Abstract
Hyperglycemia is associated with several common neurological syndromes. Chorea, however, is a rare association that has only been documented in the literature recently. The triad of chorea, non-ketotic hyperglycemia and a high signal basal ganglia lesion on the T1 weighted brain MRI (C-H-BG) is considered to be a unique syndrome. C-H-BG refers to the onset of chorea during or shortly after (days to weeks) an episode of non-ketotic hyperglycemia. There is usually a high signal lesion in the basal ganglia on T1 weighted brain MRI that corresponds to the location of the chorea. Most case reports of C-H-BG have been described in Asians. C-H-BG is considered to be a benign condition in which the clinical and MRI signs resolve quickly upon correction of blood glucose levels. Here, the authors describe a case of C-H-BG in a middle aged Caucasian in whom the chorea did not resolve with improved glycemic control.
Background
This brief case report is used to highlight the unique presentation of chorea presenting shortly after significant hyperglycemia. C-H-BG is a rare but important cause of chorea that merits greater recognition given its (usual) reversibility with improved glycemic control. Thus, prompt recognition and treatment of the hyperglycemia associated with this syndrome may be important to avoid irreversible adverse outcomes. Therefore, emphasising the discovery and clinical variations in such rare cases will enhance effective detection and management of this condition.
Case presentation
A middle aged Caucasian male with a 1 year duration of type 2 diabetes mellitus (T2DM), hypertension and depression presented to the neurology service at a tertiary care hospital in December 2007 with a 5 week history of right-sided haemichorea involving upper and lower limbs but sparing the face. The chorea had been gradually progressive over 5 weeks. The movements were involuntary, not provoked by urge, worsened with distraction and improved slightly with intentional use of the affected limbs. There were no other focal neurological symptoms or signs and the rest of the examination was normal.
In parallel with the history of haemichorea, the patient had developed worsening of his diabetic and metabolic control. He reported osmotic symptoms (polydipsia and polyuria) during the time that the chorea was developing. Objective evidence of long-standing and acute metabolic aberrancy included an HbA1c of 15.6% and a fasting glucose level of 17 mmol/l. Capillary blood glucose monitoring showed a ‘critically high level’. At this stage there was no evidence of ketoacidosis. The deterioration in his metabolic control coincided with omission of his oral hypoglycemic medications; metformin and glyburide. Adherence with his other prescribed medications including sertraline, clonidine, telmisartan and atorvastatin was also poor during this period.
Investigations
Laboratory investigations and historical timeline clearly eliminated the majority of the potential aetiologies of new onset chorea (see differential diagnosis table). Specifically, the investigations did not reveal any infectious, toxic, or paraneoplastic aetiologies for the chorea. There was no acanthocytosis, vasculitis, abnormal copper indices or positive antistrepteolysin titre (figure 1). Sequencing of the Huntington’s gene was requested and remains pending, yet his chorea has not progressed and he remains clinically unchanged after 4 years of clinical follow-up making this possibility remote. A CT scan of the brain revealed subtle hypodense areas in the left basal ganglia. An MRI of his brain showed T1 hyperintense signal in the left caudate and lentiform nuclei, with sparing of the internal capsule (figure 2). This imaging allows the possibility of a vascular event to be eliminated, as the lenticular nucleus and internal capsule are in the same vascular distribution, yet the internal capsule was unaffected.
Figure 1.
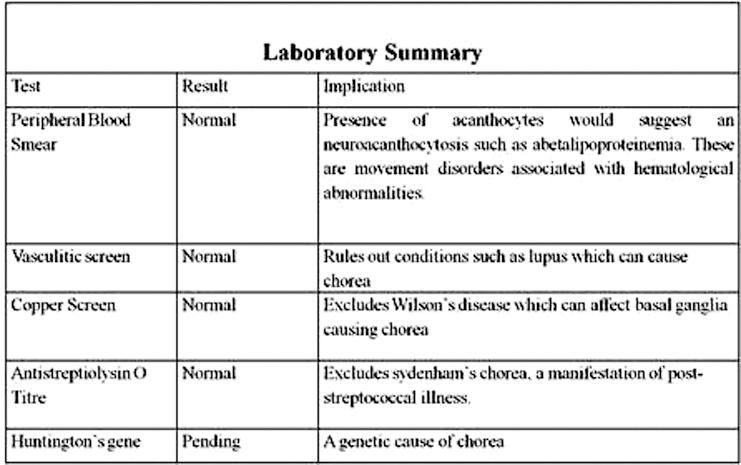
Laboratory investigations performed to address the differential diagnosis of new onset chorea.
