Abstract
Periparturient manipulation of mice is a valuable tool for modern research facilities. Although fostering and Caesarian section frequently are used to eradicate pathogens, an often overlooked use is to rescue poorly breeding strains of mice. Here we characterized the weaning success rates after fostering outbred pups of variable ages (younger than 24 h; 5 to 7 d; 10 to 12 d) with full or partial replacement of litters and multiparous dams. There were no significant differences between most groups when analyzed by full or partial replacement or age of donor pups as compared with control groups, in which pups were manipulated but returned to the birth dam or the birth dam was not disturbed. However, significant differences were associated with fostering of 10- to 12-d-old pups in combination with younger pups. Overall, these findings suggest that limiting fostering to pups that are within 48 h of age and age-matching litters when fostering are unnecessary.
Periparturient manipulation of mice is a valuable tool for the modern research facility. Techniques such as in vitro fertilization,15,19 ovarian transplantation,9 embryo transfer,15,26 Caesarian rederivation,16 and fostering7,20 are commonly used to create new animal models, rescue existing lines, and eradicate diseases. These techniques have also been used to rescue poorly breeding strains of mice and preserve litters of mice when dams neglect them or die.9,19,25,29
Fostering is the movement of pups from the birth (donor) dam to a recipient (foster) dam. Fostering has been used alone or as part of Caesarian rederivation procedures to eradicate diseases such as mouse coronavirus,14,20 Helicobacter spp.,7,30 and norovirus,1,4 although the technique is not useful for the eradication of pathogens that are passed in utero.3 Fostering techniques have advantages because they require little specialized training to perform successfully and do not always require euthanasia of the donor dam. However, the expression of various behaviors can be altered when pups are raised by unrelated dams.6,11,12
In addition, fostering has been used to save litters of rare strains of mice that are at risk of death due to neglect by the dam, agalactia, or death of the dam.29 Some valuable genetically engineered mice do not exhibit strong maternal instincts,2 and the use of fostering techniques can assist in the maintenance of these lines. However, some valuable genetically engineered mice produce pups that are unable to nurse and fail to thrive.2,21 In these cases, the use of fostering techniques will not be rewarding.
Current laboratory animal medicine practice recommends fostering pups within 48 h of birth to recipient dams with age-matched litters.13,30,31 Some resources advocate for complete replacement of the recipient dam's litter with fostered neonates,14,31 whereas others recommend inclusion of the recipient dam's neonates to facilitate acceptance of fostered neonates.7,17,30 After review of the available literature, we hypothesize that the currently recommended practice of transfer of age-matched pups has evolved from the use of fostering alone or as part of Caesarian rederivation procedures for disease control, where these recommendations are implemented to minimize potential cross-contamination of pathogens, such as Helicobacter.1,7,27,30 However, changes in the composition of the dam's milk have been hypothesized to compromise development of fostered pups potentially if they are more than a couple of days older or younger than the pups born to the foster dam.27To date, there has been no thorough scientific evaluation of the success of fostering techniques that vary from the currently recommended veterinary practice.
The current study was performed to determine whether fostering could be performed successfully as late as 12 d postpartum, without age-matching litters, and whether there was a difference in success depending on combining or replacing litters. We did not manipulate mice older than 12 d because mice of this age are able to eat solid food and are not as reliant on nursing by the dam.10 The hypothesis that female mice of most strains readily will accept litters that are not their own, regardless of age, was supported by the observations of allonursing in polygamous mating systems.28 Each female mouse can be observed nursing the litters of her cagemates, and it can be difficult to determine which mouse is the birth dam. However, other evidence indicates that female mice will cannibalize or neglect litters, including their own, underscoring the need to use care in fostering preweanling mice.27 Cannibalism and neglect in birth and foster situations commonly are ascribed to stressors (for example, noise, vibration) in the environment, competition with older pups in polygamous breeding systems, aggressive males, strain-specific factors, characteristics of genetically engineered mice, and primiparous dams.8,22,27 These differing observations necessitated the characterization of standardized procedures that may maximize the success of fostering mouse pups.
Materials and Methods
Animals.
