Abstract
The current study was performed to determine the vibration levels that were generated in cages on a ventilated rack by common construction equipment in frequency ranges likely to be perceived by humans, rats, and mice. Vibration generated by the ventilated rack blower caused small but significant increases in some of the abdominal, thoracic, and head resonance frequency ranges (RFR) and sensitivity frequency ranges (SFR) in which each species is most likely to be affected by and perceive vibration, respectively. Vibration caused by various items of construction equipment at 3 ft from the cage were evaluated relative to the RFR and SFR of humans, rats, and mice in 3 anatomic locations. In addition, the vibration levels in the RFR and SFR that resulted from the use of a large jackhammer and were measured at various locations and distances in the facility and evaluated in terms of humans, rats, and mice in 3 anatomic locations. Taken together, the data indicate that a given vibration source generates vibration in frequency ranges that are more likely to affect rats and mice as compared with humans.
Abbreviations: FFT, fast Fourier transform; RFR, resonance frequency range; SFR, sensitivity frequency range
Noise and vibration in a laboratory animal facility are cited as factors that can induce stress in animals and alter research results.8,16,24,31,32-35 The effects of noise and vibration on research animals are difficult to separate because sources of noise also can cause vibration. Noise generated in a laboratory animal facility from various sources has been quantified,24,25 but little information is available in the literature regarding how much vibration is associated with this noise. Because vibration may be as important as or more important than noise in causing physiologic changes in animals, we performed the current study to examine the levels of vibration associated with ventilated rack blowers and construction equipment in the vivarium.
Vibration is motion that is not constant but assumes an oscillatory or wave-like form. This motion is characterized as cycles, in which the structure oscillates around an equilibrium position, that is, the position of the object when no vibration is applied. The extent of the oscillations or movement determines the amplitude of the vibrations. The velocity of the movement is the speed of the motion or rate of displacement of the structure. Vibration amplitude often is measured as acceleration, the rate of change in velocity as an object moves past its resting position. Acceleration is measured with the use of an accelerometer and is expressed in meters per second squared (m/s2). The repetition rate at which the cycles occur is the frequency of the vibration. Both the amplitude and frequency of vibration influence the distance that vibration will be transmitted and the potential resultant damage.10
The effects of vibration on animals and the level of vibrations produced in the animal facility merit additional study. Vibration plays a role in communication between animal species ranging from insects to elephants.11 Vibration is important in predator–prey interactions, mother and young relationships, mate choice, and recruitment of food, suggesting that research animals may be more sensitive to vibrations than are humans. Although the role played by vibration is unknown, reports have documented changes in locomotor activity and circadian rhythm in mice several days before an earthquake occurs.15 Simulation of a severe earthquake and aftershocks caused an increase in the rates of cleft palate and fetal resorption in mice.20 Anecdotal reports of the effects of vibration include a reduction in mouse breeding efficiency in rodent breeding colonies, reductions in food intake and weight gain, and behavioral modifications.8 In other animals, low-level whole-body vibration causes cardiovascular effects in dogs and swine,7 avoidance behavior in poultry,1 physiologic changes in rats,2 and behavioral changes and increases in stress-related hormones in swine.23
As in animals, excessive vibration has deleterious effects in humans, causing headache, insomnia, fatigue, gastric disturbance, and musculoskeletal problems. In addition, vibration is thought to play a role in the motion sickness that people encounter when traveling.4,10,14 The harmful effects of vibration in humans has lead to the establishment of an exposure limit (1.15 m/s2) and action level for an 8-h reference period (0.5 m/s2).21
Similar to sound, the effects of vibration depend not only on the amplitude but the frequency (in Hertz) at which it occurs. The resonance frequency is the frequency of applied external vibration that causes an object to vibrate more readily, and even amplify the vibration, in comparison to other frequencies.9,12 Every object or part of the body has a resonance frequency (Fn), which is calculated by the formula
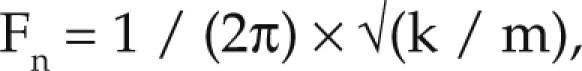 |
where k is the stiffness constant, and m is the mass.9,10 Knowledge of resonance frequencies is important because vibration near these frequencies, compared with other frequencies, will be perceived more strongly and ultimately will induce more adverse effects.9,38 The human abdomen has a resonance frequency of 4 to 8 Hz, the thorax of 5 to 10 Hz, and the head from 20 to 30 Hz.19 There is no information in the literature on resonance frequencies in mice; however, a study in rats determined that resonance frequency was 27 to 29 Hz for the abdomen, 225 to 230 Hz for the thorax, and 75 to 80 Hz for the head.36 In the current study, the resonance frequencies of rats were used to calculate the analogous frequencies in mice because the species are anatomically similar. The amount of vibration in the resonance frequency range (RFR) is a measure of the potential that the vibration will affect a human or animal physically and that the human or animal will perceive the vibration in the most sensitive frequency range.
