Abstract
The authors present a case of a 44-year-old female with unrecognised hypothyroidism consulting for heart failure symptoms. Echocardiogram revealed massive pericardial effusion with tamponade physiology, attributed to primary hypothyroidism from a previous thyroidectomy. Levothyroxine was started at a dose of 0.7 ug/kg/day followed by subxiphoid pericardiostomy. 9 h postpericardiostomy however, hypotension developed and despite hydration and inotropic support, patient succumbed to cardiogenic shock on the 14th hospital day.
Background
The case is significant because:1 hypothyroidism presenting as massive pericardial effusion is rare in this modern age;2 this case demonstrates the risk of pericardial decompression syndrome as a rare but fatal complication after pericardiostomy;3 it reviews current literature on the treatment approach of managing pericardial effusion secondary to hypothyroidism.
Case presentation
The patient had no prior illness until age 16 when she began to develop an asymptomatic goiter. After a few years she became symptomatic with palpitations, tremors, weight loss and easy fatigability, leading to a thyroidectomy at age 38. The histopathology however remains unavailable. Medications were advised after surgery, but patient could not recall what these were as she discontinued intake after 2 months. Nevertheless patient stated she felt well at that time, able to do her usual chores without difficulty and had regular monthly menstrual cycles. In that same year she got pregnant with her sixth child but suffered a spontaneous abortion. The following year she got pregnant again with her seventh child, but this time bore it to term at home, assisted by a traditional birth attendant with no feto-maternal complications. After a few months postpartum, she began to feel lethargic, constipated, dyspneic while doing the laundry, with cold intolerance and easy fatigability. Relatives observed she became less talkative and appeared sad. She also became oligomenorrheic. No consults were made and patient tolerated her condition. Four months prior to admission, she became out of breath even after just a few steps, started to gain weight, had three-pillow orthopnea and abdominal enlargement. Two months prior to admission, she became so weak that she had to be assisted in walking. At this time, facial and bipedal edema started to appear.
On consult at the out-patient clinic her blood pressure was 130/80 mm Hg, heart rate at 80 beats per min, respiratory rate at 20 cycles per min, and temperature at 35.6°C. Heart sounds were muffled but regular. She was sent to the emergency room with suspected myxedema where she was examined drowsy but cooperative, her voice gruff but coherent and slow to reply. Further examination revealed puffy eyes, anicteric sclerae, pale palpebral conjunctivae, an anterior neck scar, slightly distended neck veins with jugular venous pressure of >5 cm but no pulsus paradoxus. Kussmaul’s sign was not appreciated. Decreased breath sounds and vocal fremiti were noted on the left angle of the scapula (Ewart’s sign), but no crackles were auscultated. The apex beat could not be appreciated and the heart sounds were soft with normal rate and regular rhythm. Abdomen was distended with a fluid wave, and equal grade two non-pitting edema on both lower extremities. Her skin was dry and cool to the touch, her pulses faintly palpable. Chvostek’s sign and carpopedal spasm were not elicited and the neurologic examination unremarkable except for delayed relaxation time upon stimulation of the brachioradialis and ankle deep tendon reflexes.
Investigations
Thyroid function tests
The thyrotropin level using radioimmunoassay was high at 26.3 mIU/l (normal values 0.3–3.8 mIU/l), and the free thyroxine (FT4) low at 0.9 pmol/l (normal values 11–24 pmol/l), indicating primary hypothyroidism.
Imaging studies
Antero-posterior chest radiograph show an enlarged heart (computed cardiothoracic ratio of 1:2), with a water-bottle configuration, and bilateral pleural effusion with no infiltrates, nor congestive changes (figure 1).
Figure 1.
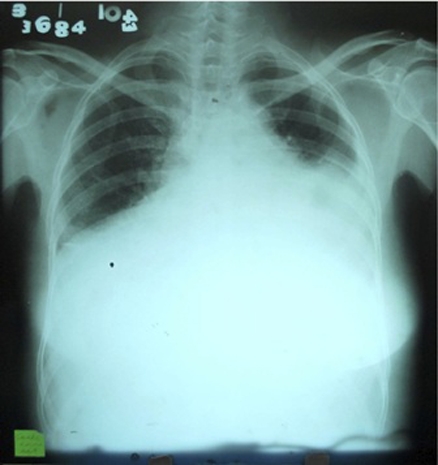
Chest radiograph taken on anteroposterior-view show an apparently enlarged heart (computed cardiothoracic ratio of 1:2), with a water-bottle configuration and bilateral pleural effusion on the left more than the right, with no infiltrates nor congestive changes.