Mice used on this project were proven breeders from experimentally naïve outbred stocks of mice (ICR, Taconic, Germantown, NY) and surplus mice from a colony with a mixed genetic background. The rotationally outbred colony originated from a heterogeneous stock that was derived from 8 inbred strains and bred to exhibit more or less severe physiologic or behavioral responses in response to ethanol administration.23 Examples of these responses to ethanol administration included enhanced (WSP1 and WSP2) or depressed (WSR1 and WSR2) seizure response; hypothermia (COLD1 and COLD2) or hyperthermia (HOT1 and HOT2); and increased (FAST1 and FAST2) or decreased (SLOW1 and SLOW2) activity as assessed in an infrared activity monitor.5,24 The associated control line for the WSP and WSR lines (WSC1 and WSC2) also was used in this study. The mixed genetic background stocks were at least in generation 77 of production. These outbred and mixed genetic background stocks were selected because the literature suggests that outbred stocks have an advantage as foster dams.28,31 All pups produced by this project were weaned at 21 d of age and used in the facility personnel training program. The colony size was maintained at approximately 20 cages per day, and the study required 2 y to complete. The Portland VA Medical Center IACUC approved all projects using these mice, in accordance with applicable federal regulations.
Husbandry.
Mice were housed in polycarbonate shoebox cages with filter tops (Thoren Caging Systems, Hazelton, PA) and corncob bedding (Bed-O'Cobs, Maumee, OH). Cages were changed at least once weekly in a laminar flow workstation (Lab Products, Seaford, DE). Animal caretakers wore gloves while changing cages and sprayed their gloves with a 10% bleach solution between cages. Soiled cages were sanitized in a mechanical cage washer with a final rinse temperature of 180 °F (82 °C). All caging equipment was autoclaved prior to reuse. The room was kept on a 12:12-h light:dark cycle, and mice were provided rodent chow (LabDiet, St Louis, MO) and tap water ad libitum. Temperature and humidity were maintained at 72 °F (22 °C) and at least 30%, respectively.
Indirect exposure sentinel mice were used to screen the colony for pathogens on a quarterly basis. Sentinel mice were 5-wk-old ICR (Taconic) mice that had been exposed to pooled dirty bedding from colony cages for a minimum of 28 d. Serum samples collected from these sentinel mice by cardiac exsanguination under isoflurane (Isothesia, Butler Animal Health Supply, Dublin, OH) anesthesia were processed in house for serologic testing (SmartSpot, Biotech Trading Partners, Encinitas, CA). Internal and external parasite screens also were performed inhouse. At the time of this clinical investigation, these lines of mice were determined to be free of Sendai virus, mouse parvovirus, minute virus of mice, reovirus type 3, pneumonia virus of mice, mouse coronavirus, Mycoplasma pulmonis, mouse rotavirus, mouse encephalomyelitis virus, and rodent pinworms and mites. The colony was not screened for Helicobacter spp. or mouse norovirus.
Fostering procedure.
Breeding pairs were allowed to mate naturally, without synchronization. Breeding pairs were established by random pairing of a male mouse from 1 of the 13 stocks with a female mouse from 1 of the other stocks. Once pregnancy was detected (by observation or palpation), dams were singly housed to avoid the confounding factors of potential male aggression, allonursing, and establishment of a new pregnancy through mating during postpartum estrus.
Donor (birth) and recipient (foster) stocks were of different coat colors to facilitate pup identification at weaning. The fostering methods selected were similar to those reported by other authors.14,17,27 In brief, donor and recipient dams were removed from their cages and placed in separate, clean cages. The litter to be fostered was picked up gently and mixed with dirty bedding, nesting material, and (if indicated by the assigned treatment group) other pups from the recipient dam's cage. This manipulation was done to transfer the recipient dam's scent. Pups were placed back in the recipient dam's nest, and the recipient dam was returned to the cage. The cage was placed on a static rack (with filter top) to allow visualization without disruption of the cage. The cage was monitored visually every 15 min for the first 60 min. If there was evidence of rejection by the dam (for example, agitation, carrying the pups around), the pups were removed from the cage and euthanized by administration of a barbiturate overdose (0.1 mL per pup IP; 260 mg/mL; Sleepaway, Fort Dodge Laboratories, Fort Dodge, IA). The cages were assessed at least once daily and not disturbed for the first 72 h after fostering to decrease potential cannibalism.17 After the first 72 h, the cages were observed daily. Any mice found dead were necropsied to determine the cause of death.
