Abstract
Administration of substances to laboratory animals requires careful consideration and planning to optimize delivery of the agent to the animal while minimizing potential adverse experiences from the procedure. The equipment selected to deliver substances to animals depends on the length of the study and the nature of the material being administered. This selection provides a significant opportunity for refining animal treatment. Similarly, when substances are administered as solutions or suspensions, attention should be given to selection of vehicles and methods used for preparing the solutions and suspensions. The research team, veterinarian, technical personnel, and IACUC members should be aware of reasons underlying selection of equipment for substance delivery and should consider carefully how substances will be prepared and stored prior to administration to animals. Failure to consider these factors during experimental planning may result in unintentional adverse effects on experimental animals and confounded results.
Abbreviation: VAP, vascular access port
Administration of substances to laboratory animals is often necessary as part of an experimental protocol or for therapeutic reasons. The considerations and procedures that surround substance administration may affect animal welfare and the scientific validity of experimental results. Animal dosing presents many opportunities for refinement. Administration of a single dose is rarely a challenge for the veterinarian, research group, or animal care committee, in terms of determining an appropriate method of delivering the material to an animal. However, treatments that must be given repeatedly over time often present special challenges, especially when little is known about the properties of the test substance or when it is administered to a new species. How the material will be prepared for delivery to animals, ensuring that the test substance or drug remains active and free of contaminants, and the actual technical details of administering the substance should be considered thoroughly. When the drug is given as a solution or suspension, the vehicle must be selected carefully, to avoid introducing confounding effects in the study, including adverse effects in animals. Similarly, the equipment and techniques used to administer substances must be assessed to minimize stress experienced by the animal and to ensure accuracy of dosing and safety of animals, research personnel, and caregivers.
This article is the second in a 2-part review of substance delivery to laboratory animals and summarizes dosing equipment and apparatus needed for substance delivery, considerations for selecting vehicles, and solute preparation and handling. The general comments contained in this article are applicable to species commonly used in research settings, including fish and amphibians.
Equipment for Administration of Substances to Animals
A wide variety of equipment is available for purchase or can be constructed in-house to assist with dosing. This equipment includes acute and chronic restraint devices, hypodermic needles, superficial or surgically implanted catheters and vascular access ports, gastric tubes, gavage needles, and infusion–delivery pumps. Consideration of hygiene, sterility, and routine device maintenance are paramount to maintaining animal health, ensuring continued patency of indwelling devices, ensuring accuracy of dosing, and minimizing disruption of the experiment, including delays in dose administration.
In all laboratory species, prior habituation to procedures and restraint devices is an important humane consideration, given that restraint is often the greatest stressor that rodents experience during procedures.12 Many animals, in particular swine, nonhuman primates, and dogs, can be trained with positive reinforcement to cooperate with vessel access and various dosing procedures. This training minimizes the need for animal restraint and sedation and decreases potential distress for animals and research staff alike.40,69 In addition, habituation to procedures may minimize stress associated with new procedures in smaller animals.82
For pair- or group-housed animals, selecting equipment or altering the home cage environment to minimize damage or disruption of indwelling devices, jackets, or bandages is essential. In addition, exposure of the other members in a test group to active test substance in urine, feces, saliva, or other body secretions should be considered, in terms of effects on pharmacokinetics. If animals must be separated for dosing, at a minimum, visual, auditory, and olfactory contact with conspecifics should be maintained.
Finally, the administration site should be prepared appropriately. For most parenteral injection sites other than intravenous, it is usually sufficient to give the injection without skin or hair preparation. Intradermal and intravenous injections often require close clipping of the hair and further skin preparation. Disinfection of the clipped puncture site includes gentle cleansing with an antibacterial scrub, such as chlorhexidine and povidone iodine, or a disinfectant, such as isopropyl alcohol.33 Care must be taken to avoid excessive wetting of small animals with disinfectants such as isopropyl alcohol, because a soaked hair coat may induce hypothermia. In addition, aggressive scrubbing or the use of rough gauze sponges can abrade and irritate the skin and should be avoided.
Vascular access equipment.
Devices and equipment used to assist with vascular access can generally be grouped by whether the access duration is acute (a single instance or a session of several hours or less), temporary (repeated or constant access of less than 2 d), or chronic (access—usually by catheter or vascular access port—needed constantly or intermittently for days and months). Minimizing pain, discomfort, infection, thrombosis, and loss of patency are primary considerations in vascular access; careful attention to anesthesia and analgesia, aseptic technique, and appropriate use of anticoagulant solutions are key to success.
Acute and temporary venous access.
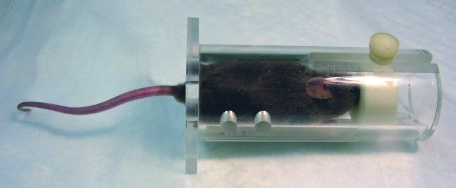
Acute and temporary vascular access most often are accomplished with percutaneous introduction of needles and catheters. Butterfly needles have the advantage over straight needles of additional flexibility that protects the inserted needle from shifting during manipulations to attach or remove syringes, and they are forgiving of some animal movement once the needle is seated in the vessel lumen. The plastic wings on either side of the butterfly needle can be bent to allow a very shallow angle of insertion, which is often necessary in accessing superficial vessels, particularly in rodents and rabbits. Once seated and secured, catheters and butterfly needles can be attached to a syringe or macro- or microdrip fluid administration set for short-term intravenous administration of fluids or other substances. For short-term (minutes to hours) fluid administration to anesthetized small species, such as rodents, inexpensive reusable spring-operated infusion devices can be used (Figure 1).
Figure 1.
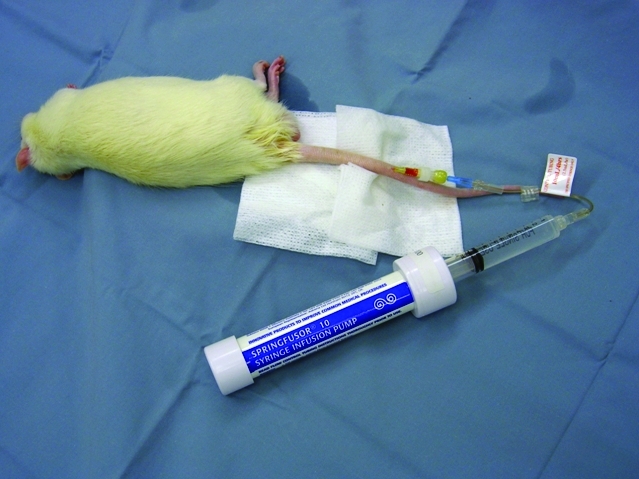
Fluids may be administered intravenously to anesthetized rats by using the lateral tail vein and a syringe infusion pump. Photo courtesy of Colette Wheler.
Chronic venous access.
For prolonged delivery of substances, surgically implanted devices facilitate repeated or continuous vessel access over days or months and minimize the pain and discomfort associated with repeated venipuncture. These devices may exit through the skin or may be completely implanted and require skin puncture to obtain access (for example, vascular access ports [VAP]). Indwelling venous catheters come in a variety of materials, each with its own benefits (Table 1). Commonly used tubing material for catheters includes polyurethane or silicone. Less commonly, polyethylene or polytetrafluoroethylene (Teflon) is used but may be required on occasion because of issues of chemical compatibility. Materials vary in cost, compliance (flexibility), strength, biocompatibility, thrombogenicity, ability to withstand high temperatures and chemicals, and ability to accept lubricating or antithrombotic coatings, such as heparin polymers. Such coatings have been shown to decrease thrombosis of intravenous catheters.19 The method of sterilization available to the researcher may determine the catheter material selected—some materials cannot be steam-sterilized and instead must be gas- or chemically sterilized. Further, some materials are easier to fabricate into catheters, depending on whether they can be heat-molded. Materials with high shape memory are less likely to stretch and slip off connections. All of these factors should be considered when selecting catheter materials.
Table 1.
Advantages and disadvantages of various catheter materials
| Silicone | Polyurethane | Polyethylene | Teflon | |
| Biocompatibility | high | high | fair | fair |
| Wall diameter | thick | thin | thick | thick |
| Bacterial adherence | low | low | high | low |
| Stiffness | flexible | somewhat flexible | rigid | rigid |
| Chemical resistance | high | high | high | high |
| Temperature resistance | high | poor | high | high, but can't be autoclaved |
| Memory | excellent | poor | poor | poor |
| Accepts antithrombotic coating | no | yes | no | no |
| Additional considerations | easy to modify | initially rigid, softens at body temperature | unlikely to kink, high tensile strength | stiffness may promote vessel irritation |
Modified from references 35, 57, 78, and 3.
The stiffness of the cannula and shape of the catheter tip play important roles in determining irritation to the vascular intima. Stiffer tubing and bevelled tips are easier to insert into blood vessels and may be easier for novice surgeons; however, ensuing friction and endothelial irritation promotes tissue proliferation and formation of microthrombi. Catheter materials that soften at body temperature, such as those that are silica-based, and tapered round catheter openings tend to cause the least vessel trauma and remain patent longer.87
In addition, the diameter of the catheter relative to the size of the vessel plays a role in minimizing tissue irritation and thrombosis. In general, a catheter diameter that permits continuing blood flow around it has a decreased chance of inducing a clot, and a catheter with a diameter smaller than that of the vessel should be used. Catheters have similar properties to blood vessels in terms of fluid flow and resistance.78 The catheter diameter must be balanced with the properties of the material to be injected, because injection resistance is directly proportional to the viscosity of the substance to be injected and inversely proportional to the fourth power of the catheter radius (r4; where radius = diameter / 2) (Poiseuille Law).84 For example, increasing the radius of a catheter by 2-fold reduces injection resistance by 16-fold. Therefore too small a catheter diameter offers increased resistance to infusion or aspiration and increased chance of clotting, because of reduced flow.78
Vascular Access Ports.