12-lead ECG showed regular sinus rhythm, normal axis at 90 degrees, low QRS voltages in both limb and chest leads, with normal PR interval of 0.14 and normal Qt interval of 0.44.
A transthoracic echocardiogram revealed massive pericardial effusion with an echo-free space measuring 43 mm posteriorly and 19 mm anteriorly on short axis M-mode, and tamponade physiology based on a swinging heart, diastolic collapse of the right ventricle, inferior vena cava (IVC) plethora and respiratory variation of valvular flow upon inspiration: 36% relative increase in tricuspid flow and a 28% relative decrease in mitral flow. The left ventricle was normal in size with good wall motion, and preserved systolic function with an ejection fraction of 73% (figure 2).
Figure 2.
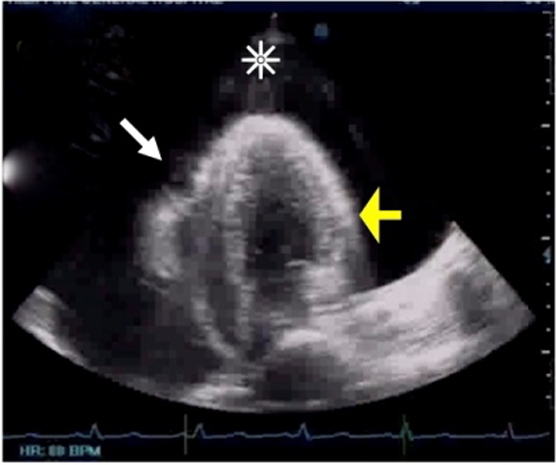
Initial transthoracic echocardiogram revealed massive pericardial effusion with an echo-free space measuring 43 mm posteriorly and 19 mm anteriorly on short axis M-mode (asterisk over black area), and tamponade physiology based on a swinging heart, diastolic collapse of the right ventricle (white arrow), IVC plethora, a 36% relative inspiratory augmentation in tricuspid (right-side) flow and a 28% relative decrease in inspiratory flow across the mitral valve. The left ventricle (yellow arrow) was normal in size with good wall motion, and an ejection fraction of 73%. The left atrium, right atrium and right ventricle were all in normal size.
Biochemical tests
Initial blood chemistry panel showed hypo-osmolar hyponatremia (Na 115 mmol/l, effective serum osmolarity 238 mmol), hypocalcemia (corrected Ca 1.67 mmol/l), elevated liver enzymes (aspartate aminotransferase 381U/l, alanine transaminase 338U/l) and normal serum creatinine levels (85 mmol/l).
Lipid profile showed a low high-density lipoprotein (0.38 mmol/l), low triglyceride (0.50 mmol/l), slightly elevated ligh-density lipoprotein (4.1 mmol/l) and normal total cholesterol (4.66 mmol/l).
Differential diagnosis
In existing studies done on well-developed countries, malignancy, uremia and idiopathic causes rank among the most common causes of significant pericardial effusion requiring intervention.1–3 However, tuberculosis (TB) remains a formidable consideration in developing countries and warrants investigation.4 In our hospital, TB is identified to be the most common aetiology of massive pericardial effusion, with malignancy coming in second at 19%.5 Hypothyroidism as a cause of cardiac tamponade has been rarely encountered.
Treatment
Given the severity and duration of hypothyroidism, low dose levothyroxine replacement at 0.7 mcg/kg/day was started with plans of slowly uptitrating the dose depending on clinical tolerability.
Despite the risk of exposing patient with severe myxoedema to general anaesthesia, the decision to proceed with subxiphoid pericardiostomy was largely driven by the echocardiographic findings of impending tamponade, supplemented by the need to do a biopsy and address the risk of recurrence.