Addition of pups to an existing litter.
For the examination of the effect of the addition of pups to an existing litter, 3 target ages were selected for evaluation. Pups younger than 48 h represented the current laboratory animal medicine practice. Pups 5 to 7 d old were more mobile, and we hypothesized they had greater likelihood of rejection by the foster dam. We considered that pups 10 to 12 d old were at the oldest age at which the dam would be required to provide lactation support, given that pups can be weaned early by day 15.10,17 In addition, we hypothesized that 10- to -12-d-old pups had an increased risk of rejection by the foster dam.
A total of 11 groups (3 control and 8 experimental) were established. In control group 1, the dam raised her own pups, and the pups were not manipulated (standard breeding control). In control group 2, pups were manipulated as though fostered but returned to the same dam (sham control), whereas in control group 3, pups that were younger than 48 h were transferred to a dam whose litter was younger than 48 h (current recommendations for laboratory animal medicine practice).1,7,30,31 In experimental group 1, pups that were younger than 48 h were transferred to a dam with a litter that was 5 to 7 d of age; experimental group 2 comprised pups that were younger than 48 h of age when transferred to a dam with a litter that was 10 to 12 d old; and experimental 3 included pups that were 5 to 7 d of age when transferred to a dam with a litter younger than 48 h. Experimental group 4 contained pups that were 5 to 7 d old when transferred to a dam with a litter that was 5 to 7 d of age; experimental group 5 comprised pups that were 5 to 7 d of age when transferred to a dam with a litter that was 10 to 12 d old; and experimental 6 included pups that were 10 to 12 d of age when transferred to a dam with a litter that was younger than 48 h. In experimental group 7, pups that were 10 to 12 d old were transferred to a dam with a litter that was 5 to 7 d of age; and experimental group 8 comprised pups that were 10 to 12 d of age when transferred to a dam with a litter that was 10 to 12 d old.
After a litter was born, it was randomly assigned to one of the described treatment groups. When they achieved the specified age of foster, the pups were removed and fostered to a dam with a litter of the specified age for the assigned treatment group. The maximal number of pups fostered per litter was 5, and the maximal total number of pups each dam reared to weaning was 10; the goal was that each litter would consist of approximately 50% fostered pups. To minimize the overall number of mice used, a dam was used as both a donor and a recipient through the exchange of partial litters (for example, dam A retained 4 pups of her own and received 4 pups from dam B; dam B retained 3 pups of her own and received 4 pups from dam A) whenever possible. If this dual use was not possible, surplus pups were euthanized as described.
Replacement of an existing litter with a new litter.
To examine the effect of removing an existing litter and replacing it with a new litter, 3 target ages were selected. Control groups 1 and 2 described for the partial replacement of litters also were used as controls for the replacement of an existing litter with a new litter. A total of 7 additional groups were established. In control group 9, pups that were younger than 48 h of age replaced the litter of a dam with pups that younger than 48 h (current recommendation). For experimental group 10 comprised pups that were younger than 48 h replaced the litter of a dam with pups that were 5 to 7 d of age; pups that were younger than 48 h of age replaced the litter of a dam with pups that were 10 to 12 d of age in experimental group 11; and in experimental group 12, pups that were 5 to 7 d old replaced the litter of a dam with pups that were younger than 48 h. With experimental group 13, pups that were 5 to 7 d old replaced the litter of a dam with pups that were 10 to 12 d of age; in experimental 14, pups that were 10 to 12 d of age replaced the litter of a dam with pups that were younger than 48 h; and for experimental group 15, pups that were 10 to 12 d of age replaced the litter of a dam with pups that were 5 to 7 d of age.
After a litter was born, it was randomly assigned to one of the described treatment groups. When it achieved the specified age of foster, the pups were removed and fostered to a female with a litter of the specified age for the assigned treatment group. The minimal total number of pups each dam reared to weaning was 5, and the maximum was 10. To minimize the overall number of mice used, a dam was used as both a donor and a recipient through the exchange of full litters (for example, a litter produced by dam C was transferred to dam D; the litter produced by dam D was transferred to dam C) whenever possible. If this exchange was not possible, surplus pups were euthanized as described earlier.