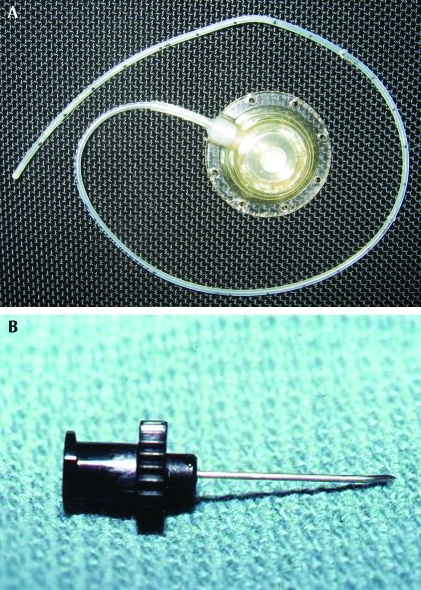
For chronic access, VAP typically are surgically implanted in a subcutaneous location, eliminating concerns of exit site infection and animal damage to the external parts of the device. The implanted cannula exits the blood vessel, often the jugular or femoral vein, and is attached to a rigid reservoir port that is secured to subcutaneous tissues by nonabsorbable sutures. The port is accessed through a penetrable silicone septum when a needle is passed through the skin. If the site where the port is anchored is poorly chosen, pressure necrosis of taut overlying skin may result. Each time the port is accessed, the skin overlying the port should be repositioned so that a new area is punctured, to decrease chances of skin irritation or necrosis. Dual lumen ports with 2 separate reservoirs and catheters are available for use in larger species, such as dogs and nonhuman primates. The use of dual lumen devices may decrease concerns of chemical compatibility of the materials being infused.89 VAP can also be used when repeated intraperitoneal dosing is required, given that subcutaneous access of the port eliminates the risk of unintentional injection into intestines, urinary bladder, or other organs, as can be associated with repeated intraperitoneal injections. Regardless of the placement site, a special noncoring Huber needle is used to access the port. Huber needles increase the life of the port by decreasing wear on the penetrable septum (Figure 2).
Figure 2.
(A) Vascular access port. (B) Noncoring Huber needle. Photo courtesy of Tanya Duke-Novakovski.
Once the animal has recovered from the initial vascular instrumentation surgery, risk of infection comes primarily from direct contamination of the site or incorrect technique during access of the catheter or port.25 Prior to access, the skin site must be disinfected gently but thoroughly, as for a surgical procedure. Periodic reshaving of the site may be needed. All needles and solutions used to access the catheter or port must be sterile, and personnel cleaning the site should wear sterile gloves. With excellent nursing support and attention to surgical site maintenance, VAP may remain patent for many months to years.21,90 Recent reviews of techniques and refinements for using catheters and VAP in animals have been published.52,58,78,79
Equipment to protect catheters and VAP.
Simple bandaging can be used to protect short-term catheters, but animals must be monitored carefully to ensure that they do not chew or otherwise disrupt the catheter. A chronic indwelling vascular catheter or line running to a subcutaneously placed VAP usually requires a sturdy form of protection to minimize contamination or disruption by the animal. For most mammalian species, customized cloth jackets and tethers are available to facilitate connection of the indwelling catheter or port with an infusion pump. Jackets are available in a range of sizes and should be fitted to the individual animal to minimize chafing and pressure sores. Animals should be acclimated to wearing a jacket prior to study initiation. For long-term studies (those over 2 wk), jackets should be changed periodically to minimize surgical site contamination and permit close observation of the catheter exit or VAP surgery site. For growing animals, jackets may have to be changed as body weight increases. Soft, stretchy liners or undershirts are available commercially or can be made for use with jackets, to minimize chafing and irritation. Jacketed and tethered animals can be group-housed successfully. Whether other animals will damage the jacket depends on the individuals in the group.70 A group-housing trial during the jacket acclimation period, prior to implant surgery, can provide information on the likelihood of success.
Implanted catheters that exit through the skin in rodents can be attached directly to a pedestal made of dental acrylic anchored on the calvarium (Figure 3).26,31,51,53,58,76,83 Pedestals permit ready access to the catheter and remove the need for jackets and other restraint devices. Regular attention must be given to keeping the apposed skin edges clean where they meet the acrylic cap and to treating any incipient infection at the site.
Figure 3.
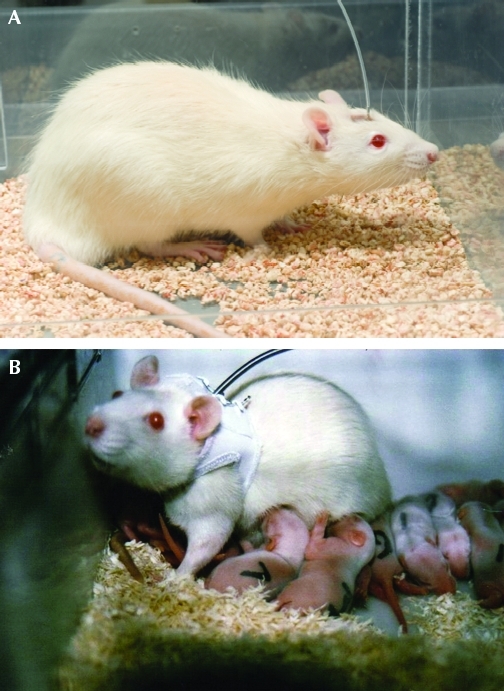
(A) A rat with an indwelling catheter attached to a head-mounted pedestal. This type of attachment eliminates the need for a jacket and tether assembly. (B) A female rat with a chronic vascular catheter is fitted with a jacket and tether assembly. This assembly permits free movement of the animal in the cage and nursing of pups. Photo courtesy of Lomir Biomedical.
Locks for indwelling catheters and VAP.
Consistent use of sterile solutions to fill catheters and ports is necessary to exclude blood and inhibit clot formation. These solutions are referred to as ‘locks.’ Anticoagulants such as heparin (at 100 to 1000 U/mL), sodium citrate, and sodium EDTA can be used as locks, and 40% to 50% dextrose solutions may be added to anticoagulant locks to inhibit bacterial growth.10,49,75 Because of concern with inducing drug-resistant bacterial infections, use of prophylactic antibiotic locks in catheters is controversial.73 If a catheter-related infection is known or suspected, an antibiotic lock may be used in combination with systemic antibiotic treatment. Early use of antibiotic locks in cases of suspected infection has been effective at eliminating catheter infection in humans and swine.7,28,59 Infected catheters and ports typically do not respond well to antimicrobial therapy and may require removal of the device followed by antimicrobial therapy or euthanasia.78
Regular flushing of catheters should occur to ensure patency and to remove substances in the catheter that should not come in contact with substances to be injected.27 The lock solution is aspirated from the lumen and discarded prior to injection of a therapeutic or test agent. Saline typically is used to flush the device after the lock solution is withdrawn; usually 3 to 5 times the volume held by the catheter and port is adequate , given that flushing does not result in an exact 1:1 volume displacement of the port contents. In addition, prolonged flushing helps to displace biofilm accumulating on the interior surface of the catheter wall.27,78 Individual vials of flush solution are recommended, because multiuse fluid bags can be a significant source of iatrogenic bacterial contamination of catheters.27 To prevent blood from collecting in the distal catheter tip, positive pressure should be maintained on the syringe when withdrawing the needle from the port. If done properly, this technique results in a small spray of fluid on the external surface of the port, which can be cleaned. A port should be flushed with saline and a new lock placed each time the port is accessed. Typically, ports are flushed every 3 to 4 d for the first 2 wk after implantation; after this time, flushing should occur every 7 to 14 d if the port is not actively being used.50,3
Catheters and ports may become occluded for a number of reasons, including a kink in the tubing, reactive tissue build-up at the end of the tubing, tubing pressed into a vessel wall, precipitation of administered substances within the tubing, the presence of a clot, or infection.17,76,89 Ability to infuse but not withdraw from a catheter suggests that either the tip is pressing against the vessel wall or that a flap of fibrin or a blood clot at the catheter tip is pulled over the opening when negative pressure is applied. If a catheter or port becomes obstructed, a number of strategies can be used to attempt to restore patency, including the use of tissue plasminogen activator, urokinase, or streptokinase as a clot-dissolving agent.34,38 Extending a limb or turning the animal's head may open a position-related obstruction in the tubing. Kinked catheters must be replaced.
Tethering equipment.
Tethering is a restraint system that permits animals some freedom of movement while protecting a fluid line running to a catheter or pedestal implanted in or on the animal. If not attached directly to an intravenous catheter or head implant, tethers often are attached to the animal through a swivel apparatus that is affixed to a jacket or harness.35,57,58 These systems are often constructed from a hollow flexible lightweight metal coil. Tethers are considered a refinement, because they permit animals to move more freely around the cage during infusion (Figures 3 and 4), although animals tethered by using jackets are unable to roll or rest in a supine position.
Figure 4.
(A) Jacket and tether assemblies are used commonly in various nonrodent species, such as nonhuman primates. (B) The catheter in this animal is attached to an infusion pump and swivel assembly, which are mounted on the exterior top of the cage for ready access.
Pumps.
Precision infusion pumps.
Electrical or battery-operated precision infusion pumps have been in use in veterinary and human medicine for many years and are a reliable means of delivering test substances subcutaneously, intraperitoneally, or intravenously. In larger species, such as dogs, pigs, and nonhuman primates, preprogrammed, battery-operated pumps loaded with the agent to be dosed can be secured in a pocket on a jacket worn by the animal. These pumps can be operated remotely to change the dose or flow rate and can be identified uniquely during use by radiofrequency signals emitted from the pump.35 If the pump is placed in a pocket on one side of the jacket, generally a counterweight is placed on the opposite side to keep the jacket balanced.24 In addition, pumps commonly are used to control dosing in combination with a tether system (Figure 4). These pumps are highly reliable and accurate and come with alarms that indicate when catheters become kinked or blocked. However, infusion pumps require regular maintenance and calibration and can be heavy and cumbersome to use, especially with smaller animals.
