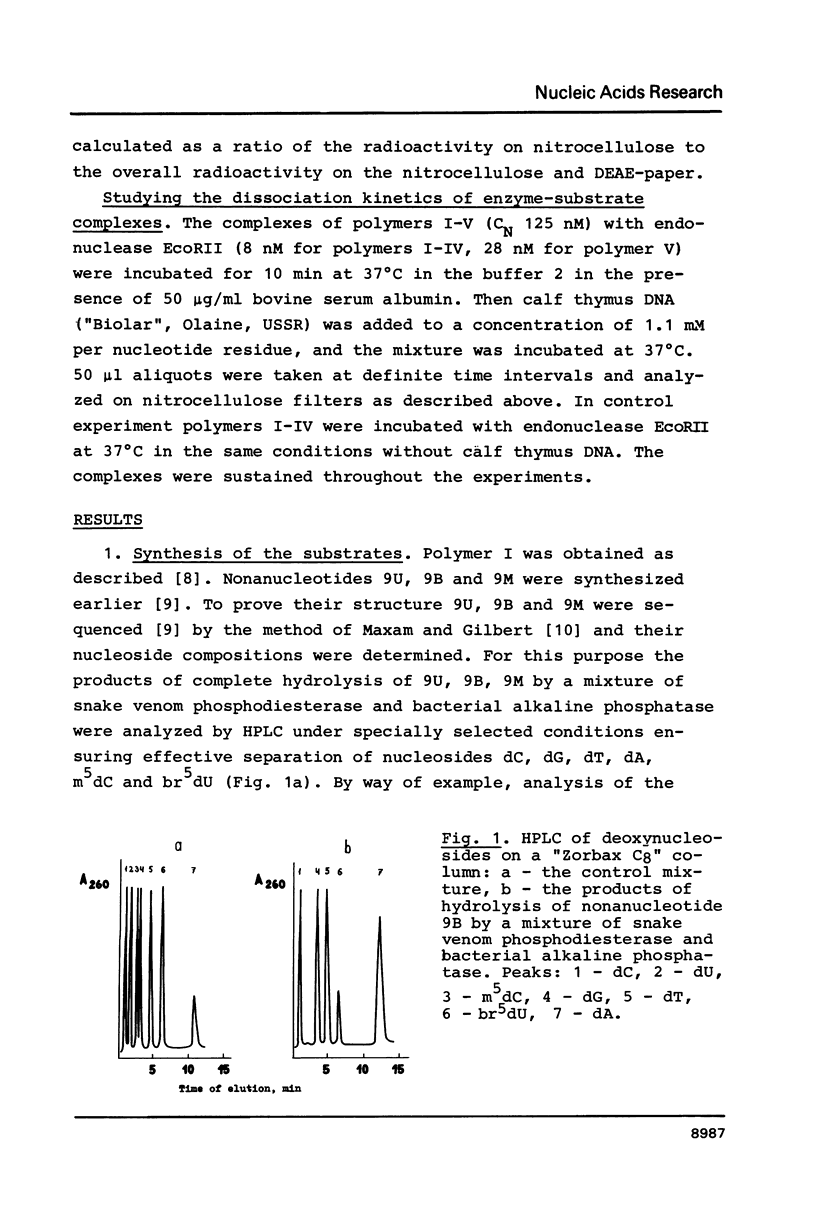
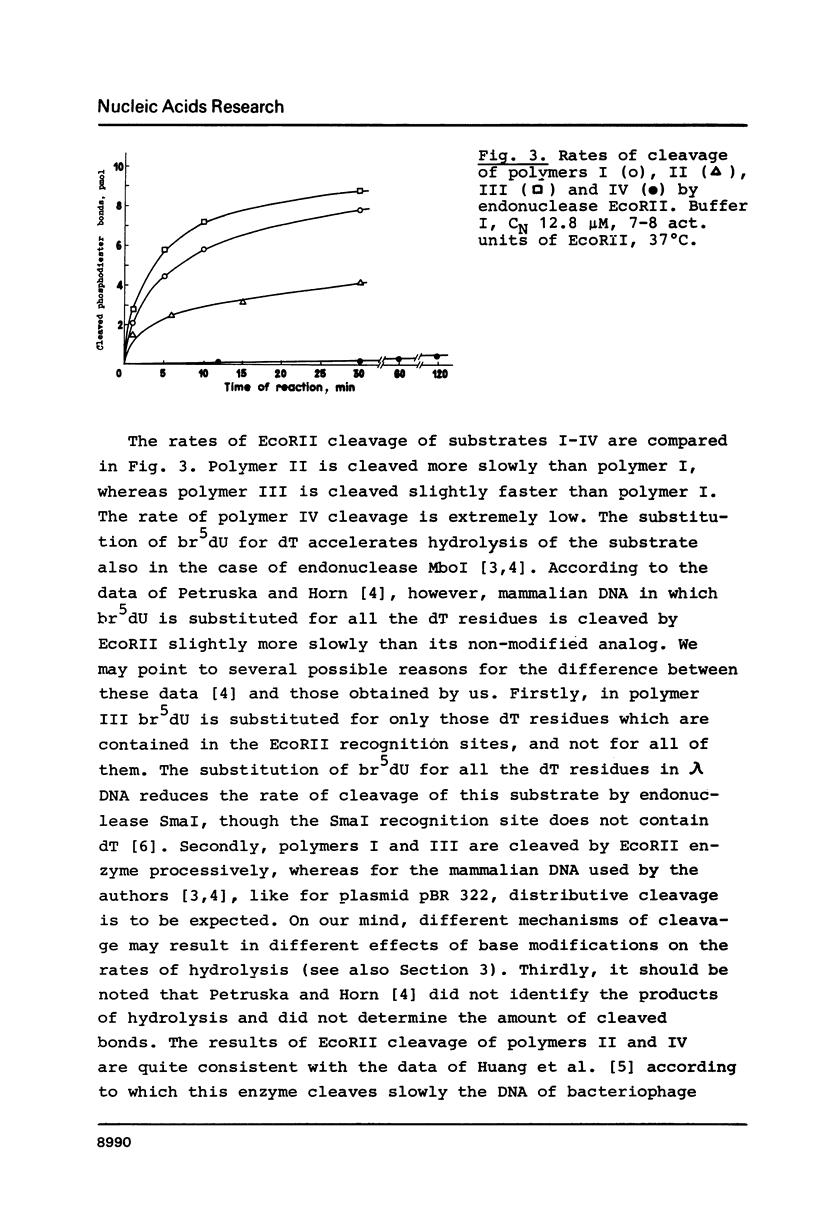
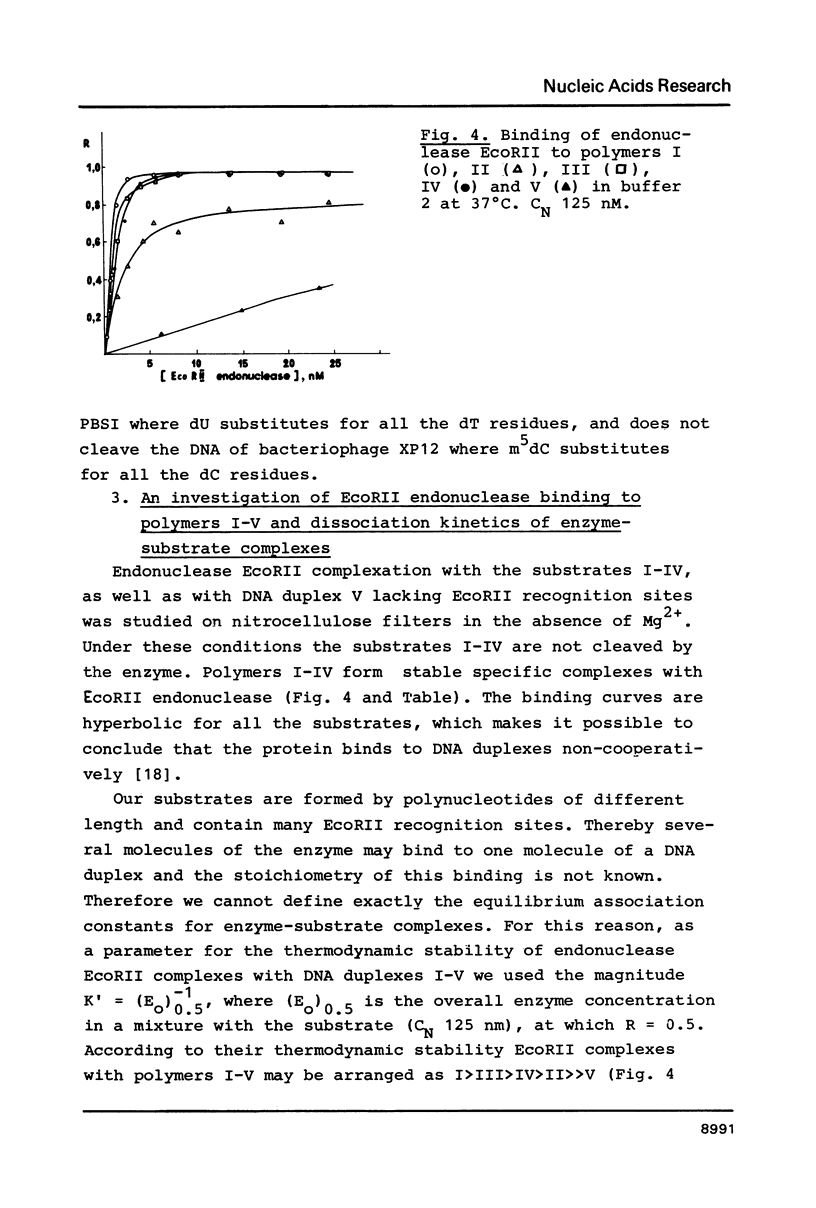
Abstract
The present study deals with the binding and cleavage by EcoRII endonuclease of concatemer DNA duplexes containing EcoRII recognition sites (formula; see text) in which dT is replaced by dU or 5-bromodeoxyuridine, or 5'-terminal dC in the dT-containing strand is methylated at position 5. The enzyme molecule is found to interact with the methyl group of the dT residue of the DNA recognition site and to be at least in proximity to the H5 atom of the 5'-terminal dC residue in dT-containing strand of this site. Modification of any of these positions exerts an equal effects on the cleavage of both DNA strands. Endonuclease EcoRII was found to bind the substrate specifically. At the same time modification of the bases in recognized sequence may result in the formation of unproductive, though stable, enzyme-substrate complexes.
Full text
PDF





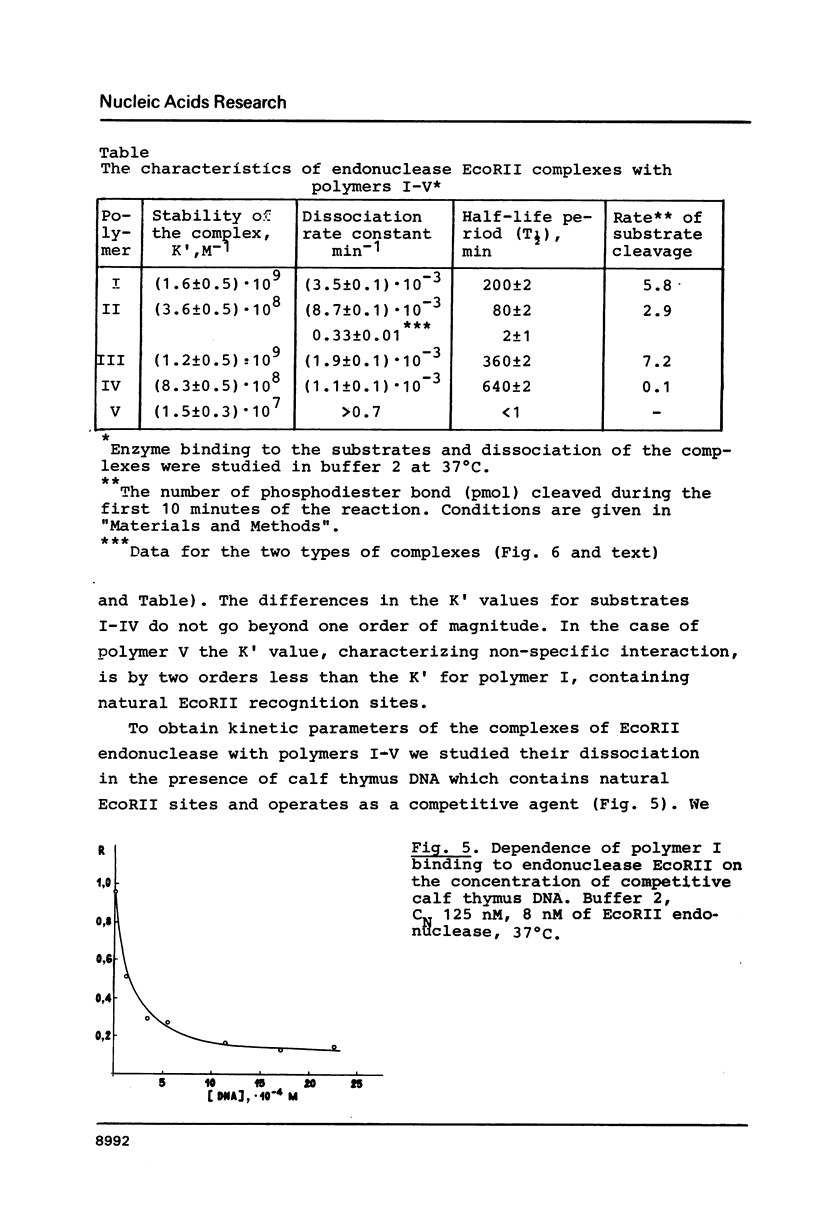
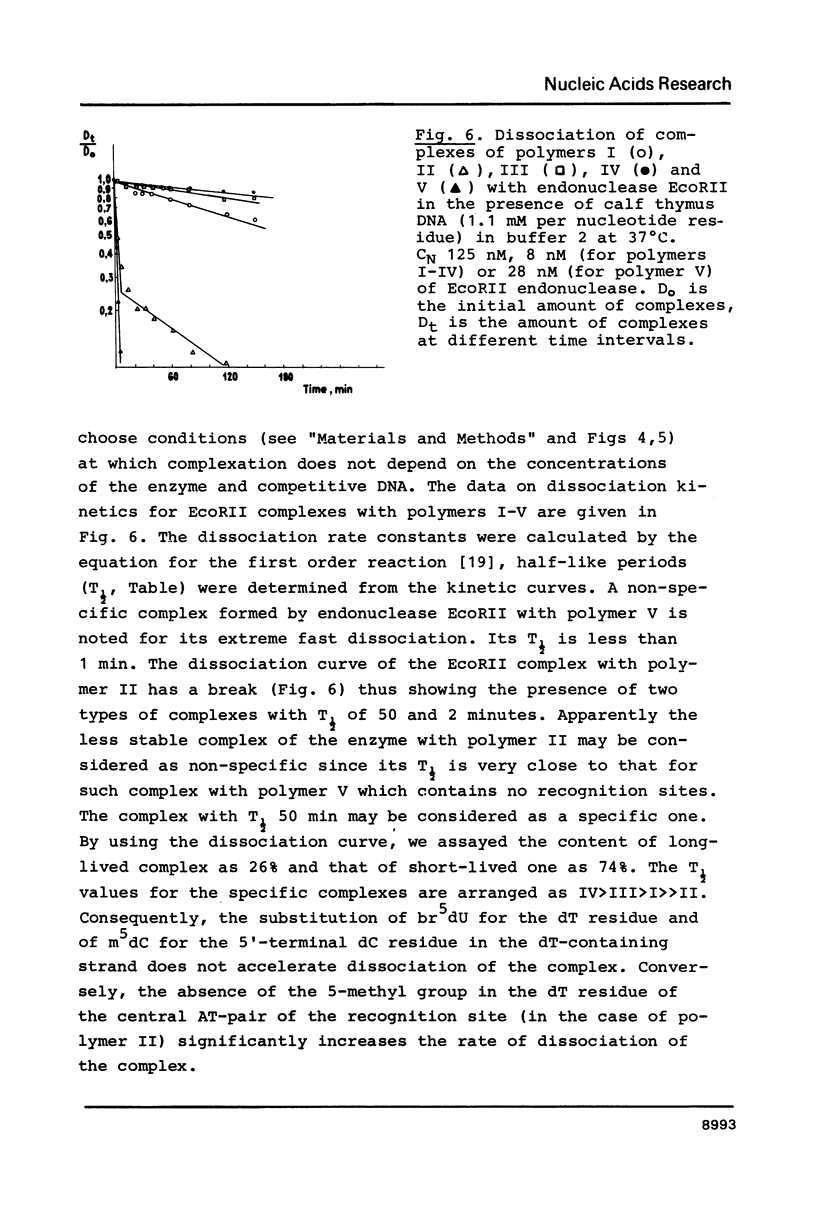





