Abstract
The authors report a case of portal vein thrombosis, with no underlying malignant cause identified, which was initially detected by fludeoxyglucose positron emission tomography/CT (FDG PET/CT) and subsequently confirmed by both contrast enhanced CT and MRI. The multimodality imaging findings are outlined, the potential clinical implications discussed and note made of the possible FDG PET/CT mimics of this disorder.
Background
Incidental findings in patients undergoing fludeoxyglucose positron emission tomography/CT (FDG PET/CT) are increasingly common, and awareness of these findings and their mimics are of importance in delivering a clinically relevant radiological report and in directing further imaging. We report a case of portal vein thrombosis, with no underlying malignant cause identified, which was initially detected by FDG PET/CT, and outline the subsequent multimodality imaging findings and discuss the implications of this diagnosis and potential FDG PET/CT mimics of this disorder.
Case presentation
A middle aged female smoker with a background of alcoholic liver disease presented for a CT scan in the course of investigation of respiratory symptoms, primarily haemoptysis.
Investigations
A left upper lobe nodule was suspicious for a bronchial malignancy. A coronal reformat of the portal venous phase CT demonstrated a normally enhancing portal vein and hepatic parenchyma (figure 1). A subsequent FDG PET/CT 7 weeks following the initial CT did not demonstrate uptake in the pulmonary abnormality, but demonstrated incidental FDG uptake in the region of the portal vein (figure 2). Subsequent measurement of α fetoprotein levels was within normal limits. A subsequent coronal sensitivity encoding MRI sequence following intravenous gadolinium (figure 3) confirmed the diagnosis of a portal vein thrombus, with an additional finding of sectorial hepatic parenchymal high signal and thought to be in keeping with a transient hepatic intensity difference (THID). No mass lesion was evident. A subsequent portal venous phase coronal reformat CT performed for follow-up of the left upper lobe pulmonary nodule demonstrated portal vein occlusion, not evident on the original CT (figure 4). No secondary complications are apparent, though only a 2 week period had elapsed since initial diagnosis. The left upper lobe pulmonary lesion is demonstrated, likely benign in the face of an unchanged CT and negative FDG PET/CT.
Figure 1.
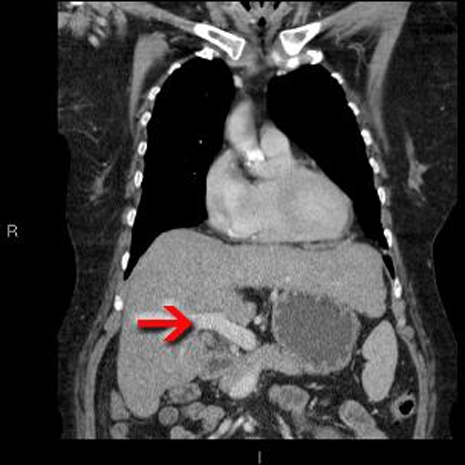
Coronal reformat of portal venous phase CT demonstrating a normally enhancing portal vein (red arrow). The liver has a slightly irregular outline, in keeping with cirrhosis, although no features of portal hypertension are present. An incidental left upper lobe nodule is not included on this slice.
Figure 2.
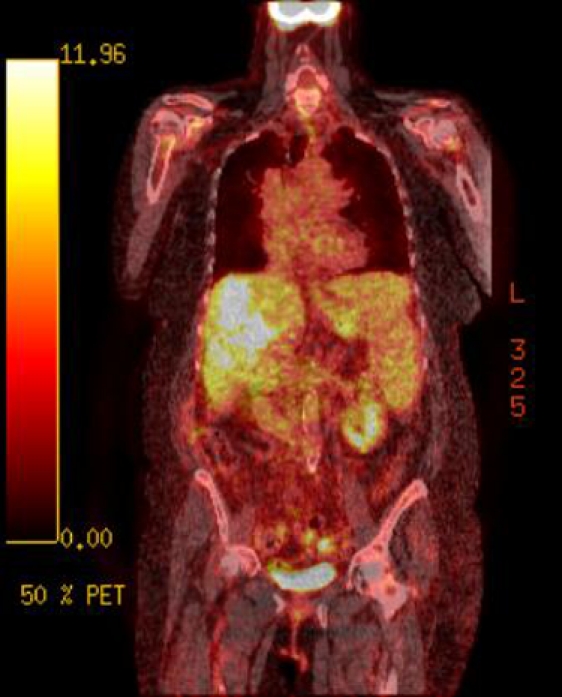
Axial PET image at level of porta hepatis demonstrating uptake in the region of the portal vein (red arrow). This is a non-specific appearance and could be in keeping with hepatocellular carcinoma, local lymphadenopathy or portal vein thrombosis due to a range of causes.
Figure 3.