Microinfusion pumps.
An electromechanical, programmable, implantable, and refillable microinfusion pump (iPRECIO MicroInfusion Pump, Data Sciences International, St Paul, MN) has become available recently for use in rats and larger species.1,79 The pump is programmed by using a PC-based platform and infrared technology, and it operates independently once activated. Flow onset, flow duration, and infusion rates can all be specified, and ultralow flow rates (for example, 1 to 30 μL/h) are achievable with this device. A separate pump program device keeps a log of pump functions and calculates the volume of agent left within the reservoir. The reservoir has a port that permits emptying of the pump and refilling by subcutaneous injection, giving much more flexibility in the duration of substance administration than that offered by an osmotic minipump. The microinfusion pump itself is implanted into a subcutaneous pocket, and a flexible catheter attached to the pump directs the outflow to a body cavity, tissue, or blood vessel.
Osmotic pumps.
Osmotic pumps are internally implanted devices that use an osmotic displacement system to infuse a preloaded substance into an animal. Use of these pumps permits constant dosing without the need to handle an animal after the initial implant surgery. Extracellular fluid is absorbed at a constant rate by an osmotic salt layer immediately beneath the permeable outer membrane. As the osmotic layer absorbs fluid, it swells and puts pressure on an impermeable reservoir in the center of the pump. The reservoir then expels the loaded substance from the pump at a constant rate through a flow moderator. The outflow can pass directly into the tissue surrounding the pump, or a cannula can be attached to the pump to direct the flow into a blood vessel or specific tissue.
Osmotic pumps are cylindrical in shape and come in sizes small enough for mice.36 These devices are surgically implanted either subcutaneously or intraperitoneally. The flow rate is fixed, and the duration of action varies from 3 d to 6 wk, depending on the size of the pump and the delivery rate selected. Pumps cannot be refilled but can be implanted sequentially to prolong dosing.
Oral dosing.
The most commonly used equipment for oral dosing are those required for gastric gavage, pilling (including administration of capsules), and powdered diet. Liquid and water-soluble substances typically are administered directly or as aqueous solutions in water bottles affixed to the caging for voluntary consumption.
Gavage.
Oral dosing typically is conducted in conscious animals to take advantage of the gag reflex and decrease the likelihood of inadvertent intratracheal dosing with subsequent aspiration. Flexible or rigid straight or curved gavage needles and pediatric nasogastric feeding tubes can be used for this purpose (Figure 5). Ball-tipped stainless steel needles are easy to clean and sterilize but carry an increased risk of esophageal rupture if their passage is forced. Softer, flexible rubber or plastic gavage tubing or needles reduce the likelihood of tissue damage and puncture but may be chewed by the animal if not placed correctly in the mouth and may require a mouth gag with use in larger animals. Nasogastric tubes can be used in larger animals for acute dosing or can be anchored in place for several days duration by using a stay suture or a drop of surgical glue.9 If nasogastric tubes are left in place, an Elizabethan collar may be needed to prevent the animal from dislodging the tube. Some animals do not readily tolerate these collars. Further, in some animals, chronic tube placement may result in repeated emesis.
Figure 5.
(A) A variety of plastic and stainless steel gavage needles are available for dosing in rodents and can be straight or curved. Flexible needles (left) reduce the likelihood of inadvertent esophageal rupture but can be chewed by the animal. Ball-tipped curved (rat-sized, middle left) and straight (mouse-sized, middle right and right) needles are readily sanitized but can induce injury if their passage is forced. (B) Measuring the gavage needle for appropriate length for dosing, from tip of nose to last rib. The needle can be marked for easy visualization of the appropriate insertion distance.
The correct length of gavage tubing is determined by measuring from the tip of the animal's nose to its last rib; when inserted this distance, the tip of the tube should be in the stomach or very distal esophagus (Figure 5). When the dose has been delivered by gavage, the syringe is left on the tube as it is withdrawn. Other options include kinking or clamping the tube to prevent fluid from leaving the tube as it is withdrawn, minimizing the risk of aspiration.
Pilling or bolus delivery.
Capsules and pills can be administered to rodents larger than 150 g (http://www.capsugel.com/products/pccaps.php), rabbits, dogs, cats, and nonhuman primates by means of pill or balling guns. These devices are long, slender, hard plastic or metal tubes with a small slot or receptacle at the end to hold the pill and a plunger that can be depressed to deliver the pill when the device is at the back of the animal's tongue. In rodents, the capsule can be placed into the distal esophagus or stomach. Another option, used with nonhuman primates, is to place the pill or capsule snugly into the tip of hollow tubing and pass the tubing as for orogastric dosing. Once the tube is in place, a bolus of air is pushed into the tubing with a syringe, to dislodge the pill. If a gelatin capsule is used, an oil lubricant such as vegetable oil is preferred over water-based lubricants, because the water-based lubricant may adversely affect the integrity of the capsule. Because the pill blocks the tube lumen, palpation is the best way to confirm correct tube placement prior to dispensing the pill.
Powdered diets.
Powdered-diet containers can be used to provide medications in the food of rodents. These are round cups, sometimes with a weighted bottom, into which precise amounts of medicated diet are placed. A round lid with a central hole is affixed to the top of the cup, permitting an animal to reach the food while minimizing spillage and waste. Two-piece stainless steel diet cups are sanitized easily and can be autoclaved, as needed.
Medicated food and food treats.
Preformulated treats containing various medications are available for many species commercially and can be custom made. In addition, pelleted diets can be purchased or made to incorporate medications or other ingredients at specific doses.
Specialized cages are available with electronically controlled feeders that open and close at preset times, to measure or limit the amount of medicated diet eaten and to record the timing and duration of feeding sessions. Similar gating systems linked to a radiofrequency-emitting collar or ear tag can be used for dogs and larger species to control or record access to medicated feed.
Restraint devices.
Restraint devices are designed to minimize injury to the animal and the handler while keeping an animal still and providing access to specific body parts for dosing. For many species, restraint is a known stressor and restraint itself is a technique specifically used to study stress responses in animals.61 As such, consideration should be given to dosing techniques that permit free movement of animals, periods of restraint should be minimized, and animals should be habituated to restraint with positive reinforcement training, whenever possible. Rigid restraint devices can be padded to improve comfort, and towels or other material can be used to provide traction on slippery surfaces of restraint containers. Furthermore, to minimize the risk of injury, escape, and respiratory crises, animals should not be left unattended in a restraint device and should be observed directly at frequent intervals if they cannot be visualized readily when placed in the restraint device. To enhance personnel safety and to minimize animal injury and stress, restraint devices may be used in combination with various sedatives or anesthetics.
Rodent restraint devices.
Broome tubes, similar rigid tubing, or even modified 60-mL syringe cases can be used to restrain rodents for access to tail vessels (Figure 6). The correct size permits the animal to enter head first for its entire body length but is not so wide that the animal can turn 180° within the tube. Tubes may be outfitted with a plug or stopper that passes over or around the tail, to secure the animal in place. Animals may enter a dark tube more readily than a brightly lit one, and variations are available that include warming plates to increase peripheral vasodilation for venipuncture or light sources to aid in blood vessel visualization.
Figure 6.
Restraint tubes can be used for tail access in small rodents to minimize movement during dose administration while optimizing handler safety.
Plastic or cloth cone-shaped bags can be used for temporary restraint of rodents. When plastic is used, a small hole must be cut into the end of the bag to permit the animal to breathe. The animal is placed head-first into the bag, and the excess material gathered to hold the animal snugly in place. Animals become hot in these bags and should only be restrained for the minimal time necessary. However, the warmth increases peripheral vasodilation, enhancing visualization and access to blood vessels for dosing. Subcutaneous, intradermal, and intramuscular injections can be made through a small hole cut in the plastic, and the animal's tail can be accessed for intravenous delivery where the tail comes out of the bag.
Rats can also be restrained in cloth wraps or towels. Rats readily enter a small dark tunnel that can be made in a fold of towel, or a rat can be rolled gently but snugly in a cloth. The cloth then is folded back to permit access to the body part needed for dosing. Stretchy tubular stockinette is another useful material for restraint of rats and other large rodents. The material is cut to a length several inches longer than the animal's body length. A short cuff is folded at one end and taped, to provide a small opening that the rat cannot push its head through. The opening at the other end of the tube is drawn back over the animal and provides close-fitting restraint.
Rabbit restraint devices.
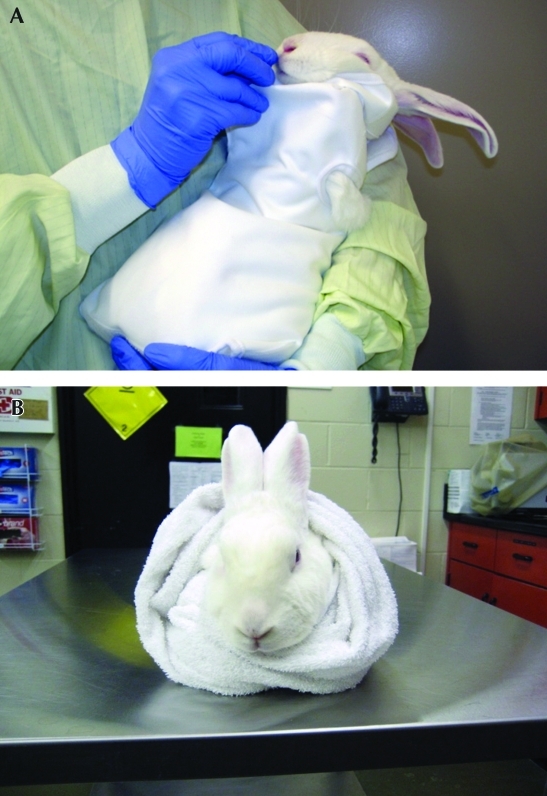
Rabbits can readily be examined and dosed by any route when snugly wrapped in a towel or commercially available cloth restraint bag (Figure 7). Covering a rabbit's eyes with a cloth during restraint may help to keep the animal calm. Soft, firm wraps that provide even pressure over the body may have a calming effect. For oral dosing, the operator can sit in a chair and hold in the lap a firmly wrapped rabbit positioned to face the operator. In addition, acrylic and stainless steel dosing restrainers are commercially available and can be tipped backward, once an animal is firmly restrained within, to hold the rabbit in an upright position, permitting a single operator to orally dose the animal.2 Plastic restrainers are warmer than metal ones and may be more comfortable to animals. Some of these devices include a head gate that secures around the rabbit's neck. Special care must be taken when using a head gate to ensure that the animal can breathe freely. Rabbits may panic and injure their spine or limbs in these restraint devices, and acclimation to the equipment is advised.