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alden C. J., Kim S. H. Solvent-accessible surfaces of nucleic acids. J Mol Biol. 1979 Aug 15;132(3):411–434. doi: 10.1016/0022-2836(79)90268-7. [DOI] [PubMed] [Google Scholar]
- Alves J., Pingoud A., Langowski J., Urbanke C., Maass G. Two identical subunits of the EcoRI restriction endonuclease Co-operate in the binding and cleavage of the palindromic substrate. Eur J Biochem. 1982 May;124(1):139–142. doi: 10.1111/j.1432-1033.1982.tb05916.x. [DOI] [PubMed] [Google Scholar]
- Frederick C. A., Grable J., Melia M., Samudzi C., Jen-Jacobson L., Wang B. C., Greene P., Boyer H. W., Rosenberg J. M. Kinked DNA in crystalline complex with EcoRI endonuclease. Nature. 1984 May 24;309(5966):327–331. doi: 10.1038/309327a0. [DOI] [PubMed] [Google Scholar]
- Halford S. E., Johnson N. P. The EcoRI restriction endonuclease with bacteriophage lambda DNA. Equilibrium binding studies. Biochem J. 1980 Nov 1;191(2):593–604. doi: 10.1042/bj1910593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. H., Farnet C. M., Ehrlich K. C., Ehrlich M. Digestion of highly modified bacteriophage DNA by restriction endonucleases. Nucleic Acids Res. 1982 Mar 11;10(5):1579–1591. doi: 10.1093/nar/10.