Although the RFR is the range at which vibration has the most potential for causing adverse physical effects on the body, the frequency range at which vibration is perceived is wider.10,18,22 Vibration outside of the RFR but still within the sensitivity frequency range (SFR), at which animals perceive vibration, may cause psychologic distress. Although studies have shown that the human perception threshold to vibration varies slightly with age, position of the body, body region, and axis of vibration,10,22 the median human vibration perception threshold is approximately 0.01 m/s2 for vertical vibration (the vibration measured in the current study) between 0 and 63 Hz.22 In humans, sensitivity to vibration decreases as the frequency of the vibration increases. No well-defined studies are available that demonstrate the perception thresholds of laboratory animals, but some information regarding the magnitude and frequency of whole-body vibration that causes pathologic or physiologic effects is known. Piglets exposed to whole-body vibration of 2 to 18 Hz at as low as 1 m/s2 had an immediate increase in plasma ACTH and cortisol levels, demonstrating that the vibration was stressful.23 As a reference, 1 m/s2 is near the human exposure limit.21 Poultry exhibited avoidance behavior at 2 Hz and 1 m/s2.1 Whole-body vibration in rats caused an increase in plasma corticosterone and brain serotonin levels at 3.9 m/s2 and 20 Hz and decreased gastric emptying time, decreased organ weight, and increased adrenal weight at 20 to 24 m/s2 and 5 to 15 Hz.2,28,33 In mice, extreme vertical whole-body vibration in the 10- to 25-Hz frequency range at approximately 147 m/s2 caused mortality within 5 to 10 min.26 Low-level whole-body vibration in mice at approximately 1 to 3 m/s2 and as much as 90 Hz over time decreased adipogenesis, lowered liver triglyceride levels,27 and increased bone volume or bone formation (or both).13,39,40
Because of the need for floor repair in a vivarium, a study was conducted to determine the level of vibration that may be perceived by mice in other parts of the facility. One option for floor repair had less potential to generate noise and vibration but was less preferred from the perspective of optimal function and aesthetics of the physical plant. Vibration generated from implements of construction equipment were measured inside a ventilated rack cage to determine the approximate levels of vibration that would be physiologically important to the mice in other parts of the facility. The resonance frequencies for mice were calculated by using those for rats.36 Because resonance frequencies for humans and rats are known, we determined the levels of vibration due to ventilated rack blowers and construction equipment in these ranges for comparison with those in mice. No information is available to determine the frequency ranges at which rats or mice perceive low-level vibration. In our study, we applied the human threshold of perception of 0.01 m/s2 within a 63-Hz range, incorporating human resonance frequencies,22 around the resonance frequencies of rats and mice to derive their respective SFR.
Materials and Methods
Vibration measurements.
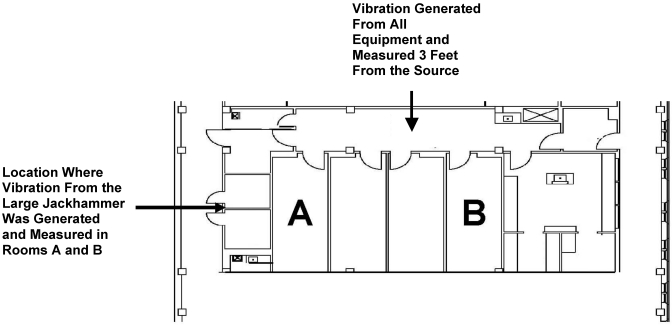
All vibration measurements were taken from inside a 75-in.2 polysulfone cage containing bedding on a fully stocked 140-cage, positive-pressure, ventilated mouse cage rack (model PNC75JU70SPSH-R, Allentown Caging, Allentown, NJ) at 60 air changes hourly per cage (blowers mounted to the rack). To minimize the exposure of rodents to vibration, no mice were present either in the module (Figure 1) where testing was conducted or in an adjacent module. Although no animals were used in this study, the Institutional Animal Care and Use Committee was made aware of the intent to perform this work before it was initiated.
Figure 1.
Physical plant configuration of vivarium module where vibration testing occurred. This module is 1 of 4 identical modules on the first floor of the animal facility, which is located at one end of 4 contiguous modules. All modules on the first floor are at grade level. This facility has 4 floors, with the second floor having the same configuration as the first floor and the third floor having a smaller animal facility with laboratory space. The fourth floor is entirely nonanimal laboratory space.
After an 8-mm circular hole was drilled into the front of a ventilated cage, the accelerometer was placed inside of the cage, and plumber's putty was used to seal the hole around the accelerometer's cable to prevent leakage of air around the cable. The end of the accelerometer where vibration is detected was placed in the middle of the cage. Consistent with operation of the rack in this vivarium, the cage contained our standard amount (approximately 200 mL) of 1/4-in. corncob bedding. The cage was placed in the middle on the bottom row of the ventilated cage rack. Remaining equipment for the study was located on a portable cart adjacent to the ventilated rack during measurements.