Figure 7.
Rabbits can be restrained calmly by using a (A) commercially available cloth restraint bag or (B) a towel.
Slings.
Slings can be used to restrain mice, rats, fish, dogs, small ruminants, and other species for periods as long as several hours. Combinations of zippers, hook-and-loop fasteners, and soft ties hold the animal resting securely on its abdomen in the sling. For dogs, pigs, and small ruminants, the feet typically extend below the sling bed through openings (Figure 8). Some sling designs use a custom jacket on the animal that then is fastened to the sling frame.71 Dogs may be permitted to keep their feet on the floor while in a sling but also are able to relax and have the sling support their body weight. Animals should be trained with positive reinforcement to enter and relax in the slings and should not be kept continuously in a sling for more than a few hours at a time.
Figure 8.
Adult sheep instrumented with an intravenous catheter and restrained in the Panepinto sling. Photo courtesy of Linda Panepinto.
Nonhuman primate restraint devices.
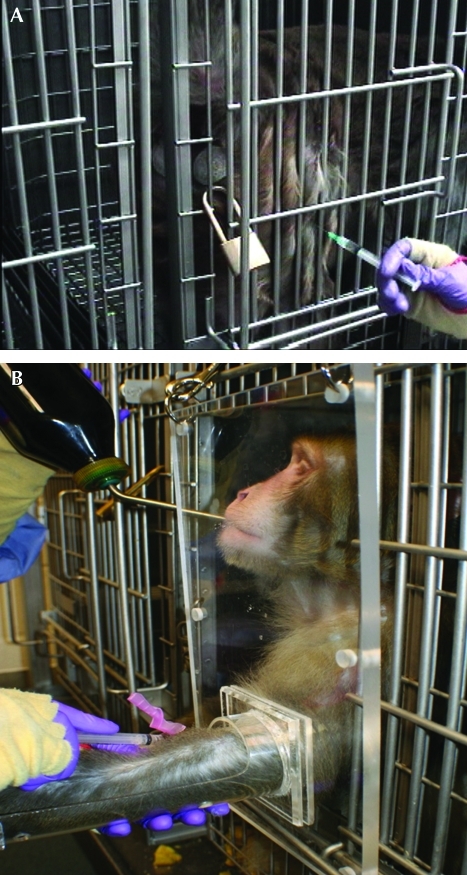
Restraint of nonhuman primates requires additional thought because of the potential for personnel injury and zoonotic disease transmission. Whenever possible, training for voluntary compliance with substance administration should be used, to minimize risk of injury to the animal and handler (Figure 9).39,64 Adaptations can be made to cages such that animals can safely present a body part, such as a forelimb, for intravenous dosing or sampling. Animals also can be trained to hold a limb inside a rigid plastic tube extending from the cage; the operator can access the limb and blood vessel through a cut-out section of the tube (Figure 9).
Figure 9.
(A) Positive reinforcement training can be used to minimize restraint needed for dosing nonhuman primates. In the image depicted, the animal has been trained to accept an intramuscular injection with minimal restraint. (B) Animals can be trained to insert a limb into a plastic sleeve for intravenous dosing. Fruit juice is being given through a water bottle as positive reinforcement during the procedure. Photos courtesy of Oregon National Primate Research Center.
For in-cage dosing, squeeze-back cages permit an animal to be pressed gently and immobilized at the front of the cage, to permit rapid dosing by injection. Correct technique has the animal pressed with its side to the front of the cage, so that the force is applied to the animal's shoulders.20 Care is taken not to entrap or damage the animal's digits or tail at the top or bottom of the moving back panel of the cage. Similar out-of-cage crank-operated squeeze devices can be used when a conscious animal must be observed closely or for dosing conscious animals by any route. Animals enter these devices by means of a transfer box.
Pole-and-collar restraint systems are used most commonly with macaques, baboons, and squirrel monkeys. A plastic or metal collar fitted to the neck of the animal is attached to catch poles by means of rings or clips. By use of the poles, the animal is guided from its cage to a chair with a yoke to which the collar is affixed, securing the animal to the chair. Typically 2 poles and 2 people are used to guide the animal to and from the chair. Animals must be conditioned and trained to accept the pole-and-collar system.6
Restraint chairs are designed to permit convenient and safe access to primates for dosing or other procedures while the animal is in a comfortable position. Chairs are made of hard plastic or metal for easy sanitation and can be adjusted for the size and anatomy of individual animals. Larger animals may be placed in a chair by using a pole and collar system, whereas smaller animals may be hand-caught and placed into a chair. Animals must be trained with positive reinforcement to acclimate to chair use and should be restrained in a chair for the shortest time necessary.68
Restraint tubes can be used for small species such as marmosets and tamarins. An animal is hand-caught and placed into the plastic or metal tube, which encases the body's trunk to hold the animal in place but permits access to limbs. The animal's head remains outside the tube. Restraint stocks are used with larger primate species, and a pole-and-collar system can be used to transfer the animal to the stock. Animals should be accustomed to this form of restraint and only be kept in stocks or tubes for the minimal time necessary for dosing.
Consideration of technique and selection of appropriate dosing equipment can contribute markedly to the success of a study and to the comfort of an animal that undergoes dosing. These factors are important for animal care committees to consider when reviewing protocols in which animals will be given substances.
Vehicle Selection for Administration of Substances
Selection of the appropriate dose form for administration to animals is both a science and an art. Many substances are administered to animals as solutions or suspensions. A solution is a homogeneous fluid mixture of 2 or more substances that cannot readily be separated, for example, table salt dissolved in water. A suspension is a heterogeneous fluid mixture that contains solid particles that are dispersed throughout the liquid phase and that may sediment, for example, flour in water. Both solutions and suspensions can be administered to animals by any common delivery route; however, both must be free of particulates when administered intravenously to minimize embolization.
The final mixture that is prepared for dosing is termed the ‘formulation.’ Formulations must be optimized for the study design, species being evaluated, and characteristics of the compound being administered. Formulation science refers to the theory and technology used to develop and understand the dose forms of medicines. This body of knowledge is applied across many disciplines including discovery chemistry, toxicology, pharmaceutical chemistry, and pharmacology, and in the design of most studies in which animals are dosed with a substance. In addition, dose formulation is an area in which often animal care committees and veterinarians do not feel comfortable or even competent reviewing and making decisions about, and yet the formulation may have as profound an effect on the animal as the test substance itself.
Excipients are the various additives used to convert pharmacologically active compounds into pharmaceutical dosage forms suitable for administration to patients. The function of excipients in the final formulation can be as solvents, binders, surfactants, stabilizers, emulsifiers, lubricants, sweeteners, anticaking ingredients, and so forth. The term ‘vehicle’ is used to describe these compounds when they become the medium in which the test substance is dissolved or suspended for administration.66 When considering how a substance can be administered to an animal, the objectives for selecting a particular dose formulation and vehicle include: 1) optimizing in vivo exposure to the test substance in the target tissue(s); 2) accuracy of dose delivery; and 3) minimizing vehicle-related side effects and toxicity.45 The homogeneity and stability of test solutions and the formulation shelf-life of admixtures are also important, particularly for long-term nonclinical safety studies. The following paragraphs discuss acceptable characteristics of excipients, recommendations for dose formulations, and an approach for vehicle selection.
Many factors contribute to vehicle tolerability, including those relating to the animal, the properties of the vehicle, the procedures that are being performed, and the nature and duration of the study.29 Animal species respond to vehicles in different ways, for example, some vehicles believed to be safe in nonhuman primates and dogs may not be well tolerated in rodents, and vice versa. For example, hydroxypropyl-β-cyclodextrin is commonly used as a vehicle for oral dosing solutions but may induce diarrhea in dogs and nonhuman primates and elevated hepatic transaminases in rats and mice.83 Even within a species, various strains can show significant differences in tolerance to vehicles. For example, at certain concentrations, hydroxypropyl-β-cyclodextrin caused soft stool and diarrhea in db/db mice but not in inbred control mice.
In addition, the fed or fasted state can affect tolerance for a vehicle. Animals may become less tolerant of vehicles as their plane of nutrition decreases on a study due to toxicity. Because clinical signs are monitored closely during the evaluation of novel compounds, the effects of the vehicles may confound the study effects, especially if not controlled for adequately.
Finally, duration of the study is an important consideration when selecting vehicles. Vehicle selection for a single-dose oral study generally is less of a concern than for chronic repeated-dose studies, because any negative side effect of the vehicle is less likely to be manifested over a short dosing period.
Characteristics of vehicles.
Careful consideration must be given to the physical and chemical nature of vehicles, including their pH, osmolality, and viscosity. Viscosity is particularly important when considering the ease of delivering the material through a small-gauge intravenous or gavage needle. The pH of the dosing solution or suspension is a critical factor to consider, given that it determines how well a substance is absorbed, where it is absorbed (if administered orally), and whether the solution or suspension induces tissue injury.37 Dosing of solutions or suspensions with a pH that is either too acidic or alkaline can cause diarrhea, vomiting, tissue ulceration or necrosis (either at the site of catheter entrance or exit or gastric ulceration), and pain on administration. Table 2 provides recommendations for pH optimization for various routes of delivery.