After strict asepsis, a small vertical incision was made from the xiphisternal junction down to a point just below the tip of the xiphoid. The pericardium was opened and the edge of the opening grasped and lifted. Intraoperative findings showed a thin normal pericardium, absence of adhesions and note of voluminous serous pericardial fluid that was collected for diagnostic tests. The pericardial fluid was continuously drained with careful monitoring of the haemodynamic status, with blood pressure stabilising at 140/100 mm Hg towards most of the procedure, and the heart rate at 90–100 bpm. Given patient’s stability, a decision to drain all of the pericardial fluid was made amounting to 1 liter in 2 h. A pericardial specimen measuring several square centimeters was then resected to create a pericardial window, which was sent for biopsy. A separate stab incision was made on the left lowermost aspect of the skin incision for drain placement, where a 28 French chest tube was tunnelled through the fascia at the entry site, through the pericardial window into the pericardial space.
A concomitant tube thoracostomy on the left chest drained 300 ml of the same characteristic fluid. Both tubes were maintained on continuous drainage, with an average pericardial fluid output of 240cc per day. Plan was to remove the tubes once fluid minimised to <100cc/day, and also to allow apposition and adhesion formation between the visceral and parietal pericardium.
Pericardial fluid assay result was suggestive of an exudative effusion with a total protein of 129 g/l. Microscopy and culture failed to grow any organisms including Mycobacterium tuberculosis. Cytology was negative for malignancy. Anti-neutrophilic antibodies were negative as well. Pericardial biopsy showed fibrocollagenous tissue with mild chronic inflammation.
Hypocalcemia due to postprocedural hypoparathyroidism was addressed with daily supplements of calcitriol at 2 mcg, calcium carbonate at 3 g and vitamin D at 2000 U on top of calcium gluconate intravenous boluses. This corrected the calcium levels to 1.95 mmol/l, with the goal of achieving levels in the low normal range.
Outcome and follow-up
Patient was intubated prior to the procedure (tube pericardiostomy) and was stable until hypotension developed 9 h after (blood pressure=70/40 mm Hg). After a trial of saline infusion, inotropes (dopamine and norepinephrine) were started which stabilised her blood pressure to 90–110/60–80 mm Hg. All throughout patient remained awake, communicative, with distinct heart sounds at a mean heart rate of 80 bpm that was regular in rhythm. There were also no episodes of hypoxia. Breath sounds were more audible except on the left base, and the neck veins appeared flattened.
She was immediately confined at the intensive care unit (ICU), closely monitoring her haemodynamic, respiratory, fluid and electrolyte status. Postpericardiostomy, a repeat chest radiograph showed resolution of the left-sided pleural effusion with persistent cardiomegaly (figure 3). A repeat transthoracic echocardiogram showed resolution of the previously noted pericardial effusion, but now with segmental wall motion abnormalities and a substantial drop in the ejection fraction to 46% (figure 4).
Figure 3.
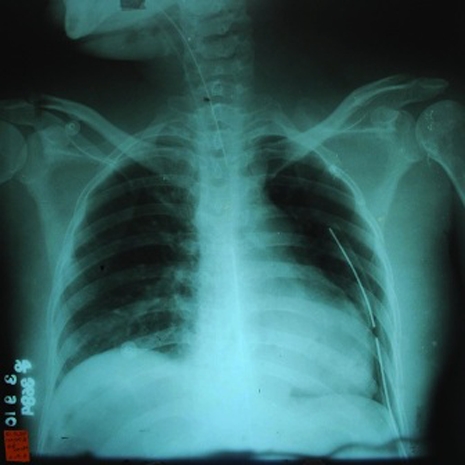
Chest radiograph postpericardostomy show resolution of pleural effusion, with cardiomegaly cardiothoracic ratio 1:2.
Figure 4.
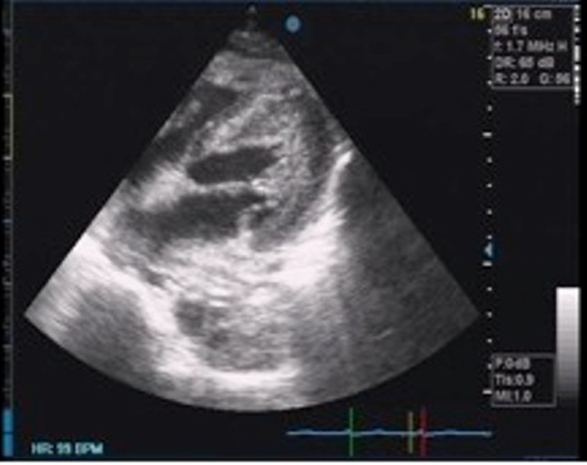
Repeat transthoracic echocardiogram shows resolution of the previously noted pericardial effusion but now with segmental wall motion abnormalities and a significant drop in ejection fraction to 46%.