Vibration measurements were made by using a Pulse Analysis System (model 3560-C with 7536 and 3110 modules, Bruel and Kjaer, Nærum, Denmark) and accelerometer (model 4508-B, Bruel and Kjaer). Measurements of vibration were made in the vertical direction on the inside base of a cage. The vibration signals were recorded unfiltered at a rate of approximately 262 kHz. The sampling rate was 3.815 µs. Fast Fourier transform (FFT) analysis was performed by using baseband analysis at a bandwidth of 3200 Hz at 800 lines, 66.67% overlap, with a frequency resolution of 4Hz. Exponential averaging (50 averages) was used to obtain the autospectra. The levels in each FFT line are the root mean square values. The total energy was calculated by summing the energy in each of the FFT lines of the spectrum.
Vibration was generated by construction equipment inside an empty module, with another empty module separating an occupied mouse housing area. Vibration was generated by a large jackhammer (Bosch Brute11304, Bosch, Farmington Hills, MI), small jackhammer (Bosch11316EVS), vacuum (Rigid 6.5-hp 2-in-1 [blower and vacuum], Emerson, St Louis, MO), grinder and vacuum (Bosch 1873-6 15-A angle grinder), terrazzo grinder (model 501S, Terrco, Watertown, SD), shot blaster (without shot; model 1-10D blaster with model 5-54 dust collector, Terrco, Watertown, SD), and the ventilated rack alone. Vibration was generated by all of the construction equipment 3 ft from the cage and measurements taken from within the corridor immediately outside the animal rooms (Figure 1). In addition, the large jackhammer was used to generate vibration immediately outside the module and was measured in rooms A and B as well as on the second and third floors directly above this site (Figure 1). Vibration generated by the ventilated racks was measured at locations in rooms A and B as well as the corridor outside of the animal rooms.
Physical plant construction.
The outer walls of the module are constructed of concrete masonry units coated with epoxy paint, whereas the inner walls and ceiling are made of 0.5-in. drywall with steel studs spaced every 16 in. No sound dampening equipment was used. Floors consist of epoxy coating over a concrete slab, and the floor-to-ceiling height is approximately 8 ft. The distance from the site where noise was generated immediately outside of the module to rooms A and B (Figure 1) is 15 and 50 ft, respectively.
Data analysis.
The natural resonance frequency ranges of the mouse body regions were calculated by using the rat resonance frequency (Fn) ranges9,10 and was based on the formula
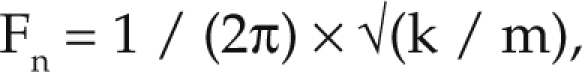 |
where k is the stiffness constant, and m is the mass. The stiffness constant (k) for mouse tissue was determined by the formula
where Fn was the resonance frequency at the limits of the range for the rat. The inherent stiffness of tissue in rats and mice was assumed to be the same and would only vary by a factor of mass, which was taken into account in the calculations. The mass of a single mouse was designated as 25 g. The resonance frequencies that were measured for mice were 88 to 92 Hz for abdomen, 712 to 724 Hz for thorax, and 240 to 252 Hz for head, with calculated resonance frequency ranges for mice of 85 to 92 Hz for abdomen, 711 to 727 H for thorax, and 237 to 253 Hz for head. These values were compared with calculated and measured values, respectively, of 4 to 8 Hz (4 to 8 Hz) for human abdomen and thorax,19 20 to 30 Hz (20 to 28 Hz) for human head, 27 to 29 Hz (28 Hz) for rat abdomen, 225 to 230 Hz (228 Hz) for rat thorax, and 75 to 80 Hz (76 Hz) for rat head.36 The values for humans were applicable to vertical vibration in the standing position.
To normalize the data for comparison of the levels of vibration likely perceived by each species, the vibration within a 63-Hz range around each species’ and body region's resonance frequency was measured. These ranges are designated as sensitivity frequency ranges and were 0 to 63 Hz for the human body regions and rat abdomen, 195 to 259 Hz for rat thorax, 45 to 109 Hz for the rat head, and 56 to 120, 687 to 751, and 213 to 277 Hz for mouse abdomen, thorax, and head, respectively. For these ranges, the actual frequencies measured, with 4-Hz resolution, were 0 to 64 Hz for the human body regions and rat abdomen, 196 to 256 Hz for rat thorax, 48 to 108 for rat head, and 60 to 120 Hz, 688 to 748, and 216 to 276 Hz for mouse abdomen, thorax, and head, respectively.
All data was analyzed by using Pulse LabShop software (version 13.1, Bruel and Kjaer). Vibration that the ventilated racks (n = 3) produced over the ambient noise was analyzed for significance by using a 2-tailed, paired t test (Excel, Microsoft, Redmond, WA). To minimize the damage to the floor from the construction equipment, one measurement of vibration was taken from each tool at each location described.
Results
Intracage vibration from the ventilated rack blower.