Table 2.
Optimal pH range for substances according to administration routea
| Administration route | pH range | References |
| Oral | 2–9 | 45 |
| 4.5–8 | 54 | |
| 5–9 | 23 | |
| Intravenous | 4–9 (bolus dosing) | 29 |
| 3–9 (continuous infusion) | 29 | |
| 2–9 | 45 | |
| physiologica | 54 | |
| Subcutaneous | physiologica | 45, 54 |
| Intramuscular | 2–9 | 45 |
| physiologica | 54 | |
| Intraperitoneal | physiologica | 45, 54 |
Administration of substances outside of the recommended pH ranges may result in tissue necrosis and vascular thrombosis.
Approximately pH 7.3–7.4.
For oral and intravenous administration, the water solubility of any compound is increased by ionization, and knowledge of the pKa of a substance can be used to determine the optimal pH of a solution to promote ionization, solubility, and absorption. For example, Bronsted–Lowry bases (proton acceptors), such as erythromycin, are ionized and therefore more soluble in low-pH solutions whereas Bronsted–Lowry acids (proton acceptors), such as penicillin–V–potassium, are ionized in higher pH conditions.42 pH varies in vivo according to species, physical location within the body, and diet.45,56 Because pH is a logarithmic scale, 1 unit difference in pH results in 10-fold variation in ionization and aqueous solubility. The gastric pH of the dog presents a problem when preparing solutions for dosing, because the gastric content of the dog stomach can vary between pH 2.7 to 8.3, depending on fed compared with fasted states.56 This changing environment can affect the ionization state of the compound, making it less soluble and resulting in precipitation in the gastric lumen. Bile salts and lipid composition greatly affect solubility and oral absorption of compounds. Differences in gastric acid production and bile composition and secretion vary greatly between species and can affect dose solubility in vivo.56 Certain biologics, such as red blood cells, insulin, coagulation factors, and antibodies may denature when exposed to nonphysiologic pH.45 Therefore the pH of vehicles is often an important consideration when preparing a substance for administration to animals.
In addition to pH, other physical and chemical properties that need to be addressed when formulating compounds include the temperature of the formulation, buffering capacity of the dose formulation (which helps to stabilize the solution pH in different body environments), the type of salt formed during purification of a new chemical substance or drug, and other factors that may enhance the potential for precipitation of the compound in various tissues (such as the kidney) before or after dosing.45,54,77 Gentle heating may assist with solubilization of substances; however, after heating or filtration, the content of the solution should be observed for the presence of particulates before being administered intravenously to a study animal. These processes can cause precipitation, physical degradation of the compound, or adsorption of the compound to the filter material.29 Ionic reactions with divalent salts, such as calcium and magnesium, tend to result in formation of insoluble precipitates with fatal consequences if a solution is administered intravenously. This precipitation can occur with changes in pH, for example, due to diet, or when different drugs or solutions are mixed and coadministered.63 The likelihood of precipitation and crystallization of administered compounds in the kidney or urinary bladder depends on the pH of the filtrate and urine, which can be influenced markedly by diet, hydration status of the animal, renal function impairment, and hypoalbumenemia.18
Approaches to Dose Formulation
Ionizing agents.
Ionizing agents are aqueous solutions that provide a buffered pH for optimal dissolution of compounds. Examples include citric acid, sodium bicarbonate, maleic acid, and lactic acid. pH buffering systems work best for weak acids and bases that ionize at physiologic pH, that is, from pH 2 to 9. Buffering solutions maintain drug solubility at the buffer pH. High buffering capacity helps prevent precipitation of the test substance; however, caution must be used, because high capacity buffers and strong acid–base controlled pH solutions can be deleterious to the animal's physiologic acid–base system in vivo. The other concern with using high capacity buffers and strong pH formulations is the potential for tissue irritation and damage, especially when administering the dose subcutaneously or intramuscularly. Strategies to administer doses with reduced buffer capacity and acceptable pH ranges are more desirable.45
Cosolvents.
Cosolvents are used to enhance the solubility of poorly water-soluble compounds.51 The most common cosolvents used in formulations include ethanol, propylene glycol, polyethylene glycol, and glycerine.80 A major limitation to using cosolvents is the potential for the drug to precipitate once it is administered, especially after intravenous bolus applications. Recommendations for minimizing this effect include: using a slow injection or infusion technique, when possible; minimizing the concentration of the drug in the dose; and incorporating a low percentage of surfactant in the formulation.45
DMSO is a powerful cosolvent and often is used for early metabolic studies when compounds are poorly characterized and difficult to solubilize. DMSO has low systemic toxicity in animals but does not provide an ideal formulation because it can cause significant local toxic effects, including, vesiculation, a sensation of burning, local allergic reactions, and dryness of skin.11,16,88,66,67 Other potential effects of DMSO include increased membrane permeability and bile secretion, which can enhance uptake and systemic exposure to lipophilic drugs. Alteration of pharmacodynamic and pharmacokinetic parameters can be misleading in early metabolic studies when comparing data between different formulations.62
Cyclodextrins are cyclic oligosaccharides with varying sugar moieties (α-, β-, and γ-cyclodextrin have 6-, 7-, and 8-member rings, respectively). These molecules form water-soluble ligand–drug complexes with hydrophobic molecules, enhancing solubility. Cyclodextrins are relatively safe for administration in animals and are used extensively for early formulation studies, particularly because a small percentage of hydrophilic polymers in cyclodextrin-based formulations can enhance drug solubility.47 A maximum tolerated dose of 3600 mg/kg daily was reported when administered orally in dogs, and 5000 mg/kg daily was tolerated in the rat. Mice dosed with as much as 1200 mg/kg daily had no observable adverse effects.74 When administered to rats parenterally, cyclodextrins induced toxicity in the lungs and kidneys because the compound accumulates in these tissues as insoluble cholesterol complexes, with lethal doses occurring between 780 and 1000 mg/kg.22
Surfactants.
Surfactants serve multiple functions, and they can act as wetting agents; reduce or eliminate drug precipitation; decrease drug degradation or modulate drug release; and facilitate drug uptake.45 Surfactants can be used at low percentages to enhance solubility alone or in combination with pH control.41,43 Commonly used surfactants include polysorbates (for example, Tween 80) and polyoxyl castor oil (Cremophor EL).44 Compared with cosolvent systems, drugs formulated with surfactants are less likely to precipitate with hemodilution in the animal.45 Micelles or bile salt micelles are another possible formulation option as a surfactant.15, 86 Commonly used bile salts include taurocholate, taurodeoxycholate, and deoxycholate. Lipids used as surfactants or emulsifying agents include egg or soy phosphatidylcholine, soy phosphatidylethanomine, oleic acid, and monoglycerides.
A negative attribute of surfactants such as Cremophor EL is that they can cause systemic toxicity including histamine release, particularly in dogs.48 Signs of this response in dogs include hyperactivity followed by depression; flushing and swelling of the muzzle, ears, and limbs; and agitation with decreased blood pressure and increased heart rate. These signs can be ameliorated by preemptive intravenous administration of an antihistamine, such as diphenhydramine.48
Suspensions.
Suspension dosing is another method for preparing substances for administration that are poorly water soluble. Suspension dosing often permits studies to be initiated while better formulations are identified. Suspensions are easily prepared by using an aqueous solution with a small percentage of hydrophilic polymers or surfactants (or both). This dosing method often is optimized by using very small particle sizes (particle size management) and by preparing the dose form immediately prior to administration to the animal.45 Physical stability can be a major problem for suspension preparation and storage. Techniques used in dose form preparation include warming, sonication, and vortex mixing. Warming by microwave should be avoided because it may destabilize molecules. Drug substances are usually available in a free or salt form for these preparations. It is important that the compound remain suspended during dosing to ensure accuracy, and this goal can be facilitated by use of a low speed stir plate with magnetic stir bars. If the compound comes out of suspension, then the quantity of administered compound can become a significant variable in the study. It is not unusual that the compound is in solution at lower doses, whereas a mix of solution and suspension exist in the same formulation at high doses. This duality is especially true when examining dose responses in a wide range.56
Particle reduction.
Particle sciences have enhanced the bioavailability and absorption of new chemical and drug compounds greatly. Micronization is a process by which the compound is milled to reduce the particle size and increase the dissolution rate prior to suspension dosing. Another method of reducing particle size is nanomilling, a process of particle size reduction to sizes smaller than 100 nm.15 This formulation strategy is used in the preparation of both oral and intravenous substances, because the minute particles can pass through capillary beds (smaller than 2 µm).55 Use of these techniques facilitates formulation of compounds previously considered near impossible to formulate.
Emulsion.
Emulsions are 2-phase systems that consist of oil and water with particles stabilized by surfactants in interfacial phases, for example, the commercial formulation of propofol. In addition, emulsions frequently are used to create nutritional products developed for parenteral administration. Emulsions typically contain soybean oil, lecithin, and glycerine and are buffered to pH 7.45 Because they may contain organic components that support bacterial colonization, emulsions must often be handled and stored carefully to minimize contamination. Other oils, such as corn and coconut, have also been used as emulsifying agents.23 Caution should always be used when administering oily substances orally to avoid aspiration and subsequent foreign body pneumonia.
Experimental considerations for vehicle selection.
Dose administration problems can occur when administering high dose concentrations or when it becomes necessary to administer large volumes. High dose suspensions often have physical stability issues such as high viscosity, aggregation, and caking, resulting in difficulties in administration and heterogeneous drug distribution.56 Guidance for dose volume is typically set by the animal care committee or institution81 (see Table 1 of reference 81 for volume recommendations by route) and can be limiting when dealing with less soluble substances that require a more dilute solution. In these cases, alternate formulation strategies may be required.