Figure 2.
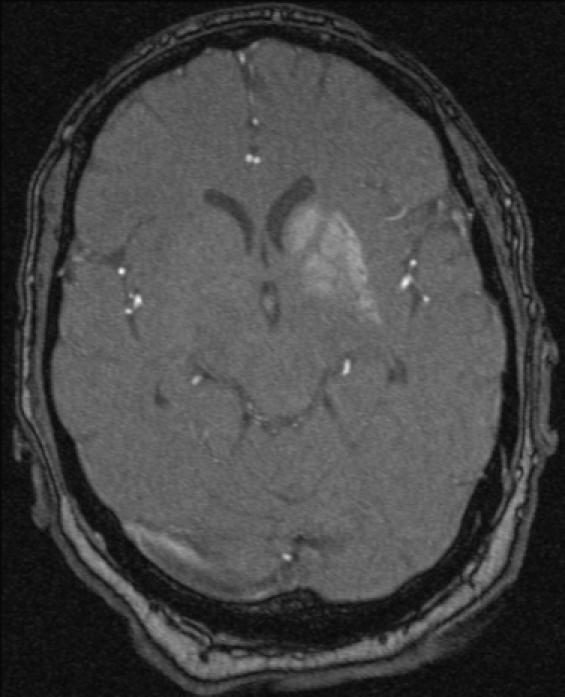
T1 weighted MRI of the brain demonstrating increased signal intensity of the left basal ganglia.
Differential diagnosis
Please refer to ‘differential diagnosis’ summary table, figure 3.
Figure 3.
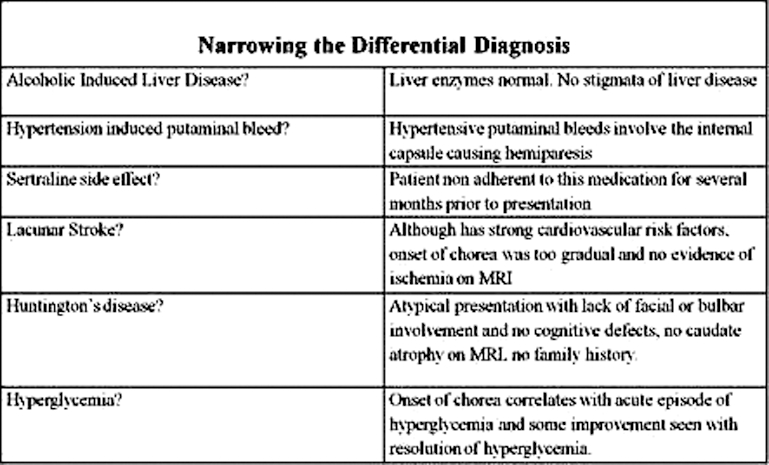
Clinical, historical and temporal considerations towards identifying the aetiology of chorea.
Treatment
The subject was prescribed respiridone for control of the chorea. His osmotic symptoms were addressed with fluid rehydration. The oral hypoglycemics were reinstated and diabetes education initiated for long-term glycemic control.
Outcome and follow-up
During follow-up visits over the subsequent year, the patient manifested clinically unresolved unilateral haemichorea, although a partial improvement was reported. The improvement correlated with better medication compliance and diabetic control (HbA1c at a target of less than 7%). Physical rehabilitation was carried out within the first 3 months of the event when such interventions are most effective. A repeat CT scan several months later showed no change in the size of the lesion but did show evolution. The patient has now continued to be followed for over 4 years, and there has been no further resolution of the haemichorea. This indicates that there is an irreversible lesion of the lenticular nucleus unresponsive to sustained and optimal glycemic control.
Discussion
The differential diagnosis for chorea is lengthy. Some of the more common aetiologies include adverse drug effects, Huntington’s disease and stroke (figure 3).
Although the results of genotyping for Huntington’s disease are still pending, this is not strongly suspected as our patient did not have a subacute presentation, and there is an absence of any family history of movement disorders typical in the history of Huntington’s disease. There was also a noted absence of caudate nucleus atrophy observed on MRI, which is a characteristic imaging feature of Huntington’s disease.1 Furthermore, after 4 years of follow-up the chorea has not progressed.