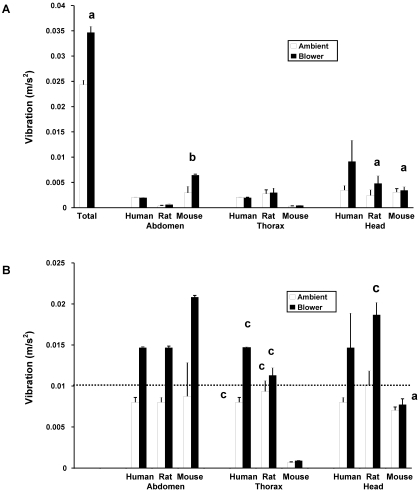
The total and rack-induced vibration within the RFR of a mouse inside of the cage, human, and rat are represented in Figure 2 A. The total vibration was significantly increased (P ≤ 0.05) to 0.035 ± 0.001 m/s2 (mean ± SEM) by the ventilated rack blower relative to ambient vibration at 0.024 ± 0.001 m/s2. Although blower-induced vibration was not increased relative to ambient vibration in the mouse abdominal RFR, mouse abdominal vibration from the ventilated rack blower was increased (P ≤ 0.01) to 0.006 ± 0.0002 m/s2 relative to the abdominal RFR of blower-induced vibration of rat (0.0006 ± 0.0002 m/s2) and human (0.002 ± 0.0001 m/s2). The vibration caused by the ventilated rack blower in rat head RFR (0.005 ± 0.001 m/s2) was increased (P < 0.05) compared with that (0.002 ± 0.001 m/s2) for ambient vibration. Similar results were found for mouse head RFR (0.0034 ± 0.0007 m/s2) relative to ambient vibration (0.0030 ± 0.0007 m/s2). In the human RFR, vibration due to the blower was not statistically different from ambient vibration in the respective ranges for abdomen, thorax, and head.
Figure 2.
Total vibration and vibration levels in the (A) RFR and (B) SFR produced from ambient sources and the ventilated rack blower measured from inside the cage. (A) This panel represents the total vibration from ambient sources (n = 3) and that generated by the ventilated rack blower (n = 3) from 0 to approximately 12.5 kHz and the levels in the RFR. The total vibration was increased significantly (a, P ≤ 0.05) by the ventilated rack blower relative to ambient vibration. Mouse abdominal vibration from the ventilated rack blower was increased (b, P ≤ 0.01) relative to the abdominal RFR of blower-induced vibration of rats and humans. The vibration caused by the ventilated rack blower in the rat and mouse head RFR was increased (a, P ≤ 0.05) relative to ambient vibration in both of these respective ranges. (B) Both ambient and ventilated rack blower-induced thoracic vibration in the SFR was greater (c, P ≤ 0.05) for both humans and rats as compared with mice. Vibration generated by the blower in the head SFR was greater (c, P ≤ 0.05) for rats than mice; the blower-induced vibration in the mouse head SFR was significantly greater (a, P ≤ 0.05) than was ambient vibration in this frequency range. The dashed line represents the human perception threshold of 0.01 m/s2.
Vibration caused by the ventilated rack blower in the SFR of human, rat, and mouse are summarized in Figure 2 B. The ventilated rack blower did not statistically increase abdominal vibration in the SFR for the human, rat, or mouse. Both ambient and blower-induced thoracic vibration in the SFR was greater (P ≤ 0.05) for human (0.008 ± 0.001 m/s2 and 0.015 ± 0.001 m/s2, respectively) and rat (0.009 ± 0.001 m/s2 and 0.011 ± 0.001 m/s2, respectively) as compared with mouse (0.0007 ± 0.0001 m/s2 and 0.0009 ± 0.0001 m/s2, respectively). Vibration generated by the blower in the head SFR was greater (P ≤ 0.05) for rat (0.019 ± 0.002 m/s2) than mouse (0.008 ± 0.0004 m/s2). The blower-induced vibration in the mouse head SFR was significantly greater (P ≤ 0.05) at 0.008 ± 0.0004 m/s2 than was ambient vibration (0.007 ± 0.0004 m/s2) in this frequency range. Although the blower-induced increase in mouse abdominal vibration relative to ambient vibration approached statistical significance (P ≤ 0.07), no other blower-induced vibration was statistically higher than its own ambient levels. However, the blower raised vibration levels above 0.01 m/s2, indicating that humans, rats, and mice may perceive this vibration.
Vibration levels generated 3 ft from the cage by construction equipment.