On the second week of ICU stay, patient tolerated extubation without hypoxia and distress but was re-intubated shortly afterwards due to worsening hypotension. Despite dopamine and norepinephrine drips, systolic blood pressure remained low at palpatory 80–90 mm Hg. Despite no documented arrhythmia on the cardiac monitor, patient was suddenly found unresponsive on the 14th hospital day, and could not be revived even after 35 min of attempted resuscitation.
Discussion
In summary we are presented with a case of hypothyroidism causing massive pericardial effusion. Elevated jugular venous pressure, diminished heart sounds and Ewart’s sign, led to the suspicion of significant pericardial effusion. Given the chronicity of the effusion and the lack of haemodynamic compromise clinically, the decision to proceed to immediate pericardial drainage was largely driven by the echocardiographic findings of cardiac tamponade, the need to do a biopsy due to the endemicity of TB in our country, and to avoid the risk of recurrence.
Pathophysiology
Current literature has elucidated for us the thyroid effects on the heart. First to identify this link was a British physician, named C. Parry in 1785, who described heart enlargement secondary to hyperthyroidism.6 This was followed in 1918 by H. Zondeck from Munich who also described that hypothyroidism can cause cardiac enlargement along with decrease pulsations and low electrocardiographic voltage, which he termed ‘myxedema heart’.7 Over the ensuing years, hypothyroidism has been associated with premature death from coronary artery occlusion (Fahr 1925), a low cardiac output state (Amidi 1968), cardiac enlargement (Aber 1968) and cardiomyopathy (Hamilton 1975).6
The active thyroid hormone tri-iodothyronine (T3) affects the haemodynamics of the cardiovascular system in three ways: First, by increasing tissue thermogenesis; Second, by decreasing systemic vascular resistance activating the renin-angiotensin-aldosterone axis to increase blood volume; Third, by increasing heart rate (chronotropy) and strength of the heart to pump (inotropy). T3 exert these effects through both genomic and non-genomic means. As T3 binds to nuclear receptors which then bind to thyroid hormone response elements, this influences gene transcription on both the structural and regulatory proteins in the heart, particularly the α-myosin heavy chain, sarcoplasmic reticulum proteins, calcium-activated adenosine triphosphatase (ATPase) (Ca2+ ATPase) and phospholamban. Also, T3 changes the performance of various sodium, potassium and calcium channels in the heart that affects heart rate and contraction.1
Consequently, the effects from a low T3 include: bradycardia, mild hypertension with a narrowed pulse pressure (due to an increase in systemic vascular resistance), and an attenuated precordial auscultation.