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosykh V. G., Puntezhis S. A., Bur'ianov Ia I., Baev A. A. Vydelenie, ochistka i kharakteristika restriktsionnoiéndonukleazy EcoRII. Biokhimiia. 1982 Apr;47(4):619–625. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langowski J., Alves J., Pingoud A., Maass G. Does the specific recognition of DNA by the restriction endonuclease EcoRI involve a linear diffusion step? Investigation of the processivity of the EcoRI endonuclease. Nucleic Acids Res. 1983 Jan 25;11(2):501–513. doi: 10.1093/nar/11.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni M. A., Roufa R. J. Digestion of 5-bromodeoxyuridine-substituted lambda-DNA by restriction endonucleases. J Biol Chem. 1978 Dec 25;253(24):9075–9081. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Modrich P. Studies on sequence recognition by type II restriction and modification enzymes. CRC Crit Rev Biochem. 1982;13(3):287–323. doi: 10.3109/10409238209114231. [DOI] [PubMed] [Google Scholar]
- Petruska J., Horn D. Bromodeoxyuridine substitution in mammalian DNA can both stimulate and inhibit restriction cleavage. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1317–1324. doi: 10.1016/0006-291x(80)90095-9. [DOI] [PubMed] [Google Scholar]
- Petruska J., Horn D. Sequence-specific responses of restriction endonucleases to bromodeoxyuridine substitution in mammalian DNA. Nucleic Acids Res. 1983 Apr 25;11(8):2495–2510. doi: 10.1093/nar/11.8.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. J Mol Biol. 1970 Nov 14;53(3):401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- Shabarova Z. A., Dolinnaya N. G., Drutsa V. L., Melnikova N. P., Purmal A. A. DNA-like duplexes with repetitions. III. Efficient template-guided chemical polymerization of d(TGGCCAAGCTp). Nucleic Acids Res. 1981 Nov 11;9(21):5747–5761. doi: 10.1093/nar/9.21.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabarova Z. A., Dolinnaya N. G., Turkin S. I., Gromova E. S. DNA-like duplexes with repetitions. I. Properties of concatemer duplexes formed by d(T-G-C-A-C-A-T-G). Nucleic Acids Res. 1980 Jun 11;8(11):2413–2429. doi: 10.1093/nar/8.11.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Nucleotide sequence specificity of restriction endonucleases. Science. 1979 Aug 3;205(4405):455–462. doi: 10.1126/science.377492. [DOI] [PubMed] [Google Scholar]