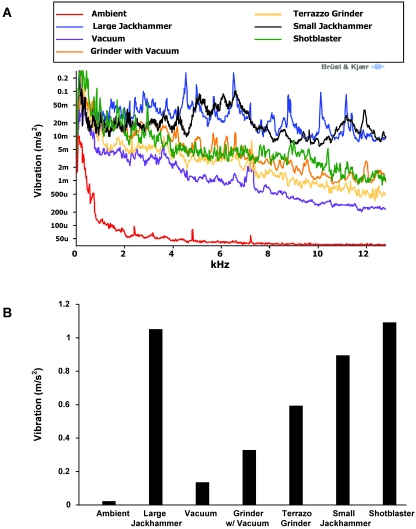
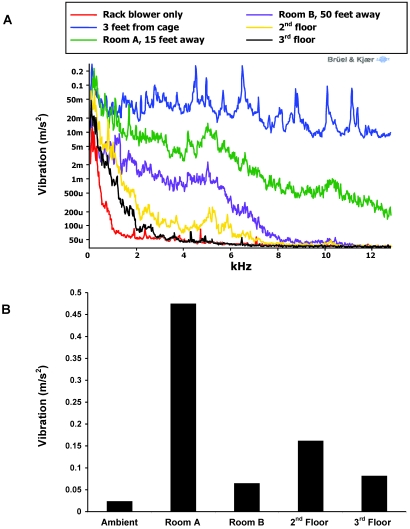
The relative vibration levels generated by each item of equipment are graphically represented in Figure 3. Vibration for the large and small jackhammers remained at relatively constant levels over the full kHz range, whereas vibration from the other implements tended to decrease as frequency increased. Compared with that for the other equipment, vibration caused by the shotblaster (used without shot) tended to be greater at lower frequencies (less than 1.5 kHz; Figure 3 A), thus contributing to the overall high level of vibration for this implement (Figure 3 B). Along with the shotblaster, the total vibration from 0 Hz to approximately 12.5 kHz was greater in the large jackhammer relative to that from the other implements.
Figure 3.
Intracage vibration levels from various types of construction equipment as measured 3 ft from the equipment represented as a (A) line graph and (B) bar graph.
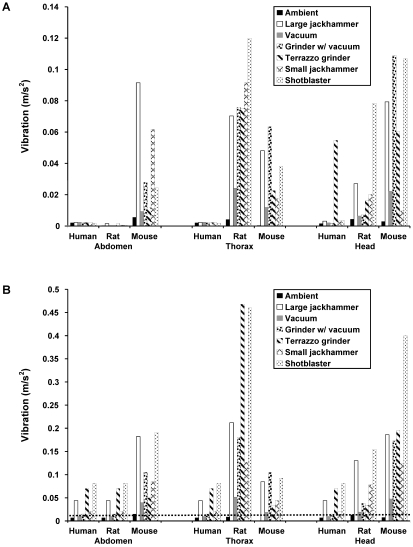
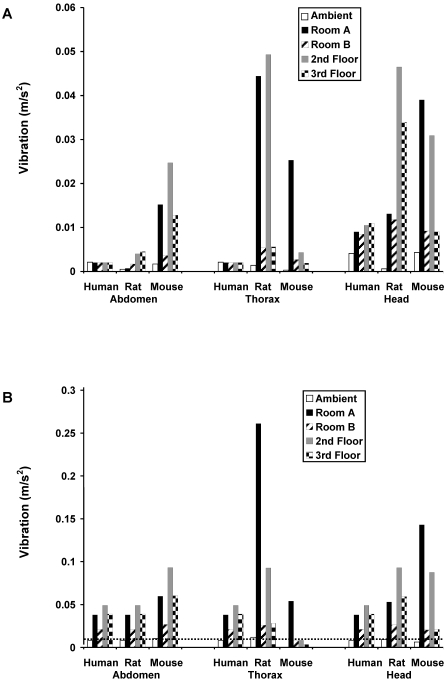
Figure 4 A shows vibration inside of the cage and anatomic location for each item of construction equipment when vibration was generated 3 ft from the cage. To preserve the integrity of the floor as much as possible, we obtained one measurement from each item of construction equipment. The most potentially damaging vibration generally appears to occur in the rat thorax and mouse head. The vibration generated by the equipment in the SFR of each species and anatomic location (Figure 4 B) followed the same general pattern as did vibration in the RFR. The human perception threshold of 0.01 m/s2 was exceeded by all of the equipment except the vacuum in the human SFR. Subjectively, personnel conducting the study and standing near the ventilated cage rack perceived the vibration from all of the equipment except the vacuum.
Figure 4.
(A) RFR and (B) SFR vibration levels for construction equipment measured inside of the ventilated rack cage at 3 ft from the equipment. Vibration from the equipment tended to be higher in both the RFR (when values for each respective item of equipment were compared) and SFR (levels above 0.01 m/s2) of mouse abdomen, rat and mouse thorax, and rat and mouse head relative to those of humans. The dashed line represents the human perception threshold of 0.01 m/s2.
Vibration levels inside of the cage at various distances from the noise source.
The large jackhammer generated vibration that could be measured at various locations within the facility. Room A was 15 ft away from the vibration source, whereas Room B was 50 ft away (Figure 1). The large jackhammer was used on the floor immediately adjacent to a support column to create the most potential for upward movement of vibration, and vibration was measured on the second and third floors directly above this site. As the distance from the large jackhammer increased, the level of vibration fell more quickly at higher frequencies (Figure 5 A). Although vibration levels on the second and third floors fell most rapidly, high levels of vibration persisted at lower frequencies. Because of vibration at lower frequencies, the total level of vibration generated by the large jackhammer on the second and third floors (Figure 5 B) exceeded the total vibration in room B.
