 1H NMR and distance geometry with restrained molecular dynamics. Protein Eng. 1993;6:149–156. doi: 10.1093/protein/6.2.149. [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Schafer H, Zheng J, Morys-Wortmann C, Folsch UR, Schmidt WE. Structural motifs of pituitary adenylate cyclase-activating polypeptide (PACAP) defining PAC1-receptor selectivity. Regul Pept. 1999;79:83–92. doi: 10.1016/s0167-0115(98)00147-5. [DOI] [PubMed] [Google Scholar]
- Sexton PM, Poyner DR, Simms J, Christopoulos A, Hay DL. Modulating receptor function through RAMPs: can they represent drug targets in themselves? Drug Discov Today. 2009;14:413–419. doi: 10.1016/j.drudis.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, Arata S, Kitamura S, Okuda H, Takenoya F, Kitamura Y. Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann NY Acad Sci. 2006;1070:550–560. doi: 10.1196/annals.1317.080. [DOI] [PubMed] [Google Scholar]
- Shioda S, Ozawa H, Dohi K, Mizushima H, Matsumoto K, Nakajo S, Takaki A, Zhou CJ, Nakai Y, Arimura A. PACAP protects hippocampal neurons against apoptosis: involvement of JNK/SAPK signaling pathway. Ann NY Acad Sci. 1998;865:111–117. doi: 10.1111/j.1749-6632.1998.tb11169.x. [DOI] [PubMed] [Google Scholar]
- Siatskas C, Bernard CC. Stem cell and gene therapeutic strategies for the treatment of multiple sclerosis. Curr Mol Med. 2009;9:992–1016. doi: 10.2174/156652409789712774. [DOI] [PubMed] [Google Scholar]
- Siatskas C, Payne NL, Short MA, Bernard CC. A consensus statement addressing mesenchymal stem cell transplantation for multiple sclerosis: it's time! . Stem Cell Rev. 2010;6:500–506. doi: 10.1007/s12015-010-9173-y. [DOI] [PubMed] [Google Scholar]
- Sigalov E, Fridkin M, Brenneman DE, Gozes I. VIP-Related protection against lodoacetate toxicity in pheochromocytoma (PC12) cells: a model for ischemic/hypoxic injury. J Mol Neurosci. 2000;15:147–154. doi: 10.1385/JMN:15:3:147. [DOI] [PubMed] [Google Scholar]
- Sorensen PS, Deisenhammer F, Duda P, Hohlfeld R, Myhr KM, Palace J, Polman C, Pozzilli C, Ross C. Guidelines on use of anti-IFN-beta antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFN-beta antibodies in multiple sclerosis. Eur J Neurol. 2005;12:817–827. doi: 10.1111/j.1468-1331.2005.01386.x. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Spong CY, Abebe DT, Gozes I, Brenneman DE, Hill JM. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J Pharmacol Exp Ther. 2001;297:774–779. [PubMed] [Google Scholar]
- Sreedharan SP, Patel DR, Huang JX, Goetzl EJ. Cloning and functional expression of a human neuroendocrine vasoactive intestinal peptide receptor. Biochem Biophys Res Commun. 1993;193:546–553. doi: 10.1006/bbrc.1993.1658. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Straub SG, Sharp GW. A wortmannin-sensitive signal transduction pathway is involved in the stimulation of insulin release by vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide. J Biol Chem. 1996;271:1660–1668. doi: 10.1074/jbc.271.3.1660. [DOI] [PubMed] [Google Scholar]
- Sun C, Song D, Davis-Taber RA, Barrett LW, Scott VE, Richardson PL, Pereda-Lopez A, Uchic ME, Solomon LR, Lake MR, Walter KA, Hajduk PJ, Olejniczak ET. Solution structure and mutational analysis of pituitary adenylate cyclase-activating polypeptide binding to the extracellular domain of PAC1-RS. Proc Natl Acad Sci USA. 2007a;104:7875–7880. doi: 10.1073/pnas.0611397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Hong J, Zang YC, Liu X, Zhang JZ. Altered expression of vasoactive intestinal peptide receptors in T lymphocytes and aberrant Th1 immunity in multiple sclerosis. Int Immunol. 2006;18:1691–1700. doi: 10.1093/intimm/dxl103. [DOI] [PubMed] [Google Scholar]