- Woodbury C. P., Jr, von Hippel P. H. On the determination of deoxyribonucleic acid-protein interaction parameters using the nitrocellulose filter-binding assay. Biochemistry. 1983 Sep 27;22(20):4730–4737. doi: 10.1021/bi00289a018. [DOI] [PubMed] [Google Scholar]
- Yolov A. A., Gromova E. S., Kubareva E. A., Potapov V. K., Shabarova Z. A. Interaction of EcoRII restriction and modification enzymes with synthetic DNA fragments. V. Study of single-strand cleavages. Nucleic Acids Res. 1985 Dec 20;13(24):8969–8981. doi: 10.1093/nar/13.24.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolov A. A., Gromova E. S., Romanova E. A., Oretskaya T. S., Oganov A. A., Buryanov YaI, Shabarova Z. A. Interaction of EcoRII restriction and modification enzymes with synthetic DNA fragments. EcoRII endonuclease cleavage of substrates with repeated natural and modified recognition sites. FEBS Lett. 1984 Feb 13;167(1):147–150. doi: 10.1016/0014-5793(84)80850-9. [DOI] [PubMed] [Google Scholar]
- Yolov A. A., Gromova E. S., Shabarova Z. A. Processive cleavage of concatemer DNA duplexes by Eco RII restriction endonuclease. Mol Biol Rep. 1985 Apr;10(3):173–176. doi: 10.1007/BF00778525. [DOI] [PubMed] [Google Scholar]