Figure 5.
Intracage vibration levels from the large jackhammer as measured at various locations from the jackhammer represented as a (A) line graph and (B) bar graph.
Figure 6 A shows vibration levels in the RFR at various distances from the large jackhammer with respect to values at each anatomic location for human, rat, and mouse. Vibration from the large jackhammer in the SFR (Figure 6 B) exceeded 0.01 m/s2 in human and rats at all distances regardless of anatomical location. For the mouse, vibration levels exceeded 0.01 m/s2 for all distances in the abdomen and head, but only at 15 ft from the vibration source for the mouse thorax. Study personnel confirmed they could perceive vibration from the large jackhammer at all locations.
Figure 6.
(A) RFR and (B) SFR vibration levels for the large jackhammer measured from inside of the ventilated rack cage at various locations in the facility. RFR and SFR levels for both rats and mice tended to be higher than those for humans at the various locations, especially for rat thorax and head and mouse abdomen and head when compared to the value for each location for the human and values exceeding 0.01 m/s2, respectively.
Discussion
Evidence found in the literature overwhelmingly demonstrates that vibration can have detrimental effects in both humans and animals.4,21,23,29,33 To ensure that vibration caused by construction equipment during floor repair would not adversely affect mice in other parts of the animal facility, we here assessed the level of vibration that would reach mice housed in ventilated cages and whether the mice might perceive the vibration or suffer other adverse effects. For comparative information, we measured the vibration levels from the ventilated racks used in this facility to which these mice were exposed. Vibration from ambient sources, ventilated racks (data not shown), and construction equipment all conformed to the same pattern, in which vibration was highest at lower frequencies and decreased as frequency increased to approximately 12.5 kHz. The formula for the resonance frequency (Fn), where vibration is most likely to cause physical damage to objects, animals or humans, is
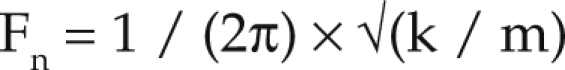 |
where k is the stiffness constant, and m is the mass.9,10 If the tissue stiffness constant is assumed to be the same for humans, rats, and mice, the resonance frequency increases as the mass decreases, thus explaining why resonance frequencies increase from human to rat and from rat to mouse. Although the level of vibration for most equipment does decrease as frequency increases, the vibration levels in the frequencies that most affect rats and mice are still high and tend to be higher than for humans.
The ventilated rack blower changed some of the relative vibration levels in both the RFR and SFR of the various anatomic locations in the species. In most cases, the ventilated rack blower caused an average upward trend above the perception level of humans in all 3 species, but what effect this increase would have on animals is unclear. Adaption to any perceived vibration likely occurs and may not be a factor in ventilated caging. Many caging companies now manufacture ventilated racks that likely cause less vibration than do the ventilated racks used in the current study, and blowers that are not attached to ventilated racks may cause less cage vibration.
The total vibration levels inside of the cage approached the human exposure limit of 1.15 m/s2 for the large jackhammer and shot blaster located at 3 ft from the cage. Levels exceeded 1.0 m/s2 and, as a reference, vibration above 0.8 m/s2 is perceived as uncomfortable for humans.3 Vibration levels at all locations and distances from the vibration source showed the same general trends in both the RFR and SRF for the construction equipment. The trend for all of the construction equipment at 3 ft from the cage and for the large jackhammer measured at various facility locations was an increase in vibration in mouse abdomen, rat thorax, mouse thorax, rat head, and mouse head compared with human levels. Because high-frequency vibration is attenuated more readily than lower frequency vibration,6 high-frequency vibration became less of a component of the total level of large jackhammer-induced vibration as the distance from the jackhammer increased. However, at more distant locations, the amplitude of this high-frequency vibration still tended to remain within the RFR and SFR of mice and rats. These results indicate that depending on the distance from the source, construction equipment may expose rats and mice to more vibration than would be experienced by humans.
In people, the RFR and SFR can vary slightly with body position and the axis of vibration.19,22 In the current study, we measured our reference frequencies according to those applicable to vertical vibration. Because the normal posture and anatomy of humans differ from those of mice, the mouse resonance frequencies used in the current study were derived from published resonance frequencies for rats. 36 In that study, 36 resonance frequencies in rats were estimated to be 4 to 5 times higher than in humans. Our work indicates that the resonance frequencies of mice would need to range from 100 to more than 3000 times higher in mice than humans for there to be similar vibration within resonance frequencies for humans and mice when large jackhammer vibration was measured at the various locations (data not shown). Considering that resonance frequencies in rats were only 4 to 5 times higher than those in humans, this difference between humans and mice is extremely unlikely. The study in rats36 was conducted with the rats on their backs, but studies in humans have shown that resonance frequencies for supine subjects are similar to the ranges for those who stand.17,35,37 Because resonance frequency ranges are similar for standing and supine humans,10 resonance frequencies are also likely to be comparable for standing and supine mice and rats.