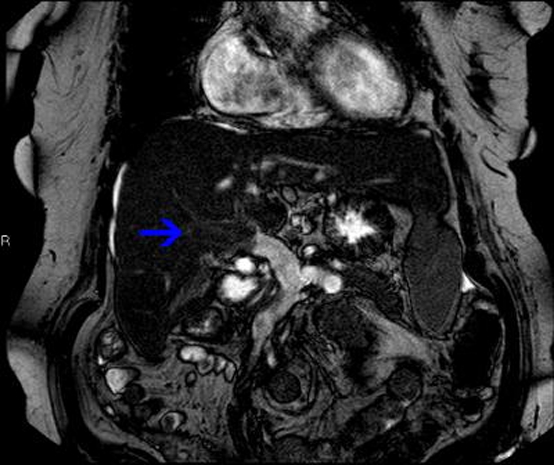
Coronal sensitivity encoding MRI sequence following intravenous gadolinium of the liver demonstrating lack of enhancement of the portal vein (blue arrow). Initially performed to evaluate the liver parenchyma and porta hepatis to investigate for malignancy, the portal vein appearances are pathognomonic of thrombosis and resulted in commencement of appropriate therapy.
Figure 4.
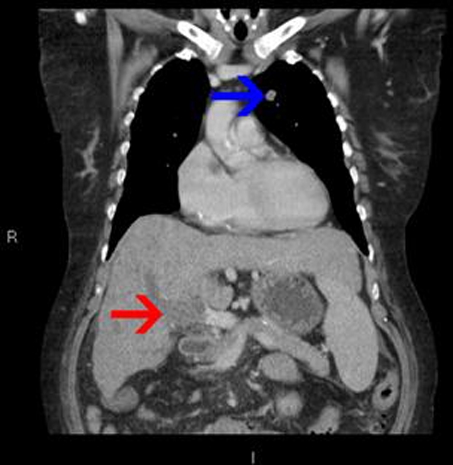
Coronal reformat of the second portal venous phase CT, performed for follow-up of the unchanged left upper lobe nodule (blue arrow), confirming the presence of the occluded portal vein (red arrow).
Differential diagnosis
Given the clinical history of cirrhosis and corresponding hepatic appearances on the CT, the prospect of a primary hepatic malignancy such as hepatocellular carcinoma (HCC) or extensive lymphadenopathy was raised and further imaging advised.
Treatment
Not applicable.
Outcome and follow-up
The patient died shortly following completion of investigations through the complications of alcoholic liver disease. No further interval follow-up consultation or imaging studies were therefore performed, but no evidence of malignancy was observed on the imaging to date.
Discussion
Incidental findings at FDG PET/CT are common1 given FDG uptake is a marker of glycolysis, and in addition to initiate further investigation, may adversely impact the patient’s prognosis, rendering interpretation and recognition of normal variants and important incidental findings critical.2 Although portal vein thrombosis resulting in 18F-FDG uptake is previously described, these cases are associated with a range of malignancies including lymphoma, pancreatic cancer and HCC coexisting with cirrhosis.3–8 To our knowledge, this case describes the only FDG-avid portal vein thrombosis with no underlying malignancy, cirrhosis the likely causative factor in this case.
Thrombus which is not metabolically active is a simple thrombus with no signs of infection, and usually reflects a hypercoagulable state or may simply be idiopathic. Metabolically active thrombus usually results from inflammation with or without infection, or tumour thrombus. Phlebitis can arise as a result of vessel involvement with thrombus, though is also associated with formation of thrombus secondary to circulating inflammatory mediators including tumour necrosis factor or endotoxins.9 10 Systemic inflammatory disorders may also raise the risk of venous thromboembolism, including inflammatory bowel disease and rheumatoid arthritis, although not a factor in our case. Though HCC is the most serious likely cause of hepatic FDG uptake, particularly in the context of cirrhosis, potential causes of peri-hepatic or hepatic FDG uptake include portal vein thrombosis (PVT), haemorrhagic cysts, pancreatic pseudocysts, or peripancreatic lymph nodes.9 11 Suspicion of PVT on PET/CT necessitates contrast-enhanced cross sectional imaging if no contemporaneous intravenous contrast-enhanced cross sectional imaging is available for direct comparison.
Venous thrombosis development is multifactorial with gene-environment interaction playing a key role, with acquired risk factors including immobilisation, surgery, trauma, pregnancy, malignancy and circulating female hormones.12 PVT itself it is a source of morbidity and mortality, with splenomegaly, varices, portal hypertension, haemorrhage and ascites all recognised sequelae.13 With a lifetime incidence of 1%,14 diagnosis is most commonly made by abdominal Doppler ultrasound but may be incidentally detected by PET/CT not infrequently given the risk factors attendant to the group of patients under investigation, as well as by contrast-enhanced CT or MRI.
Though spontaneous resolution of thrombosis can occur, antithrombotic treatment increases the incidence of partial recanalisation, and is used to limit the development of portal hypertension. Although mortality due to uncomplicated PVT is relatively low (8%), this increases to 26% in patients with underlying malignancy or cirrhosis,15 making this an important diagnosis to raise in this clinical context, with timely initiation of antithrombotic therapy potentially impacting on prognosis.