Although we cannot exclude a contribution from his psychotropic medication, it is more common for this to result in bilateral chorea.1 In addition, he was non-adherent to most of his medications, including psychotropics, for several months prior to the onset of the chorea.
Haemorrhagic stroke was excluded since there was no internal capsule involvement or hemiparesis, both of which would be expected with a basal ganglia bleed2 as the lenticular nucleus and internal capsule are dependent on the same vascular distribution.
There are many other rarer causes of chorea. However, C-H-BG is a syndrome that, because of its association with hyperglycemia,1 3 should be considered in this case. The subject’s onset of chorea coincided with the exacerbation of hyperglycemia and there was a temporal relationship between restoration of euglycemia and partial improvement of the chorea. Furthermore, the imaging findings were typical for this syndrome which characteristically demonstrates a unilateral, often persistent, increased signal within the striatum on T1 weighted MRI.2–5 However, we cannot neglect the possibility that some of the improvement that seemed to coincide with glycemic control may also have been due to the effect of respiridone6 and early physical rehabilitation.
In previous case reports of C-H-BG, the clinical duration and location of chorea have been varied.7–9 Significantly, almost all cases have been reported in the presence of T2DM, and frequently after a new diagnosis of T2DM has been made.3 In many cases, the chorea develops a few weeks after the blood glucose levels are actively treated, suggesting a delayed reaction to hyperglycemia.3
Our case report is similar to 53 cases of C-H-BG systematically reviewed from 1985 to 2001 by Oh et al.3 in that there was haemichorea rather than bilateral chorea, and the serum glucose at presentation was remarkably elevated (mean 26.5 mmol/l) as was the HbA1c (mean HbA1c 14.4%). In contrast to other reported cases, our patient was not Asian (91%), female or older (mean age 71 years). Although 75% of reported cases resolved within the first 5.5 days, ours did not. The lesion within the lenticular nucleus appears to be irreversible in this case.
The precise aetiology of C-H-BG has yet to be determined, however there is ongoing research with a focus being directed to the cause of the hyperintensity of the basal ganglia on T1 weighted brain MRIs. Theories include putaminal petechial haemorrhage,10 hyperviscosity,11–13 calcification and deficiency of basal ganglia γ-aminobutyric acid (GABA) due to its use as an alternative energy source during hyperglycemia.3 8 Some authors have questioned the GABA theory given the persistence of the chorea beyond the episode of hyperglycemia.7 Calcification as an aetiology also seems unlikely since the imaging findings, whether MRI or CT, often resolve with disappearance of the chorea. Basal ganglia calcification would be expected to persist once established.
Petechial haemorrhage with blood brain barrier breakdown has been proposed as the most plausible mechanism.10 Although the acute-subacute nature of the chorea in C-H-BG would support haemorrhage as a mechanism, there are several arguments that can be posed against this aetiology. Namely, haemorrhage usually resolves with time. In a few case reports, the imaging findings persist even after resolution of the chorea.12 In those cases with bilateral basal ganglia hyperintensity,3 it would be even more difficult to support haemorrhage as the aetiology as it is extremely rare to develop bilateral basal ganglia bleeds.7 Indeed, a recent autopsy report of C-H-BG by Ohara et al. showed no haemorrhage or calcification.11 In fact, multiple infarcts were seen associated with a reactive astrocytic response. It is thus proposed that hyperviscosity, induced by hyperglycemia, causes transient ischemia of vulnerable striatal neurons in predisposed individuals. This could cause astrocytic hypertrophy and oedema resulting in the characteristic MRI changes. This reversible mechanism would explain those cases where transient MRI and clinical alterations occur. To date, only this one autopsy study in addition to three biopsy results9 14 have given us this vital histological information regarding the pathogenesis of C-H-BG. Although all three studies demonstrate hypertrophic astrocytes to be the main abnormal finding within the basal ganglia, clearly more histological studies are required to substantiate these results.
There are fewer theories in the literature regarding the aetiology of persistent chorea in C-H-BG. This is due to the paucity of cases,7 such as the presentation we are communicating, in which chorea fails to resolve. As mentioned, the prognosis for C-H-BG is usually good with complete resolution of clinical and MRI signs after blood glucose correction.3 So why are there some cases where these changes persist?