Statistical analysis.
The percentage pup survival of each litter (number of pups weaned compared with number of pups in litter at time of fostering) in each treatment group was compared with that of the traditional foster control group (litter younger than 48 h fostered to a dam with a litter younger than 48 h) by using one-way ANOVA (Excel, Microsoft, Redmond, WA) with a power of 0.05. In addition, the results of positive (at least one pup weaned) compared with negative (no pups weaned) litter success were analyzed for foster and birth pups by using a single maximally restricted likelihood logistic regression (JMP, Cary, NC). We hypothesized that there would be no statistical significance between these groups, suggesting that all age combinations are appropriate alternatives to the control group.
Results
Addition of pups to an existing litter.
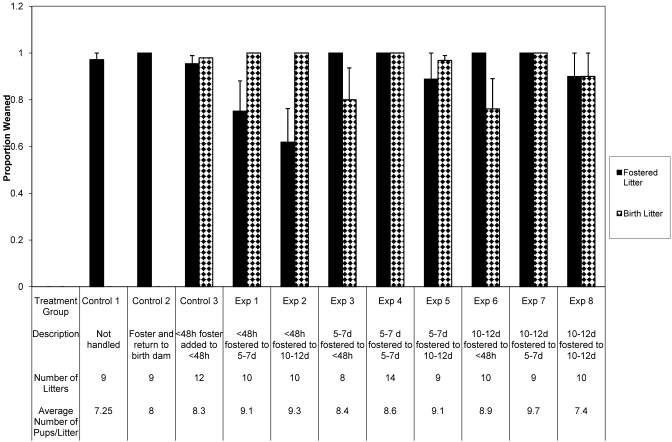
A total of 110 litters were evaluated in this phase of the project (Figure 1). ANOVA of the percentage of fostered pups weaned according to current laboratory animal medicine practices (control group 3) and in all experimental groups compared with those of the nonmanipulated (control group 1) and sham (control group 2) groups revealed a significant difference (F = 2.83, P = 0.0039). Posthoc analysis identified that the percentage of pups weaned did not differ between the treatment group in which pups were younger than 48 h were transferred to dams with litters that were 10 to 12 d of age (experimental group 2) and any other group (Figure 1), except for the nonmanipulated (P = 0.043) and sham (P = 0.043) controls (groups 1 and 2, respectively) and experimental groups 3 (pups that were 5 to 7 d old were transferred to dams with pups younger than 48 h, P = 0.048), 4 (pups that were 5 to 7 d of age were transferred to dams with pups that were 5 to 7 d of age, P = 0.026), 6 (pups that were 10 to 12 d old were transferred to dams with pups that were younger than 48 h, P = 0.026), and 7 (10- to 12-d-old pups were transferred to dams with pups that were 5 to 7 d of age, P = 0.024).
Figure 1.
Percentages of pups successfully weaned after the addition of fostered pups to existing litters. Percentages of fostered pups weaned as compared with the number of pups fostered and percentages of birth pups weaned as compared with the number of birth pups at time of weaning are depicted. Statistical significance between successful weaning of fostered pups was present between experimental (Exp) group 2 (pups that were younger than 48 h were transferred to a dam with a litter that was 10 to 12 d old) and multiple treatment groups. No statistically significant differences were noted between treatment groups with regard to birth litter. Data are presented in the graph as mean ± SEM. *, P < 0.05.
ANOVA showed that the percentage of birth pups weaned according to the current laboratory animal medicine practiced (control group 3) and in all experimental groups did not differ (F = 1.90, P = 0.054) from those of the nonmanipulated (control group 1) and sham (control group 2) groups.
Of the 110 litters evaluated, only 4 groups of fostered pups were lost (missing at daily check, presumed cannibalized). Of these 4 groups of fostered pups, 2 were in experimental group 2 (pups that were younger than 48 h were transferred to dams with litters that were 10 to 12 d of age), 1 was in experimental group 1 (pups younger than 48 h were transferred to dams with litters that were 5 to 7 d old), and the remaining 1 was in experimental group 5 (pups that were 5 to 7 d of age were transferred to dams with litters that were 10 to 12 d old). In all 4 cases, the birth dams’ pups were weaned without loss.