- Sun Y, McGarrigle D, Huang XY. When a G protein-coupled receptor does not couple to a G protein. Mol Biosyst. 2007b;3:849–854. doi: 10.1039/b706343a. [DOI] [PubMed] [Google Scholar]
- Tan YV, Abad C, Lopez R, Dong H, Liu S, Lee A, Gomariz RP, Leceta J, Waschek JA. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YV, Couvineau A, Laburthe M. Diffuse pharmacophoric domains of vasoactive intestinal peptide (VIP) and further insights into the interaction of VIP with the N-terminal ectodomain of human VPAC1 receptor by photoaffinity labeling with [Bpa6]-VIP. J Biol Chem. 2004;279:38889–38894. doi: 10.1074/jbc.M404460200. [DOI] [PubMed] [Google Scholar]
- Tan YV, Couvineau A, Murail S, Ceraudo E, Neumann JM, Lacapere JJ, Laburthe M. Peptide agonist docking in the N-terminal ectodomain of a class II G protein-coupled receptor, the VPAC1 receptor. Photoaffinity, NMR, and molecular modeling. J Biol Chem. 2006;281:12792–12798. doi: 10.1074/jbc.M513305200. [DOI] [PubMed] [Google Scholar]
- Tan YV, Couvineau A, Van Rampelbergh J, Laburthe M. Photoaffinity labeling demonstrates physical contact between vasoactive intestinal peptide and the N-terminal ectodomain of the human VPAC1 receptor. J Biol Chem. 2003;278:36531–36536. doi: 10.1074/jbc.M304770200. [DOI] [PubMed] [Google Scholar]
- The IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- Thornton K, Gorenstein DG. Structure of glucagon-like peptide (7–36) amide in a dodecylphosphocholine micelle as determined by 2D NMR. Biochemistry. 1994;33:3532–3539. doi: 10.1021/bi00178a009. [DOI] [PubMed] [Google Scholar]
- Toscano MG, Delgado M, Kong W, Martin F, Skarica M, Ganea D. Dendritic cells transduced with lentiviral vectors expressing VIP differentiate into VIP-secreting tolerogenic-like DCs. Mol Ther. 2010;18:1035–1045. doi: 10.1038/mt.2009.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida D, Tatsuno I, Tanaka T, Hirai A, Saito Y, Moro O, Tajima M. Maxadilan is a specific agonist and its deleted peptide (M65) is a specific antagonist for PACAP type 1 receptor. Ann NY Acad Sci. 1998;865:253–258. doi: 10.1111/j.1749-6632.1998.tb11185.x. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- Vandermeers A, Vandenborre S, Hou X, de Neef P, Robberecht P, Vandermeers-Piret MC, Christophe J. Antagonistic properties are shifted back to agonistic properties by further N-terminal shortening of pituitary adenylate-cyclase-activating peptides in human neuroblastoma NB-OK-1 cell membranes. Eur J Biochem. 1992;208:815–819. doi: 10.1111/j.1432-1033.1992.tb17252.x. [DOI] [PubMed] [Google Scholar]
- Vassiliou E, Jiang X, Delgado M, Ganea D. TH2 lymphocytes secrete functional VIP upon antigen stimulation. Arch Physiol Biochem. 2001;109:365–368. doi: 10.1076/apab.109.4.365.4245. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Basille M, Pamantung TF, Fontaine M, Fournier A, Vaudry H, Gonzalez BJ. Pituitary adenylate cyclase-activating polypeptide prevents C2-ceramide-induced apoptosis of cerebellar granule cells. J Neurosci Res. 2003;72:303–316. doi: 10.1002/jnr.10530. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fontaine M, Fournier A, Vaudry H. The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells is mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc Natl Acad Sci USA. 2000;97:13390–13395. doi: 10.1073/pnas.97.24.