Several issues commonly arise when initiating investigations with novel substances or drugs. Often compounds are poorly characterized and may be unstable or degrade in the vehicle selected. Research personnel, including students and technical staff, administering the substance need to be familiar with this problem and ready to reject any substance that is to be administered intravenously that contains visible particulates or is too viscous for administration. Other more polar compounds may require special heating or mixing prior to dose administration. If the compound becomes supersaturated when heated, different physical phases may separate when cooled. Doses should only be administered at temperatures equal to or less than core body temperature. Many formulations are viscous, making oral or intravenous dosing difficult. Viscous solutions or suspensions may adhere to the outside walls of the dosing needle, resulting in vehicle and compound being aspirated during oral gavage. Good communication between personnel dosing the animals and those preparing the dosing solutions or suspensions is critical to minimize these adverse effects.
Veterinary and animal care personnel should be familiar with study protocols and be vigilant for signs relating to vehicle intolerance. Cosolvents, surfactants, and lipid-based vehicles can have laxative effects, resulting in diarrhea and vomiting. Long-term diarrhea can create fecal matting on the rectum and abdomen, resulting in scalding and eventually erosion of the skin. If fecal matting persists, the animal can become obstructed and die. Understanding the dose preparation history can be key to understanding findings on necropsy. It is not uncommon to find precipitated materials in the gastrointestinal tract of the animal at necropsy if the formulation has not been optimized.
Animal care committees have varying requirements for reviewing vehicle selection in study protocols. No vehicle is truly without biologic effects, and even innocuous vehicles, such as sterile water and 0.9% saline, can cause deleterious unintended consequences, such as fluid overload, when given intravenously, or unintended diuresis.29 At minimum, committees should request and consider information related to the volume and rate of administration of vehicles, the species being dosed, the route of administration, and in vitro solubility testing, because all of these factors determine the maximum dose that can be administered to animals and the likelihood of problems occurring during dose administration. The lowest possible dose or rate of delivery that ensures adequate exposure to the substance should be used, and the total volume of vehicle being administered must be kept in mind to avoid vehicle toxicity. Solubility testing of a substance in the proposed vehicle is an easy in vitro test that should be used before any novel substance is administered to an animal, to reduce unnecessary animal use, ensure accuracy of dose calculations, and minimize waste of the novel substance or drug being administered.30 For institutions and facilities that test many novel substances or compounds, creation of a database that documents information on vehicle selection and any resulting toxicity or safety concerns can be useful when new experiments are being planned. Pilot studies should be performed before the use of any new vehicle or vehicle application. Formulation experts, such as pharmaceutical chemists, should be contacted prior to beginning a study to address concerns about the dose solution or suspension. Experimental designs should include vehicle controls that serve as comparators, so that study outcomes attributed to treatment are not confused with effects of the vehicle. Animal health concerns resulting from inappropriate formulations should be discussed by the research group, veterinary staff, and animal care committee, with consideration given for finding alternative formulations.
Formulation Strategies
Comprehensive reviews regarding specific vehicles, their properties, applications, and toxicities have been published.23,45,46,56,66,72,85 The strategy used will be determined in large part by the route of substance administration. Overviews of approaches for oral and parenteral dose preparations are summarized in the following sections.45
Oral formulations.
For oral solution formulations, vehicle selection should begin with consideration of aqueous buffers. For substances with poor solubility, small volumes of cosolvents, cyclodextrins, or micelles can be added. Potentially, the combination of pH adjustment with these vehicles can be helpful for dissolution. If solubility precludes preparation of a solution, a (micronized) suspension in methylcellulose and water, with or without an added surfactant such as Tween 80, may be prepared. If these approaches are not successful, more novel formulations, such as nanosuspensions, solid dispersions, and emulsions, can be considered.
Parenteral formulations.
The sequence for preparing intravenous solutions is similar to that for oral formulations: aqueous buffers, followed by cosolvents, cyclodextrins and micelles, or the combination of these technologies with pH adjustment. The combination of cyclodextrins or cosolvents with pH adjustment is especially effective. An important concern regarding intravenous dose preparation lies in the potential for drug precipitation after injection. It is recommended that the dose preparation be evaluated in vitro prior to dosing by using a serial dilution method30 to ensure safety. For intravenous studies, solutions should be the primary dosing method. When a solution cannot be formulated, nanosuspension or microemulsion can be considered.
In summary, when preparing substances for administration to animals, consideration needs to be given to factors such as the species, study type, duration, and the vehicle used. Several different formulation strategies can be used to optimize dose preparation, including the use of ionizing agents, cyclodextrins, cosolvents, surfactants, suspension dosing, emulsions, and combinations of these methodologies. Recommendations regarding appropriate pH ranges should be followed, and attention must be given to other physical and chemical properties of both the substance to be administered and the vehicle that may contribute to irritation. Newly proposed formulations should be scrutinized carefully prior to dosing to prevent animal health issues relating to the vehicle. Specific toxicities relating to vehicles exist, and research and animal care personnel should be made familiar with the potential adverse clinical signs. Collaboration with scientists experienced with novel dose preparations is invaluable when new substances must be administered to laboratory animals.
Considerations Regarding Solute Preparation
Preparation of substances for administration.
Careful attention to sample preparation is necessary whenever substances are administered to animals. A clear workspace should be used for preparation of doses and substances. This preparation includes clearing the site of used vials, other medications, and other doses of the substance, if multiple doses are being administered in a study, to minimize dosing errors. Similarly, for studies with multiple dose groups, an organized system of administering substances to animals should be developed to minimize dosing errors. For example, control animals frequently are dosed first and high dose animals last, and cage cards can be color-coded for different dose groups to assist with visual recognition of different groups.
All substances being administered parenterally should be free of particulates and other forms of contamination, such as bacteria, unless these organisms are specifically being administered to animals. Whenever possible, purchased or synthesized materials for administration should be of pharmaceutical grade, as designated in the US Pharmacopeia National Formulary (http://www.usp.org/USPNF/), and free of impurities, including foreign bodies, concomitant substances, and signal impurities, such as chemical intermediates, isomers, and by-products, organic volatile agents, residual solvents, and toxic impurities.4,32 Impurities can be introduced or arise from a number of sources, such as during synthesis of the substance, during formulation (when various excipients and vehicles are added), during heating, or secondary to degradation. Degradation occurs commonly with many compounds over time, for example, with anesthetic agents exposed to soda lime,5 but the process can be accelerated if lack of attention is given to correct storage of the substance.
Samples prepared as suspensions or solutions should be shaken well prior to use. The administration of pills that need to be cut or crushed to achieve the correct dose should be considered carefully. Many drugs are produced in slow-release or enteric formulations that require the full protective coating for efficacy. Other substances become bitter when cut or crushed and unpalatable to the animal or contain toxic or carcinogenic substances that put the operator at risk.60
Multiuse vials and other storage devices.
When administering a liquid by injection, whenever possible, a sterile single-dose vial should be used for administering substances to animals. Practically, this goal is not always possible, such as when batching surgeries to rodents or dosing large numbers of animals; however, a number of strategies can be used to minimize contamination. If a multidose vial must be used, dosing should not occur out of the stock bottle. Rather, a small aliquot, for example, enough for one to several days of experimentation, should be transferred to a sterile multiuse vial. This way, if the bottle is contaminated inadvertently, it will be replaced quickly, minimizing the effect of cross-contamination between animals. The bottle should always be inspected closely before withdrawing fluid to ensure that the substance is free of particulates and unexpected cloudiness and that the expiration date has not been surpassed. Cracked vials or those with precipitate around the outside of the stopper should not be used. Further, when using multiuse vials, the rubber stopper should always be cleaned with alcohol and allowed to dry prior to use. A sterile needle should be inserted to puncture the septum and should not be left in the septum between uses. Because pushing a small-gauge needle through a rubber stopper dulls the needle and may introduce burrs or other irregularities on the needle tip, both of which can increase injection pain in patients with delicate skin, it is preferable to put a clean needle on the syringe prior to injection. An appropriately sized new, sterile needle should always be used for each injection. Any remaining material left after a multidose vial is no longer in use must be discarded appropriately.
Storage.
Substances should be stored securely, in accordance with laws, regulations, and organizational policies, so that unauthorized persons cannot access them. Controlled substances must be stored properly by using a double-lock system to prevent diversion, according to state or provincial and federal laws and regulations.13 Leaving the drug storage keys in the cabinet locks, when not in use, is unacceptable. Medication storage areas should be inspected during routine facility and laboratory visits. Until returned to the pharmacy for disposal, expired substances should be marked clearly and kept separated from unexpired substances.
Labeling.
Correct labeling of substances administered to animals is critical. Appropriate labels contain the substance name, strength, and amount; preparation and expiration dates; and vehicle (as appropriate).65 Bags of sterile intravenous fluids do not require additional labels if they are being used as is; however, the bag should be labeled with the date when first opened and discarded within a week of that date. Very small economies are realized by keeping opened fluid bags for protracted periods of time, and the risk of contamination outweighs any potential savings.
Record-keeping.
Doses, volumes, and frequency of administration should be calculated, verified, and documented, whenever possible, by 2 people. Prescribing errors are very common in human and veterinary medicine and often linked to external factors, such as overwork, heavy caseload, multiple people recording in the same record—all factors that occur commonly in vivaria.8,14
If substances are dispensed to investigators, dispensing must adhere to laws, regulations, and licensure requirements, including any legal requirements for record keeping. Substances administered should be noted clearly in the animal's medical record, a log book, or the experimental record, depending on the species and substance being administered.
Adverse effects should always be documented. In addition to good record-keeping, having a written record of the doses and calculations used for substance administration can be very useful when trying to determine the cause of an unexpected effect.