In addition, 4 groups of birth pups were lost (missing at daily check, presumed cannibalized) after foster. Of these 4 groups of birth pups, 2 were in experimental group 6 (pups that were 10 to 12 d of age were transferred to dams with litters that were younger than 48 h), 1 was in experimental group 8 (pups that were 10 to 12 d of age were transferred to dams with litters that were also 10 to 12 d old), and the remaining 1 was in experimental group 3 (pups that were 5 to 7 d old were transferred to dams with litters that were younger than 48 h). In all 4 cases, the fostered pups were weaned without loss.
The effect likelihood of weaning any pups was analyzed for both birth and fostered litters. For fostered litters, the area under the receiver operating curve was 90.4% (that is, the analysis identified positive weaning of pups in 90.4% of litters). The positive weaning of fostered litters was not significantly different between treatment groups (effect likelihood ratio: χ2 = 11.58, P = 0.3143). For the birth litters, the area under the receiver operating curve was 88.9% (that is, the analysis identified positive weaning of pups in 88.9% of litters). The positive weaning of birth litters was not significantly different between treatment groups (effect likelihood ratio: χ2 = 10.37, P = 0.2401).
Replacement of an existing litter with a new litter.
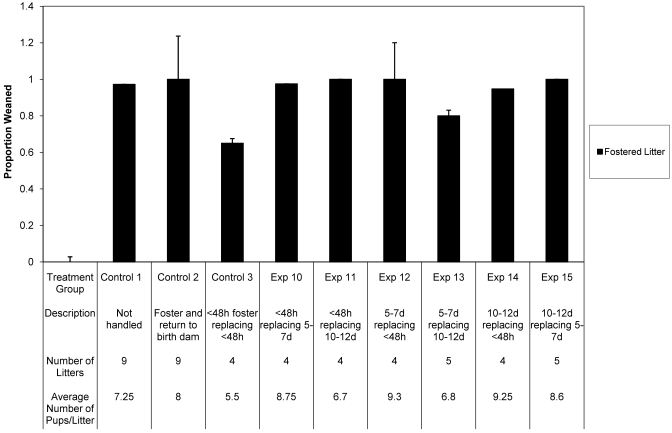
A total of 48 litters were evaluated in this phase of the project (Figure 2). ANOVA revealed that the percentage of fostered pups weaned according to the current laboratory animal medicine practice (control group 3) and in all experimental groups did not differ significantly (F = 1.66, P = 0.1379) from those of the nonmanipulated (control group 1) and sham (control group 2) groups.
Figure 2.
Percentages of fostered pups successfully weaned after complete replacement of existing litters. Exp, experimental. No statistically significant differences were noted for any group. Data are presented as mean ± SEM.
Of the 48 litters evaluated, only 2 litters were lost (missing at daily check, presumed cannibalized). One of these litters was in control group 3 (pups younger than 48 h replaced the litter of a dam with pups that were younger than 48 h); the other was in experimental group 12 (pups that were 5 to 7 d of age replaced the litter of a dam with pups that were younger than 48 h).
The effect likelihood of weaning any pups was analyzed for fostered litters. The area under the receiver operating curve was 92.29% (that is, the analysis identified positive weaning of pups in 92.29% of litters). The positive weaning of fostered litters was not significantly (effect likelihood ratio: χ2 = 7.125, P = 0.5232) different between treatment groups.
Discussion
The results of the current study suggest that age may not necessarily be a limiting factor when fostering mouse pups. The replacement of complete litters with fostered litters appeared to be the most reliable method of fostering, consistent with the hypothesis that survival is greater if the fostered pups are not competing with or in danger of being injured by older pups. However, the sample size for these groups was small, so that only large effects on survivability would be detectable, due to the limited power.
Comingling of litters was generally successful, although survival fell significantly in foster paradigms that involved pups that were 10 to 12 d of age. The combination of pups that were younger than 48 h with a birth litter that was 10 to 12 d old (experimental group 2) had the lowest survival rate. We suspect that the older pups were more competitive than were the neonates, increasing the likelihood of neonatal loss. Alternatively, the dams of pups that were 10 to 12 d old may have produced less milk than did dams with younger pups, as the older pups were beginning to eat solid foods. If this age differential is the only one available to save a valuable litter, we recommend removing the older pups and transferring the younger pups to the foster dam. If comingling of litters is preferred, we recommend attempting to arrange the group so that the fostered pups are older than are the birth pups.