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Pamantung TF, Basille M, Rousselle C, Fournier A, Vaudry H, Beauvillain JC, Gonzalez BJ. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur J Neurosci. 2002a;15:1451–1460. doi: 10.1046/j.1460-9568.2002.01981.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, Fontaine M, Fournier A, Vaudry H, Gonzalez BJ. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc Natl Acad Sci USA. 2002b;99:6398–6403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, Goetzl EJ. c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol. 2004;172:7289–7296. doi: 10.4049/jimmunol.172.12.7289. [DOI] [PubMed] [Google Scholar]
- Voice JK, Dorsam G, Lee H, Kong Y, Goetzl EJ. Allergic diathesis in transgenic mice with constitutive T cell expression of inducible vasoactive intestinal peptide receptor. FASEB J. 2001;15:2489–2496. doi: 10.1096/fj.01-0671com. [DOI] [PubMed] [Google Scholar]
- Vomhof-DeKrey EE, Dorsam GP. Stimulatory and suppressive signal transduction regulates vasoactive intestinal peptide receptor-1 (VPAC-1) in primary mouse CD4 T cells. Brain Behav Immun. 2008;22:1024–1031. doi: 10.1016/j.bbi.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomhof-DeKrey EE, Hermann RJ, Palmer MF, Benton KD, Sandy AR, Dorsam ST, Dorsam GP. TCR signaling and environment affect vasoactive intestinal peptide receptor-1 (VPAC-1) expression in primary mouse CD4 T cells. Brain Behav Immun. 2008;22:1032–1040. doi: 10.1016/j.bbi.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, Wu JC, Chen X. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. 2009;27:1548–1558. doi: 10.1002/stem.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschek JA. Vasoactive intestinal peptide: an important trophic factor and developmental regulator? Dev Neurosci. 1995;17:1–7. doi: 10.1159/000111268. [DOI] [PubMed] [Google Scholar]
- Waschek JA, Casillas RA, Nguyen TB, DiCicco-Bloom EM, Carpenter EM, Rodriguez WI. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci USA. 1998;95:9602–9607. doi: 10.1073/pnas.95.16.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray V, Kakoschke C, Nokihara K, Naruse S. Solution structure of pituitary adenylate cyclase activating polypeptide by nuclear magnetic resonance spectroscopy. Biochemistry. 1993;32:5832–5841. doi: 10.1021/bi00073a016. [DOI] [PubMed] [Google Scholar]
- Xia M, Sreedharan SP, Bolin DR, Gaufo GO, Goetzl EJ. Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. J Pharmacol Exp Ther. 1997;281:629–633. [PubMed] [Google Scholar]
- Yadav M, Huang MC, Goetzl EJ VPAC1. (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis. Cell Immunol. 2011;267:124–132. doi: 10.1016/j.cellimm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Yadav M, Rosenbaum J, Goetzl EJ. Cutting edge: vasoactive intestinal peptide (VIP) induces differentiation of Th17 cells with a distinctive cytokine profile. J Immunol. 2008;180:2772–2776. doi: 10.4049/jimmunol.180.5.2772. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Danielsen N, Sundler F, Mulder H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport. 1998;9:2833–2836. doi: 10.1097/00001756-199808240-00027. [DOI] [PubMed] [Google Scholar]
- Zhou X, Rodriguez WI, Casillas RA, Ma V, Tam J, Hu Z, Lelievre V, Chao A, Waschek JA. Axotomy-induced changes in pituitary adenylate cyclase activating polypeptide (PACAP) and PACAP receptor gene expression in the adult rat facial motor nucleus. J Neurosci Res. 1999;57:953–961. [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]