In the current study, vibration from the large jackhammer at various locations was an average of 3.4 times higher at 60 Hz than 20 Hz. In a standing position, human legs have a resonance frequency of about 20 Hz,19 and the mouse leg has a resonance frequency of approximately 60 Hz.5 Therefore, vibration again likely would affect mice more than humans. Mouse and human legs may absorb some vibration, because the resonance frequencies for legs are different than those for other body regions. However, the impact of vibration dampening by the legs is complex. Secondary resonances in the legs (a broad spectrum of frequencies produced by the legs when vibrated at the legs’ resonance frequency) may coincide with other body resonances. This overlap would cause the legs to amplify, rather than absorb, vibration.
The results of this work demonstrate that vibration produced by the same source may have different effects on the abdomen, thorax, and head of various species. The effects of whole-body vibration on these anatomic areas of humans include gastrointestinal alterations, headache, and increased respiratory rate due to oscillation of oxygen in the lungs.4,10,14,30 Due to the interspecies differences in the susceptibility of body regions to vibration depending on their particular critical frequency values, the physiologic and pathologic effects of vibration from the same vibration source are likely to differ between species.
Vibration and noise have long been known to have potentially detrimental effects in rodents, but the relative contribution of each to these adverse effects is unknown. We measured sound at the same time as vibration was measured in the current study. The results of the sound analysis due to the construction equipment indicated that mice actually hear less construction noise than do humans.25 Therefore, the study in our facility suggests that vibration due to construction equipment would be of greater concern than would the associated noise. Further work needs to be done to determine the resonance frequencies of mice directly and to correlate levels of vibration with physiologic and pathologic effects.
Acknowledgments
We thank Jesse Degraff (Division of Laboratory Animal Resources, Duke University Medical Center) and Ned Leverage (Life Science Products) for their technical expertise in logistical matters involving the ventilated racks and construction equipment, respectively.
References
- 1.Abeyesinghe SM, Wathes CM, Nicol CJ, Randall JM. 2001. The aversion of broiler chickens to concurrent vibrational and thermal stressors. Appl Anim Behav Sci 73:199–215 [DOI] [PubMed] [Google Scholar]
- 2.Ariizumi M, Okada A. 1983. Effect of whole-body vibration on the rat brain content of serotonin and plasma corticosterone. Eur J Appl Physiol Occup Physiol 52:15–19 [DOI] [PubMed] [Google Scholar]
- 3.Branner AJ. 2010. Human response to shock and vibration, p 41.1–41.39 : Piersol AG, Paez TL. Harris’ shock and vibration handbook. New York (NY): McGraw Hill [Google Scholar]
- 4.Candian Center for Occupational Health and Safety [Internet] Vibration—health effects. [Cited 09 December 2010]. Available at: http://www.ccohs.ca/oshanswers/phys_agents/vibration/vibration_effects.html
- 5.Christiansen BA, Bayly PV, Silva MJ. 2008. Constrained tibial vibration in mice: a method for studying the effects of vibrational loading of bone. J Biomech Eng 130:044502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Construction Industry Research and Information Association [Internet] Vibration. [Cited 09 December 2010]. Available at: http://www.ciria.org.uk/complianceplus/4_guidance2.htm?g_id=I
- 7.Edwards RG, McCutcheon EP, Knapp CF. 1972. Cardiovascular changes produced by brief whole-body vibration of animals. J Appl Physiol 32:386–390 [DOI] [PubMed] [Google Scholar]
- 8.Faith RF, Miller SJ. 2007. The need for sound and vibration standards in US research animal rooms. ALN Magazine. July/August:31–38 [Google Scholar]
- 9.Frankovich D. [Internet] The basics of vibration isolation using elastomeric materials. Aearo AR specialty composites. [Cited 09 December 2010]. Available at: http://www.earsc.com/pdfs/engineering/BasicsofVibrationIsolation.pdf
- 10.Griffin MJ. 1996. Whole-body vibration and health. : Griffin MJ. Handbook of human vibration. San Diego (CA): Elsevier Academic Press [Google Scholar]
- 11.Hill PSM. 2001. Vibration and animal communication: a review. Integr Comp Biol 41:1135–1142 [Google Scholar]
- 12.Jackson KE. Basic impact procedures. DoctorKnow application paper. [Cited 09 December 2010]. Available at: http://www.mhm.assetweb.com/DRKNOW/APLPAPR.NSF/apweb/3D751D9716F8F06F852565A200606A64?OpenDocument
- 13.Judex S, Donahue LR, Rubin C. 2002. Genetic predisposition to bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J 16:1280–1282 [DOI] [PubMed] [Google Scholar]