The secondary MRI finding of hepatic parenchymal high signal is thought to represent a THID, likely due to PVT, though this finding can variously be seen in hepatic vein thrombosis, biliary obstruction or an arterioportal shunt. A THID is induced by three pathological processes, namely inflammation of the biliary vessels, flow diversion as a result of an arterioportal shunt, or, as in this case, portal hypoperfusion secondary to portal branch thrombosis/compression. These defects have varying appearances on contrast-enhanced MRI, and may be sectorial (as in the case, commonly due to portal hypoperfusion and demonstrating a triangular shape with a straight border conforming to the portal supply), polymorphous, or diffuse.16
In conclusion, this case highlights a non-malignant coincidental finding from a PET/CT initially performed for investigation of an unrelated lung nodule. This finding has the potential for significant clinical repercussions, with prompt initiation of appropriate medical therapy potentially of prognostic importance. These findings emphasise the need for the reporting clinician to consider benign (though potentially serious) hepatic aetiologies as well as malignant causes in incidental abnormalities of the liver in FDG PET/CT, and direct consequential imaging appropriately.
Learning points.
-
▶
Incidental findings at FDG-PET/CT are important source of pathology in their own right.
-
▶
Such findings may require immediate action – failure to consider a potentially serious aetiology for such a finding may result in a delay or failure in initiation of treatment.
-
▶
Knowledge of potential causes of incidental findings and their repercussions is important both for the reporting radiologist and the referring clinician.
Footnotes
Competing interests None.
Patient consent Not obtained.
References
- 1.Ozkol V, Alper E, Aydin N, et al. The clinical value of incidental 18F-fluorodeoxyglucose-avid foci detected on positron emission tomography/computed tomography. Nucl Med Commun 2010;31:128–36 [DOI] [PubMed] [Google Scholar]
- 2.Sopov V, Bernstine H, Stern D, et al. The metabolic spectrum of venous thrombotic disorders found on PET/CT. AJR Am J Roentgenol 2009;193:W530–9 [DOI] [PubMed] [Google Scholar]
- 3.Sun L, Guan YS, Pan WM, et al. Highly metabolic thrombus of the portal vein: 18F fluorodeoxyglucose positron emission tomography/computer tomography demonstration and clinical significance in hepatocellular carcinoma. World J Gastroenterol 2008;14:1212–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen BD. Pancreatic neuroendocrine tumor with portal vein tumor thrombus: PET demonstration. Clin Nucl Med 2005;30:628–9 [DOI] [PubMed] [Google Scholar]
- 5.Kurtovic J, Van Der Wall H, Riordan SM. FDG PET for discrimination between tumor extension and blood thrombus as a cause for portal vein thrombosis in hepatocellular carcinoma: important role in exclusion of transplant candidacy. Clin Nucl Med 2005;30:408–10 [DOI] [PubMed] [Google Scholar]
- 6.Hanajiri K, Mitsui H, Maruyama T, et al. 18F-FDG PET for hepatocellular carcinoma presenting with portal vein tumor thrombus. J Gastroenterol 2005;40:1005–6 [DOI] [PubMed] [Google Scholar]
- 7.Natsuizaka M, Kudo M, Suzuki M, et al. Diffuse large B-cell lymphoma with massive portal vein tumor thrombosis in a patient with alcoholic cirrhosis: a case report and literature review. Intern Med 2009;48:805–8 [DOI] [PubMed] [Google Scholar]
- 8.Roldán-Valadez E, Ortega-López N, Valdivieso-Cárdenas G, et al. (18)F-FDG PET/CT for discrimination between tumor extension and blood thrombus in pancreatic adenocarcinoma associated with portal vein thrombosis. Rev Esp Med Nucl 2008;27:40–4 [DOI] [PubMed] [Google Scholar]
- 9.Friess H, Langhans J, Ebert M, et al. Diagnosis of pancreatic cancer by 2[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Gut 1995;36:771–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorospe L, Raman S, Echeveste J, et al. Whole-body PET/CT: spectrum of physiological variants, artifacts and interpretative pitfalls in cancer patients. Nucl Med Commun 2005;26:671–87 [DOI] [PubMed] [Google Scholar]
- 11.Bares R, Klever P, Hauptmann S, et al. F-18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology 1994;192:79–86 [DOI] [PubMed] [Google Scholar]
- 12.Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet 1999;353:1167–73 [DOI] [PubMed] [Google Scholar]
- 13.Valla DC, Condat B. Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol 2000;32:865–71 [DOI] [PubMed] [Google Scholar]
- 14.Ogren M, Bergqvist D, Björck M, et al. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol 2006;12:2115–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sogaard KK, Astrup LB, Vilstrup H, et al. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol 2007;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colagrande S, Centi N, Galdiero R, et al. Transient hepatic intensity differences: part 2, Those not associated with focal lesions. AJR Am J Roentgenol 2007;188:160–6 [DOI] [PubMed] [Google Scholar]