As hypothyroidism progresses undetected however, pericardial effusion can occur, with an incidence of 80% in severe and long-standing hypothyroidism,2 and 3–6% in milder cases.3 Despite this, there has been no correlation with thyroid stimulating hormone levels and the existence or severity of the effusions.7 The mechanism has been attributed to an increase in capillary permeability, with effusion of fluid rich proteins and glycaminoglycans into the pericardial sac8; a decrease in lymphatic drainage9; and an increased retention of salt and water.10
The progression of effusion to cardiac tamponade in hypothyroidism is best understood along a spectrum of severity. From the moderate to large pericardial effusions without tamponade, into echocardiographic tamponade without paradoxical pulse and once pericardial pressure persistently exceeds intracavitary pressure, ensuing haemodynamic compromise.11
Epidemiology
The effusion rarely causes tamponade because it accumulates slowly and allows time for the pericardial sac to distend.12 13 So rare that as of 1992, the world literature has only about 30 case reports of hypothyroidism presenting as cardiac tamponade.14 Today a search in PUBMED will yield 81 case records, the first one by Martin and Spathis published in the British Medical Journal in 1965.15
Management
Unlike other causes of cardiac tamponade, the following considerations should be made during treatment: First, cardiomyopathy or ischemic heart disease may be concomitantly present due to hypothyroidism-induced changes in the cardiac myocytes and a predisposition to an atherogenic profile (increased total cholesterol and low-density cholesterol), thereby increasing cardiovascular risk respectively; Second, hypothyroidism is characterised by lowered metabolic demands, and that despite a decrease in cardiac output in the presence of tamponade, cardiac function may remain sufficient to sustain the demands imposed on the heart16; Lastly, given the rarity of hypothyroidism-induced tamponade, other precipitating factors such as infection, spontaneous haemorrhage, thyroid treatment or abdominal paracentesis,17 should be considered during investigation.10 17
Thyroxine replacement alone has been found sufficient to resolve mild to moderate effusions,6 7 and even large effusions with echocardiographic evidence of cardiac tamponade.11 Although there is evidence that starting levothyroxine replacement at 100 mcg/day outright may be tolerated,12 18 in most cases it is recommended to start at a lower dose of 25–50 mcg per day with gradual uptitration,12 15 17 19 20 especially in cases wherein an underlying heart disease cannot be ruled out. This is to prevent the possibility of precipitating cardiac ischemia, as well as new effusions, or decompensation towards cardiac tamponade.17 20 Onset of resolution often precedes achievement of euthyroidism and ranges up to 1–21 months.6 11 Recurrence however can still occur,12 21 thus close monitoring is imperative.
As to when to drain and what method to use when the cause of tamponade is due to hypothyroidism remains an area of debate. The largest series studying hypothyroid cardiac tamponade favour drainage only in the presence of cardiovascular compromise such as hypotension and presence of pulsus paradoxus,11 in purulent pericarditis, or when biopsy is helpful to determine the aetiology.12 22 Options range from non-surgical: pericardiocentesis done blindly or guided by echocardiography or CT, percutaneous catheter drainage or sclerotherapy; to surgical: subxiphoid pericardial drainage, video-assisted thoracoscopy, pericardial-peritoneal drainage, pericardial window, or partial of complete pericardiotomy by sternotomy or thoracotomy.12
Existing case reports, each with various aetiologic causes of hypothyroidism, show that while pericardiocentesis along with thyroxine replacement proved successful in both echocardiographic tamponade18 23 24 and overt tamponade,11 surgical procedures are favoured in the presence of haemodynamic compromise.11 12 25–27 This is in contrast to the management of cardiac tamponade of variable causes where pericardiocentesis is favoured for haemodynamically unstable patients and the subxiphoid approach for stable ones.28
Subxiphoid pericardiostomy to address cardiac tamponade is usually successful.17 26 27 In our hospital, it carries with it very low morbidity 1–2% and mortality <1%,5 that is comparable to other centers.29 Although recurrence remains a possibility,12 26 this is lower in comparison to pericardiocentesis.29
Death following subxiphoid pericardiostomy is usually attributed to the underlying cause of cardiac tamponade, but in some cases acute cardiac failure may be directly related to the drainage itself, and has been termed ‘pericardial decompression syndrome’.30
This severe systolic cardiac dysfunction occurring soon after decompression from pericardial tamponade (within 24 h) has been cited as a major predictor of early mortality aside from the underlying aetiology.31 Most cases do not have a clear cause for this paradoxical ventricular dysfunction but numerous mechanisms include haemodynamic, ischemic and sympathetic overdrive hypotheses. Reportedly rare and very unusual,32 33 it can occur in both pericardiocentesis33 and subxiphoid pericardiostomy, the latter having an incidence of 4.8%.31
It has been manifested both in patients with underlying cardiac pathology,34 and those with normal myocardium and haemodynamic status preoperatively.35 Such transient heart failure may be fatal even after relief from a benign causing effusion, and reported cases although limited, indicate very high mortality. Some suggest that slow decompression, close monitoring and inotropic support may result in recovery,33 36 although this has been contested.34
Learning points.
-
▶
Adequate thyroxine replacement often results in the resolution of pericardial effusion due to hypothyroidism
-
▶
Drainage should be reserved in haemodynamically compromised cases with clinical evidence of cardiac tamponade.