Although the results of the current study suggest that age may not necessarily be a limiting factor when fostering pups, the acceptance of a fostered litter by the dam is only one part of the fostering process. Here, the stocks we used were genetically robust and successful breeders, as evidenced by the average litter sizes and the low losses of individual pups and whole litters. If working with genetically engineered mice with pups that are unable to nurse, the fostering technique is unlikely to lead to the same level of success. However, fostering of genetically engineered mice to outbred stocks of mice1,14,20 or inbred strains of mice7,30 for the eradication of pathogens has been reported as a successful practice.
Furthermore, the age of the pups to be fostered will continue to be a limiting factor when using fostering techniques to eradicate pathogens. As has been repeatedly demonstrated, early foster is required to successfully eradicate pathogens such as Helicobacter,1,7, mouse coronavirus,1,14,20 and norovirus.1,4 However, our study suggests that the age of the litter of the foster recipient may be less important than previously believed.
Overall, the results of the current study suggest alternatives to recommendations to foster mice to a dam with an age-matched litter.27 We found that pups could be added to an existing litter of any variety of ages with the expectation that at least 65% would be weaned successfully. In the current study, less than 5% of litters were rejected by the foster dam. Given that many facilities house multiple strains of mice, we anticipate that a recipient dam could be identified from a strain that tends to do well rearing pups. Although outbred mice typically are recognized as being superior mothers, some inbred strains of mice (for example, C57BL/6J and FVB/NJ) are equally good at rearing pups.18 Our results suggest that when working with nonfragile pups, a proven nursing dam from an existing colony may be a potential recipient dam and live pups will be weaned after the transfer.
References
- 1.Artwohl JE, Purcell JE, Fortman JD. 2008. The use of cross-foster rederivation to eliminate murine norovirus, Helicobacter spp., and murine hepatitis virus from a mouse colony. J Am Assoc Lab Anim Sci 47:19–24 [PMC free article] [PubMed] [Google Scholar]
- 2.Berry ML, Linder CC. 2007. Breeding systems: considerations, genetic fundamentals, genetic background, and strain types. : Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research: vol 1, 2nd ed Burlington (MA): Academic Press [Google Scholar]
- 3.Carthew P, Wood MJ, Kirby C. 1985. Pathogenicity of mouse hepatitis virus for preimplantation mouse embryos. J Reprod Fertil 73:207–213 [DOI] [PubMed] [Google Scholar]
- 4.Compton SR. 2008. Prevention of murine norovirus infection in neonatal mice by fostering. J Am Assoc Lab Anim Sci 47:25–30 [PMC free article] [PubMed] [Google Scholar]
- 5.Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. 1990. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res 14:141–151 [DOI] [PubMed] [Google Scholar]
- 6.Crews D. 2010. Epigenetics, brain, behavior, and the environment. Hormones (Athens) 9:41–50 [DOI] [PubMed] [Google Scholar]
- 7.Crisler-Roberts R, Ge Z, Kearney MT, Singletary KB, Fox JG, Roberts CS, Baker DG. 2005. Evaluation of Helicobacter hepaticus bacterial shedding in fostered and sex-segregated C57BL/6 mice. Comp Med 55:515–522 [PubMed] [Google Scholar]
- 8.Curtin L. 2006. Choosing the right foster dam. Tech Talk 11:1–2 [Google Scholar]
- 9.Dawes J, Liu B, Mars W, Michalopoulos G, Khillan JS. 2010. Multiple ovarian transplants to rescue a transgenic line of mice. Lab Anim (NY) 39:191–193 [DOI] [PubMed] [Google Scholar]
- 10.Duysen EG, Fry DL, Lockridge O. 2002. Early weaning and culling eradicated Helicobacter hepaticus from an acetylchoinesterase knockout 129S6/SvEvTac mouse colony. Comp Med 52:461–466 [PubMed] [Google Scholar]