Conclusions
When substances must be administered to animals for therapeutic or experimental purposes, careful consideration should be given to a number of factors to optimize dose delivery while minimizing adverse effects that may be experienced by laboratory animals. Experimental planning should always include selecting the most appropriate equipment and restraint method for route of administration and duration of the study. In addition, much thought should be given to the suitability of the vehicle for dosing and to methods of optimizing handling materials that will be administered to the animals. When evaluating new substances in animals, in vitro solubility testing is important for minimizing adverse effects related to substance precipitation, and pilot studies should be considered for novel vehicles or vehicle combinations.
Acknowledgments
Many thanks to David Bailey and Ron Brashear for comments and guidance regarding vehicle selection and to C Terrance Hawk and Jeffrey Everitt for review and comments.
References
- 1.Abe C, Tashiro T, Tanaka K, Ogihara R, Morita HJ. 2009. A novel type of implantable and programmable infusion pump for small laboratory animals. J Pharmacol Toxicol Methods 59:7–12 [DOI] [PubMed] [Google Scholar]
- 2.Abell P, Pangilinan GN, Chellman GJ. 1995. Novel restraint device for oral dosing of rabbits. Contemp Top Lab Anim Sci 34:86–87 [PubMed] [Google Scholar]
- 3.Access Technologies [Internet] Technical bulletin: vascular access port technical guide. [Cited 23 May 2010]. Available at: http://www.norfolkaccess.com/TechnicalInfo.html
- 4.Ahuja SS. 2007. Assuring quality of drugs by monitoring impurities. Adv Drug Deliv Rev 59:3–11 [DOI] [PubMed] [Google Scholar]
- 5.Anders MW. 2005. Formation and toxicity of anesthetic degradation products. Annu Rev Pharmacol Toxicol 45:147–176 [DOI] [PubMed] [Google Scholar]
- 6.Anderson JH, Houghton P. 1983. The pole and collar system. A technique for handling and training nonhuman primates. Lab Anim 12:47–49 [Google Scholar]
- 7.Bestul MB, VandenBussche HL. 2005. Antibiotic lock technique: review of the literature. Pharmacotherapy 25:211–227 [DOI] [PubMed] [Google Scholar]
- 8.Boothe DM. 2006. Veterinary compounding in small animals: a clinical pharmacologist's perspective. Vet Clin Small Anim 36:1129–1173 [DOI] [PubMed] [Google Scholar]
- 9.Brown C. 2010. Nasogastric tube placement in the rabbit. Lab Anim (NY) 39:14–15 [DOI] [PubMed] [Google Scholar]
- 10.Caruso F, Darnowski JW, Opazo C, Goldberg A, Kishore N, Agoston ES, Rossi M. 2010. Taurolidine antiadhesive properties on interaction with E. coli: its transformation in biological environment and interaction with bacteria cell wall. PLoS ONE 5:e8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavaletti G, Oggioni N, Sala F, Pezzoni G, Cavalletti E, Marmiroli P, Petruccioli MG, Frattola L, Tredici G. 2000. Effect in the peripheral nervous system of systemically administered dimethylsulfoxide in the rat: a neurophysiological and pathological study. Toxicol Lett 118:103–107 [DOI] [PubMed] [Google Scholar]
- 12.Cinelli P, Rettich A, Seifert B, Bürki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184 [DOI] [PubMed] [Google Scholar]
- 13.Controlled Substances Act 39 CFR 3674, § 1301.75: Physical security and controls for practitioners. [Google Scholar]
- 14.Dean B, Schachter M, Vincent C, Barber N. 2002. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet 359:1373–1378 [DOI] [PubMed] [Google Scholar]
- 15.De Jong WH, Borm PJ. 2008. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 3:133–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De la Torre JC, Surgeon JW, Ernest T, Wollmann R. 1981. Subacute toxicity of intravenous dimethylsulfoxide in rhesus monkeys. J Toxicol Environ Health 7:49–57 [DOI] [PubMed] [Google Scholar]
- 17.Ege CA, Parra NC, Johnson TE. 2006. Noninfectious complications due to vascular access ports (VAP) in Yucatan minipigs (Sus scrofa domestica). J Am Assoc Lab Anim Sci 45:27–34 [PubMed] [Google Scholar]
- 18.Fogazzi GB, Cantu M, Saglimbeni L, Daudon M. 2003. Amoxycillin, a rare but possible cause of crystalluria. Nephrol Dial Transplant 18:212–214 [DOI] [PubMed] [Google Scholar]
- 19.Foley PL, Barthel CH, Brausa HR. 2002. Effect of covalently bound heparin coating on patency and biocompatibility of long-term indwelling catheters in the rat jugular vein. Comp Med 52:243–248 [PubMed] [Google Scholar]
- 20.Fortman JD, Hewett TA, Bennett BT. 2002. Experimental methodology, p 141–154 : The laboratory nonhuman primate. Boca Raton (FL): CRC Press [Google Scholar]
- 21.Francis PC, Hawkins BL, Houchins JO, Cross PA, Cochran JA, Russell EL, Johnson WD, Vodicnik MJ. 1992. Continuous intravenous infusion in Fischer 344 rats for 6 months: a feasibility study. Toxicol Mech Methods 2:1–13 [Google Scholar]
- 22.Frank DW, Gray JE, Weaver RN. 1976. Cyclodextrin nephrosis in the rat. Am J Pathol 83:367–374 [PMC free article] [PubMed] [Google Scholar]
- 23.Gad SC, Cassidy CD, Aubert N, Spainhour B, Robbe H. 2006. Nonclinical vehicle use in studies by multiple routes in multiple species. Int J Toxicol 25:499–521 [DOI] [PubMed] [Google Scholar]
- 24.Gleason TR, Chengelis CP. 2000. The ambulatory model in dog multidose infusion toxicity studies, p 148–160 : Healing G, Smith D. Handbook of preclinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 25.Graham ML, Rieke EF, Wijkstrom M, Dunning M, Aasheim TC, Graczyk MJ, Pilon KJ, Hering BJ. 2008. Risk factors associated with surgical site infection and the development of short-term complications in macaques undergoing indwelling vascular access port placement. J Med Primatol 37:202–209 [DOI] [PubMed] [Google Scholar]
- 26.Green OP, Patten D. 2000. Femoral cannulation using the jacket/harness model in the rat, p 7–19 : Healing G, Smith D. Handbook of preclinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 27.Hadaway L. 2009. Flushing vascular access catheters: risks for infection transmission. Infection Control Resources 14:1–7 [Google Scholar]
- 28.Henderson KK, Mokelke EA, Turk JR, Rector RS, Laughlin MH, Sturek M. 2003. Maintaining patency and asepsis of vascular access ports in Yucatan miniature swine. Contemp Top Lab Anim Sci 42:28–32 [PubMed] [Google Scholar]
- 29.Hickling K, Smith D. 2000. The contribution of vehicles, rates of administration, and volumes to infusion studies. : Healing G, Smith D. Handbook of preclinical intravenous infusion. New York (NY): Taylor & Francis [Google Scholar]
- 30.Higuchi T, Connors KA. 1965. Phase-solubility techniques. Adv Anal Chem Inst 4:117–212 [Google Scholar]
- 31.Horii I. 2000. Jugular cannulation and efficacy studies in the mouse, p 71–80 : Healing G, Smith D. Handbook of pre-clinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 32.Institute of Laboratory Animal Resources 2010. Guide for the care and use of laboratory animals, chapter 2: animal care and use program. Washington (DC): National Academies Press [Google Scholar]
- 33.Huerkamp MJ. 2002. Alcohol as a disinfectant for aseptic surgery of rodents: crossing the thin blue line? Contemp Top Lab Anim Sci 41:10–12 [PubMed] [Google Scholar]
- 34.Hurtubise MR, Bottino MD, Lawson M, McCredie KB. 1980. Restoring patency of occluded central venous catheters. Arch Surg 115:212–213 [DOI] [PubMed] [Google Scholar]
- 35.Jacobson A. 2000. Equipment for continuous intravenous infusion. : Healing G, Smith D. Handbook of pre-clinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 36.Knedla A, Riepl B, Kistella S, Grifka J, Straub RH, Gay S, Scholmerich J, Muller-Ladner U, Neumann E. 2009. The therapeutic use of osmotic minipumps in the severe combined immunodeficiency (SCID) mouse model for rheumatoid arthritis. Ann Rheum Dis 68:124–129 [DOI] [PubMed] [Google Scholar]
- 37.Kostewicz ES, Brauns U, Becker R, Dressman JB. 2002. Forecasting the oral absorption behavior of poorly soluble weak bases using solubility and dissolution studies in biorelevant media. Pharm Res 19:345–349 [DOI] [PubMed] [Google Scholar]
- 38.Krinke GJ. 2000. Procedures, routes of administration, p 478 : Suckow MA, Weisbroth SH, Franklin CL. The laboratory rat. San Diego (CA): Academic Press [Google Scholar]
- 39.Laule GE, Bloomsmith MA, Schapiro SJ. 2003. The use of positive reinforcement training techniques to enhance the care, management, and welfare of primates in the laboratory. J Appl Anim Welf Sci 6:163–173 [DOI] [PubMed] [Google Scholar]
- 40.Laule G, Whittaker M. 2007. Enhancing nonhuman primate care and welfare through the use of positive reinforcement training. J Appl Anim Welf Sci 10:31–38 [DOI] [PubMed] [Google Scholar]
- 41.Lee Y, Zocharski PD, Samas B. 2003. An intravenous formulation decision tree for discovery compound formulation development. Int J Pharm 253:111–119 [DOI] [PubMed] [Google Scholar]