-
▶
Subxiphoid pericardiostomy offers the advantage of doing pericardial biopsy as an aid to diagnosis, as well as lower the risk of recurrence in cases of chronic pericardial effusions.
-
▶
Pericardial decompression syndrome can occur after pericardial drainage, either pericardiocentesis or subxiphoid pericardiostomy, resulting in paradoxical ventricular dysfunction or pulmonary oedema.
-
▶
The mechanism of this rare but fatal complication remains to be elucidated, but has been observed regardless of the presence of an underlying systolic dysfunction.
Acknowledgments
Lawrence Tria, Resident-in-training, Department of Internal Medicine. Gisel Catalan MD, Consultant, Department of Thoraco-cardiovascular surgery. Barney Garcia, Fellow, Department of Thoraco-cardiovascular surgery.
Footnotes
Competing interests Fellow-in-training, research requirements
Patient consent Obtained.
References
- 1.Irwin K, Kaie O. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501–9 [DOI] [PubMed] [Google Scholar]
- 2.Hardisty CA, Naik DR, Munro DS. Pericardial effusion in hypothyroidism. Clin Endocrinol (Oxf) 1980;13:349–54 [DOI] [PubMed] [Google Scholar]
- 3.Kabadi UM, Kumar SP. Pericardial effusion in primary hypothyroidism. Am Heart J 1990;120:1393–5 [DOI] [PubMed] [Google Scholar]
- 4.Jain S, Sharma N, Varma S, et al. Profile of cardiac tamponade in the medical emergency ward of a North Indian hospital. Can J Cardiol 1999;15:671–5 [PubMed] [Google Scholar]
- 5.Aleta KNR, Bautista ER, Catalan GT, et al. Massive pericardial effusion in the Philippine General Hospital: clinical profile of patients over a five-year experience. Philipp J Thoracic Cardiovas Surg 2004;11:30–7 [Google Scholar]
- 6.Khaleeli AA, Memon N. Factors affecting resolution of pericardial effusions in primary hypothyroidism: a clinical, biochemical and echocardiographic study. Postgrad Med J 1982;58:473–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerber RE, Sherman B. Echocardiographic evaluation of pericardial effusion in myxedema. Circulation 1975;52:823–7 [DOI] [PubMed] [Google Scholar]
- 8.Kronenberg H, Shlomo M, Kenneth P, Reed LP. Chapter 12. William’s Textbook of Endocrinology. 11th edition, Philadelphia, PA, Edinburgh: Saunders Elsevier; 2008:379 [Google Scholar]
- 9.Parving HH, Hansen JM, Nielsen SL, et al. Mechanisms of edema formation in myxedema–increased protein extravasation and relatively slow lymphatic drainage. N Engl J Med 1979;301:460–5 [DOI] [PubMed] [Google Scholar]
- 10.Manolis AS, Varriale P, Ostrowski RM. Hypothyroid cardiac tamponade. Arch Intern Med 1987;147:1167–9 [PubMed] [Google Scholar]
- 11.Wang JL, Hsieh MJ, Lee CH, et al. Hypothyroid cardiac tamponade: clinical features, electrocardiography, pericardial fluid and management. Am J Med Sci 2010;340:276–81 [DOI] [PubMed] [Google Scholar]
- 12.Karu AK, Khalife WI, Houser R, et al. Impending cardiac tamponade as a primary presentation of hypothyroidism: case report and review of literature. Endocrine Practice 2005;11:265–71 [DOI] [PubMed] [Google Scholar]
- 13.Smolar EN, Rubin JE, Avramides A, et al. Cardiac tamponade in primary myxedema and review of the literature. Am J Med Sci 1976;272:345–52 [DOI] [PubMed] [Google Scholar]
- 14.Jiménez-Nácher JJ, de Alonso N, Vega B, et al. [Cardiac tamponade as a presentation of primary hypothyroidism in a young woman]. Rev Clin Esp 1993;193:290–2 [PubMed] [Google Scholar]
- 15.Martin L, Spathis GS. Case of myxedema with a huge pericardial effusion and cardiac tamponade. Brit Med J 1965;2:83–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seely EW, Williams GH. The Cardiovascular System and Endocrine Disease. Principles and Practice of Endocrinology and Metabolism. Third edition Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1857–64 [Google Scholar]