- 11.Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. 2010. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 68:408–415 [DOI] [PubMed] [Google Scholar]
- 12.Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. 2010. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science 329:643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen AK. 2003. Health status and health monitoring. : Hau J, Schapiro SJ. Handbook of laboratory animal science, 2nd ed Boca Raton (FL): CRC Press [Google Scholar]
- 14.Hickman DL, Thompson KJ. 2004. Multiphase approach to eradicate enzootic mouse coronavirus infection. Contemp Top Lab Anim Sci 43:22–28 [PubMed] [Google Scholar]
- 15.Hogan B. 1994. Manipulating the mouse embryo: a laboratory manual, 2nd ed Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press [Google Scholar]
- 16.Homberger FR. 1997. Enterotropic mouse hepatitis virus. Lab Anim 31:97–115 [DOI] [PubMed] [Google Scholar]
- 17.Jackson Laboratories. [Internet] 2006. Chapter 4.4. The postnatal period. [Cited 2 February 2011]. Available at: http://www.informatics.jax.org/silver/chapters/4-4.shtml
- 18.Jackson Laboratories. [Internet] 2011. Breeding considerations. [Cited 2 February 2011]. Available at: http://jaxmice.jax.org/support/husbandry/breeding-considerations.html
- 19.Kong FY, Zhang G, Zhoung ZS, Li YL, Sun QY, Chen DY. 2005. Transplantation of male pronucleus derived from in vitro fertilization of enucleated oocyte into parthenogentically activated oocyte results in live offspring in mouse. Zygote 13:35–38 [DOI] [PubMed] [Google Scholar]
- 20.Lipman NS, Newcomer CE, Fox JG. 1987. Rederivation of MHV and MEV antibody-positive mice by crossfostering and use of the microisolator caging system. Lab Anim Sci 37:195–199 [PubMed] [Google Scholar]
- 21.Lu Q, Hasty P, Shur BD. 1997. Targeted mutation in beta1,4-galactoxyltransferase leads to pituitary insufficiency and neonatal lethality. Dev Biol 181:257–267 [DOI] [PubMed] [Google Scholar]
- 22.Marques-de-Araujo S, Cardoso MA. 1999. A laboratory cage for foster nursing newborn mice. Braz J Med Biol Res 32:319–321 [DOI] [PubMed] [Google Scholar]
- 23.McClearn GE, Wilson JR, Meredith W. 1970. The use of isogenic and heterogenic mouse stocks in behavioral research, p 3–22. : Lindzey MG, Thiessen DD. Contributions to behavior—genetic analysis—the mouse as a protoype. New York (NY): Appleton–Century–Crofts [Google Scholar]
- 24.Meyer PJ, Phillips TJ. 2003. Sensitivity to ketamine, alone or in combination with ethanol, is altered in mice selectively bred for sensitivity to ethanol's locomotor effects. Alcohol Clin Exp Res 27:1701–1709 [DOI] [PubMed] [Google Scholar]
- 25.Mochida K, Ohkawa M, Inoue K, Valdez DM, Jr, Kasai M, Ogura A. 2005. Birth of mice after in vitro fertilization using C57BL/6 sperm transported within epididymides at refrigerated temperatures. Theriogenology 64:135–143 [DOI] [PubMed] [Google Scholar]
- 26.Morrell JM. 1999. Techniques of embryo transfer and facility decontamination used to improve the health and welfare of transgenic mice. Lab Anim 33:201–206 [DOI] [PubMed] [Google Scholar]
- 27.Pritchett KR, Taft RA. 2007. Reproductive biology of the laboratory mouse. : Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research: vol 3, 2nd ed Burlington (MA): Academic Press [Google Scholar]
- 28.Roulin A. 2002. Why do lactating females nurse alien offspring? A review of hypotheses and empirical evidence. Anim Behav 63:201–208 [Google Scholar]
- 29.Silver LM. 1995. Reproduction and breeding. : Mouse genetics: concepts and applications. New York (NY): Oxford University Press [Google Scholar]
- 30.Singletary KB, Kloster CA, Baker DG. 2003. Optimal age at fostering for derivation of Helicobacter-hepaticus-free mice. Comp Med 53:259–264 [PubMed] [Google Scholar]
- 31.Watson J, Thompson KN, Feldman SH. 2005. Successful rederivation of contaminated immunocompetent mice using neonatal transfer with iodine immersion. Comp Med 55:465–469 [PubMed] [Google Scholar]