- 42.Lehninger A, Nelson Dl, Cox MM. 2008. Lehninger principles of biochemistry, 5th ed Gordonsville (VA): WH Freeman [Google Scholar]
- 43.Li P, Patel H, Tabibi SE, Vishnuvajjala R, Yalkowsky SH. 1999a. Evaluation of intravenous flavopiridol formulations. PDA J Pharm Sci Technol 53:137–140 [PubMed] [Google Scholar]
- 44.Li P, Tabibi SE, Yalkowsky SH. 1999c. Solubilization of ionized and unionized flavopiridol by ethanol and polysorbate 20. J Pharm Sci 88:507–509 [DOI] [PubMed] [Google Scholar]
- 45.Li P, Zhao L. 2007. Developing early formulations: practice and perspective. Int J Pharm 341:1–19 [DOI] [PubMed] [Google Scholar]
- 46.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46:3–26 [DOI] [PubMed] [Google Scholar]
- 47.Loftsson T, Fridfriksdottir H, Sigurdardottir AM, Ueda H. 1994. The effect of water-soluble polymers on drug–cyclodextrin complexation. Int J Pharm 110:169–177 [Google Scholar]
- 48.Lorenz W, Reimann HJ, Schmal A, Dormann P, Schwarz B. 1977. Histamine release in dogs by cremophor EL and its derivatives: oxethylated oleic acid is the most effective constituent. Agents Actions 7:63–67 [DOI] [PubMed] [Google Scholar]
- 49.Luo YS, Luo YL, Ashford EB, Morin RR, White WJ, Fisher TF. 2000. Comparison of catheter lock solutions in rats. Contemp Top Lab Anim Sci 39:83 [Google Scholar]
- 50.Mann WA, Landi MS, Woodward P, Campbell S, Kintner LB. 1987. Simple procedure for direct blood pressure measurement inconscious dogs. Lab Anim Sci 37:105–108 [PubMed] [Google Scholar]
- 51.McCarthy AM. 2000. Femoral cannulation using the tail cuff exteriorisation method in the rat, p 20–25 : Healing G, Smith D. Handbook of preclinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 52.Mendenhall V, Cornell S, Scalaro MA. 2000. Surgical preparation and infusion of the large primate, p 163–180 : Healing G, Smith D. Handbook of preclinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 53.Miedel ES. 2000. Tethering and the implanted button model in the rat, p 26–30 : Healing G, Smith D. Handbook of preclinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 54.Morton DB, Jennings M, Buckwell A, Ewbank R, Godfrey C, Holgate B, Inglis I, James R, Page C, Sharman I, Verschoyle R, Westall L, Wilson AB; Joint Working Group on Refinement 2001. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab Anim 35:1–41 [DOI] [PubMed] [Google Scholar]
- 55.Muller R, Peters K, Becker R, Kruss B. 1996. Nanosuspensions for the IV administration of poorly soluble drugs—stability during sterilization and long-term storage. Proc Int Symp Control Release Bioact Mater 22:574–575 [Google Scholar]
- 56.Neervannan S. 2006. Preclinical formulations for discovery and toxicology: physicochemical challenges. Expert Opin Drug Metab Toxicol 2:715–731 [DOI] [PubMed] [Google Scholar]
- 57.Nolan TE, Loughnane M, Jacobson A. [Internet] 2008. Tethered infusion and withdrawal in laboratory animals. ALN Magazine. [Cited 23 May 2010]. Available at: http://www.alnmag.com/article/tethered-infusion-and-withdrawal-laboratory-animals?page=0,0
- 58.Nolan TE, Klein HJ. 2002. Methods in vascular infusion biotechnology in research with rodents. ILAR J 43:175–179 [DOI] [PubMed] [Google Scholar]
- 59.Onder AM, Chandar J, Billings AA, Simon N, Diaz R, Francoeur D, Abitbol C, Zilleruelo G. 2008. Comparison of early versus late use of antibiotic locks in the treatment of catheter-related bacteremia. Clin J Am Soc Nephrol 3:1048–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paparella S. 2010. Identified safety risks with splitting and crushing oral medications. J Emerg Nurs 36:156–158 [DOI] [PubMed] [Google Scholar]
- 61.Pare WP, Glavin GB. 1986. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev 10:339–370 [DOI] [PubMed] [Google Scholar]
- 62.Peterson CG, Robertson RD. 1967. A pharmacodynamic study of dimethyl sulfoxide. Ann N Y Acad Sci 141:273–276 [DOI] [PubMed] [Google Scholar]
- 63.Reedy JS, Kuhlman JE, Voytovich M. 1999. Microvascular pulmonary emboli secondary to precipitated crystals in a patient receiving total parenteral nutrition: a case report and description of the high resolution CT findings. Chest 115:892–895 [DOI] [PubMed] [Google Scholar]
- 64.Reinhardt V. 2003. Working with rather than against macaques during blood collection. J Appl Anim Welf Sci 6:189–197 [DOI] [PubMed] [Google Scholar]
- 65.Rich DS. 2004. New JCAHO medication management standards for 2004. Am J Health Syst Pharm 61:1349–1358 [DOI] [PubMed] [Google Scholar]
- 66.Rowe RC, Sheskey P, Quinn M. 2009. Handbook of pharmaceutical excipients, 2nd ed: preface X Washington (DC): American Pharmaceutical Association [Google Scholar]
- 67.Rubin LF. 1983. Toxicologic update of dimethyl sulfoxide. Ann N Y Acad Sci 411:6–10 [DOI] [PubMed] [Google Scholar]
- 68.Ruys JD, Mendoza SP, Mason WA. 2004. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiol Behav 82:205–213 [DOI] [PubMed] [Google Scholar]
- 69.Schapiro SJ, Perlman JE, Thiele E, Lambeth S. 2005. Training nonhuman primates to perform behaviors useful in biomedical research. Lab Anim (NY) 34:37–42 [DOI] [PubMed] [Google Scholar]
- 70.Schnell CR. 2000. Surgical preparation and multidose infusion toxicity studies in the marmoset, p 210–222 : Healing G, Smith D. Handbook of preclinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 71.Schultz-Darken NJ, Pape RM, Tannenbaum PL, Saltzman W, Abbott DH. 2004. Novel restraint system for neuroendocrine studies of socially living common marmoset monkeys. Lab Anim 38:393–405 [DOI] [PubMed] [Google Scholar]
- 72.Smolinske SC. 1992. Handbook of food, drug and cosmetic excipients, 1st ed Boca Raton (FL): CRC Press [Google Scholar]
- 73.Snaterse M, Ruger N, Scholte Op Reimer WJ, Lucas C. 2010. Antibiotic-based catheter lock solutions for prevention of catheter-related bloodstream infection: a systematic review of randomised controlled trials. J Hosp Infect 75:1–11 [DOI] [PubMed] [Google Scholar]
- 74.Stella VJ, He Q. 2008. Cyclodextrins. Toxicol Pathol 36:30–42 [DOI] [PubMed] [Google Scholar]
- 75.Stoll LJ, Moran NM. [Internet] 2009. Taurolidine–citrate as a venous access port lock solution: a review. ASR Newsletter July. [Cited 25 May 2010]. Available at: http://www.surgicalresearch.org/requestPage.asp?ID=69
- 76.Stripling JS. 1981. A simple intravenous catheter for use with a cranial pedestal in the rat. Pharmacol Biochem Behav 15:823–825 [DOI] [PubMed] [Google Scholar]
- 77.Sweetana S, Akers MJ. 1996. Solubility principles for parenteral dosage form development. PDA J Pharm Sci Technol 50:330–342 [PubMed] [Google Scholar]
- 78.Swindle MM, Nolan TJ, Jacobson A, Wolf P, Dalton MJ, Smith AC. 2005. Vascular access port (VAP) usage in large animal species. Contemp Top Lab Anim Sci 44:7–17 [PubMed] [Google Scholar]
- 79.Tan T, Tsuru Y. [Internet] 2009. Infusion pumps for small laboratory animals. ALN Magazine. [Cited 23 August 2010]. Available at: http://www.alnmag.com/article/infusion-pumpssmall-laboratory-animals?page=0,0
- 80.Thackaberry EA, Kopytek S, Sherratt P, Trouba K, McIntyre B. 2010. Comprehensive investigation of hydroxypropyl methylcellulose, propylene glycol, polysorbate 80, and hydroxypropyl-β-cyclodextrin for use in general toxicology studies. Toxicol Sci 117:485–492 [DOI] [PubMed] [Google Scholar]
- 81.Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 82.Turner PV, Vaughn E, Sunohara J, Junkin A, Ovari J, Leri F. Oral gavage in rats: an animal welfare issue? J Am Assoc Lab Anim Sci. 2011 In press. [PMC free article] [PubMed] [Google Scholar]
- 83.van Wijk H, Robb D. 2000. Femoral cannulation using the tail cuff model in the mouse, p 61–70 : Healing G, Smith D. Handbook of preclinical continuous intravenous infusion. New York (NY): Taylor and Francis [Google Scholar]
- 84.Walker HK, Hall WD, Hurst JW. 1990. Clinical methods: the history, physical, and laboratory examinations, 3rd ed Boston (MA): Butterworths; [PubMed] [Google Scholar]
- 85.Weiner ML, Kotkoskie LA. 2000. Excipient toxicology and safety, 1st ed New York (NY): Marcel Dekker [Google Scholar]
- 86.Wiedmann TS, Kamel L. 2002. Examination of the solubilization of drugs by bile salt micelles. J Pharm Sci 91:1743–1764 [DOI] [PubMed] [Google Scholar]
- 87.Wojnicki FH, Bacher JD, Glowa JR. 1994. Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci 44:491–494 [PubMed] [Google Scholar]
- 88.Worthley EG, Schott CD. 1969. A toxicity of 4 concentrations of DMSO. Toxicol Appl Pharmacol 15:275–281 [DOI] [PubMed] [Google Scholar]
- 89.Yalkowsky SH, Valvani SC. 1977. Precipitation of solubilized drugs due to injection or diution. Drug Intell Clin Pharm 11:417–419 [Google Scholar]
- 90.Yang J, Maarek J-MI, Holschneider DP. 2005. In vivo quantitative assessment of catheter patency in rats. Lab Anim 39:259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]