- 17.Rachid A, Caum LC, Trentini AP, et al. Pericardial effusion with cardiac tamponade as a form of presentation of primary hypothyroidism. Arq Bras Cardiol 2002;78:580–5 [DOI] [PubMed] [Google Scholar]
- 18.Lin CT, Liu CJ, Lin TK, et al. Myxedema associated with cardiac tamponade. Jpn Heart J 2003;44:447–50 [DOI] [PubMed] [Google Scholar]
- 19.Usalan C, Atalar E, Vural F. Pericardial tamponade in a 65-year old woman. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1741156/pdf/v075p00183.pdf (accessed 27 July 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auguet T, Vazques J. Cardiac tamponade and hypothyroidism. Intensive Care Med 1993;19:241. [DOI] [PubMed] [Google Scholar]
- 21.Stephan A. Pericardial tamponade in a patient with treated myxedema. Arch Int Med 1983;143:1983–4 [PubMed] [Google Scholar]
- 22.Chou SH, Chern CH, How CK, et al. A rare case of massive pericardial effusion secondary to hypothyroidism. J Emerg Med 2005;28:293–396 [DOI] [PubMed] [Google Scholar]
- 23.Retnam VJ, Chichgar JA, Patkar LA, et al. Myxedmea and pericardial effusion with cardiac tamponade (a case report) J Postgrad Med 1983;29:188–90 [PubMed] [Google Scholar]
- 24.Vazquez A. Cardiac tamponade and hypothyroidism. http://resources.metapress.com (accessed February 2011). [Google Scholar]
- 25.Gupta R, Munyak J, Jaydock T, et al. Hypothyroidism presenting as acute cardiac tamponade with viral pericarditis. Am J Emergency Med 1999;17:176–8 [DOI] [PubMed] [Google Scholar]
- 26.Stephen A, Fitzgerald J, Loke I, et al. Hypothyroidism presenting with recurrent pericardial tamponade. BMJ Case Rep 2009: doi:10.1136/bcr.03.2009.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marijon E, Jani D, Aubert S, et al. Cardiac tamponade in Hashimoto’s disease. Int J Cardiol 2006;111:470–1 [DOI] [PubMed] [Google Scholar]
- 28.Meyerson S, Amico T. Pericardial Procedures. 2006 WebMD, Inc. Chapter 12. http://www.acssurgery.com/acs/pdf/ACS0412.pdf (accessed 27 July 2011). [Google Scholar]
- 29.Allen KB, Faber LP, Warren WH, et al. Pericardial effusion: subxiphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg 1999;67:437–40 [DOI] [PubMed] [Google Scholar]
- 30.Angouras DC, Dosios T. Pericardial decompression syndrome: a term for a well-defined but rather underreported complication of pericardial drainage. Ann Thorac Surg 2010;89:1702–3 [DOI] [PubMed] [Google Scholar]
- 31.Dosios T, Theakos N, Angouras D, et al. Risk factors affecting survival of patients with pericardial effusion submitted to subxiphoid pericardiostomy. Chest 2003;124:242–6 [DOI] [PubMed] [Google Scholar]
- 32.Karamichalis JM, Gursky A, Valaulikar G, et al. Acute pulmonary edema after pericardial drainage for cardiac tamponade. Ann Thorac Surg 2009;88:675–7 [DOI] [PubMed] [Google Scholar]
- 33.Ligero C, Leta R, Bayes-Genis A. Transient biventricular dysfunction following pericardiocentesis. Eur J Heart Fail 2006;8:102–4 [DOI] [PubMed] [Google Scholar]
- 34.Dosios T, Angouras D. Low cardiac output syndrome complicating subxiphoid pericardiostomy for pericardial effusion. J Thorac Cardiovasc Surg 1997;113:220. [DOI] [PubMed] [Google Scholar]
- 35.Neelakandan B, Jayanthi N, Kanthimathi R. Subxiphoid drainage for pericardial tamponade. J Thorac Cardiovasc Surg 1996;111:489. [DOI] [PubMed] [Google Scholar]
- 36.Sunderji I, Kuhl M, Mathew T. Post pericardiocentesis low cardiac output syndrome in a patient with malignant thymoma. BMJ Case Rep 2009: doi:10.1136/bcr.12.2008.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]