- 14.Kjellberg A. 1990. Psycological aspects of occupational vibration. Scand J Work Environ Health 16:39–43 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Liu Y, Jiang Z, Guan J, Yi G, Cheng S, Yang B, Fu T, Wang Z. 2009. Behavioral changes related to Wenchuan devastating earthquake in mice. Bioelectromagnetics 30:613–620 [DOI] [PubMed] [Google Scholar]
- 16.Lipman NS, Perkins SE. 2002. Factors that may influence animal research, p 1153 : Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine. San Diego (CA): Academic Press [Google Scholar]
- 17.Liu JZ, Kubo M, Aoki H, Terauchi F. 1996. The transfer function of the human body on vertical sinusoidal vibration. Japanese Journal of Ergonomics 32:28–29 [Google Scholar]
- 18.Ljunggren F, Wang J, Agren A. 2007. Human vibration perception from single- and dual-frequency components. J Sound Vib 300:13–24 [Google Scholar]
- 19.MacMillian R. 2009. Occupational health and safety practitioner, p 10–16 : Guo J. Human vibration: basic characteristics. West Perth (Australia): Worksafe [Google Scholar]
- 20.Montenegro MA, Palomino H, Palomino HM. 1995. The influence of earthquake-induced stress on human facial clefting and its simulation in mice. Arch Oral Biol 40:33–37 [DOI] [PubMed] [Google Scholar]
- 21.Occupational Health and Safety Authority [Internet] Protection of workers from risks arising from exposure vibration regulations 2005. [Cited 09 December 2010]. Available at: http://www.ohsa.org.mt /showpage.asp?pageid=165
- 22.Parsons KC, Griffin MJ. 1988. Whole-body vibration perception thresholds. J Sound Vib 121:237–258 [Google Scholar]
- 23.Perremans S, Randall JM, Rombouts G, Decuypere E, Geers R. 2001. Effect of whole-body vibration in the vertical axis on cortisol and adrenocorticotropic hormone levels in piglets. J Anim Sci 79:975–981 [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen S, Glickman G, Norinsky R, Quimby FW, Tolwani RJ. 2009. Construction noise decreases reproductive efficiency in mice. J Am Assoc Lab Anim Sci 48:363–370 [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds RP, Kinard WL, Degraff JJ, Leverage N, Norton JN. 2010. Noise in a laboratory animal facility from the human and mouse perspectives. J Am Assoc Lab Anim Sci 49:592–597 [PMC free article] [PubMed] [Google Scholar]
- 26.Romans J. 1958. Effect of severe whole-body vibration on mice and methods of protection from vibration injury. WADC Technical Report 58-107, ASTIA Document No. AD 151070. Wright–Patterson Air Force Base (OH): Wright Air Development Centre [Google Scholar]
- 27.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, Mittal V, Rosen CJ, Pessin JE, Judex S. 2007. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA 104:17879–17884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sackler AM, Weltman AS. 1966. Effects of vibration on the endocrine system of male and female rats. Aerosp Med 37:158–166 [PubMed] [Google Scholar]
- 29.Seidel H. 1993. Selected health risks caused by long-term, whole-body vibration. Am J Ind Med 23:589–604 [DOI] [PubMed] [Google Scholar]
- 30.Seidel H, Griffin MJ. 1998. Whole-body vibration, p 50.2–50.5 : Stellman JM. Encyclopedia of occupational health and safety, vol 2. Switzerland (Geneva): International Labor Office [Google Scholar]
- 31.Shenaeva TA. 1990. Effects of vibration and noise on the reproductive function in experimental animals. Gig Tr Prof Zabol 9:16–21 [PubMed] [Google Scholar]
- 32.Small JD, Dietrich T. 2007. Environmental and equipment monitoring, p 409–436 : Fox J, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smoth AL. The mouse in biomedical research, vol 3. San Diego (CA): Academic Press [Google Scholar]
- 33.Toraason MA, Badger DW, Wright GL. 1980. Gastrointestinal response in rats to vibration and restraint. Environ Res 23:341–347 [DOI] [PubMed] [Google Scholar]
- 34.Turner JG, Bauer CA, Rybak LP. 2007. Noise in animal facilities and why it matters. J Am Assoc Lab Anim Sci 46:10–13 [PubMed] [Google Scholar]
- 35.Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM. 2005. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med 55:12–23 [PMC free article] [PubMed] [Google Scholar]
- 36.Ushakov IB, Soloshenko NV, Koslovskij AP. 1983. The examination of resonance frequencies of vibration in rats. Kosm Biol Aviakosm Med 17:65–68 [Article in Russian] [PubMed] [Google Scholar]
- 37.Vogt L, Mertens H, Krause HE. 1978. Model of supine human body and its reactions to external forces. Aviat Space Environ Med 49:270–278 [PubMed] [Google Scholar]
- 38.Wasserman DE, Wasserman JF. 1999. [Internet] From the Institute for Study of Human Vibration. Occupational vibration: a brief overview. [Cited on 10 December 2010]. Available at: http://www.engr.utk.edu/ishv/newpaper.html
- 39.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miler LM, Rubin CT, Judex S. 2006. Low-level mechanical vibrations can influence bone resorption in the growing skeleton. Bone 39:1059–1066 [DOI] [PubMed] [Google Scholar]
- 40.Xie L, Rubin C, Judex S. 2008. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol 104:1056–1062 [DOI] [PubMed] [Google Scholar]