There may be a threshold effect where if the insult is sufficiently great, or sustained, that irreversible damage results. Underlying co-morbidities may play an important role in the duration of C-H-BG. Our patient had hypertension, which, in combination with diabetes, is a large risk factor for atherosclerotic disease. This may augment the ischemia induced by hyperviscosity, causing a lesion which takes a longer time to resolve or even be so severe as to cause irreversible damage. In addition, our patient had a longer duration of hyperglycemia, given the report of polydipsia and polyuria over months rather than days and the elevated HbA1c. It is therefore possible that prolonged exposure to hyperglycemia enabled more severe vascular damage to occur, making the striatum that much more susceptible to damage from transient ischemia.
In conclusion, C-H-BG appears to be a rare but potentially important cause of chorea since although generally benign and self-limiting, the chorea, as demonstrated in this report, can persist. It is likely that C-H-BG is under-recognised and underdiagnosed given the prevalence of diabetes and the fact that hyperglycemia may have resolved by the time the individual seeks medical attention. Prompt recognition and management of the hyperglycemia associated with this syndrome may be important to avoid adverse outcomes. Further clinicopathological studies of this syndrome would provide important insights into the pathogenesis of this disorder. However the identification of cases will depend upon recognition of this syndrome by attentive, thoughtful clinicians who become aware as a result of careful reports of cases in the medical literature.
Learning points.
-
▶
Hyperglycemia (C-H-BG) is a rare but important cause of chorea.
-
▶
Early recognition can lead to initiation of glycemic control and potentially reverse the condition.
-
▶
C-H-BG may be underdiagnosed as the hyperglycemia often corrects by the time the patient presents with chorea.
-
▶
Rarely, the chorea does not resolve with treatment, perhaps due to prolonged hyperglycemia and irreversible neuronal damage.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Cardoso F, Seppi K, Mair KJ, et al. Seminar on choreas. Lancet Neurol 2006;5:589–602 [DOI] [PubMed] [Google Scholar]
- 2.Iwata A, Koike F, Arasaki K, et al. Blood brain barrier destruction in hyperglycemic chorea in a patient with poorly controlled diabetes. J Neurol Sci 1999;163:90–3 [DOI] [PubMed] [Google Scholar]
- 3.Oh SH, Lee KY, Im JH, et al. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci 2002;200:57–62 [DOI] [PubMed] [Google Scholar]
- 4.Lai PH, Tien RD, Chang MH, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol 1996;17:1057–64 [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu JL, Wang HC, Hsu WC. Hyperglycemia-induced unilateral basal ganglion lesions with and without hemichorea. A PET study. J Neurol 2004;251:1486–90 [DOI] [PubMed] [Google Scholar]
- 6.Evidente VG, Gwinn-Hardy K, Caviness JN, et al. Risperidone is effective in severe hemichorea/hemiballismus. Mov Disord 1999;14:377–9 [DOI] [PubMed] [Google Scholar]
- 7.Ahlskog JE, Nishino H, Evidente VG, et al. Persistent chorea triggered by hyperglycemic crisis in diabetics. Mov Disord 2001;16:890–8 [DOI] [PubMed] [Google Scholar]
- 8.Nagai C, Kato T, Katagiri T, et al. Hyperintense putamen on T1-weighted MR images in a case of chorea with hyperglycemia. AJNR Am J Neuroradiol 1995;16:1243–6 [PMC free article] [PubMed] [Google Scholar]
- 9.Shan DE, Ho DM, Chang C, et al. Hemichorea-hemiballism: an explanation for MR signal changes. AJNR Am J Neuroradiol 1998;19:863–70 [PMC free article] [PubMed] [Google Scholar]
- 10.Chang MH, Chiang HT, Lai PH, et al. Putaminal petechial haemorrhage as the cause of chorea: a neuroimaging study. J Neurol Neurosurg Psychiatr 1997;63:300–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohara S, Nakagawa S, Tabata K, et al. Hemiballism with hyperglycemia and striatal T1-MRI hyperintensity: an autopsy report. Mov Disord 2001;16:521–5 [DOI] [PubMed] [Google Scholar]
- 12.Chu K, Kang DW, Kim DE, et al. Diffusion-weighted and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: a hyperviscosity syndrome? Arch Neurol 2002;59:448–52 [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Kim JY, Kim JM, et al. Oro-bucco-lingual dyskinesia associated with nonketotic hyperglycaemia. J Clin Neurosci 2006;13:947–9 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Akamine T, Makihara S. Hemiballism presenting with high intensity at lentiform nuclei on short spin echo of serial MRI. A case report. Clin Neurol (Tokyo) 1992;36:203–6 [Google Scholar]


