Abstract
The Zebrafish International Resource Center (ZIRC) supplies wildtype, mutant, and transgenic zebrafish (Danio rerio) to the international research community. In 2005, the ZIRC halted shipment of adult Tübingen (TU) zebrafish, a popular wildtype line, after diagnosis of asymptomatic Mycobacterium chelonae infections in a high proportion of the TU stock. Mycobacterium presents a zoonotic risk to fish handlers. In addition, the presence of underlying chronic disease in a model organism is unacceptable. The TU stock was depopulated and replaced by a new import of TU with the intent of reducing disease prevalence. In the current study, we sampled the new population of TU and fish of the AB, Tupfel long-fin (TL), TAB5 and TAB14 (2 AB × TU hybrid lines), and wildtype-in-Kalkutta (WIK) lines for histologic evaluation and acid-fast staining and compared the prevalence of subclinical mycobacteriosis between these lines. Although prevalence in the new TU stock was lower than that of the original TU stock, asymptomatic infections with Mycobacterium remained high (10%) in the new TU stock held in 20-gal tanks. The prevalence was similar (10%) in the TAB5 line compared with other wildtype lines held in similar conditions. Prevalence of infections in TU can be minimized by husbandry adjustments, including tank size, population density, and cleaning method. Application of these findings has allowed us to decrease mycobacteriosis in TU zebrafish and resume shipment of TU adults to the research community.
Abbreviation: AB, wildtype line; TAB5, TU × AB hybrid line, TAB14, TU × AB hybrid line; TU, Tübingen line; TL, Tupfel long-fin line; WIK, wildtype in Kalkutta line; ZIRC, Zebrafish International Resource Center
In a 2005 surveillance study at the Zebrafish International Resource Center (ZIRC), Mycobacterium detected in fish and biofilms was further characterized as M. chelonae by sequencing the hsp65 gene.14 M. chelonae is a low-virulence environmental pathogen in aquatic systems.4 Unlike M. marinum and M. haemophilum, which have been associated with outbreaks in zebrafish colonies,11,13 M. chelonae usually is associated with incidental infections in zebrafish.4 In fact, the overwhelming majority of cases of mycobacteriosis at the ZIRC are asymptomatic. The initial study used histology, PCR, and bacterial culture to identify infected zebrafish.14 Combining data from all 3 diagnostic methods, the highest prevalence of mycobacteriosis (34%) occurred in the Tübingen (TU) line. Lower prevalence was observed in Tupfel long-fin (TL; 5%, 1 of 20), wildtype in Kalkutta (WIK; 10%, 2 of 20), and AB (5%, 1 of 20) fish. These data were obtained from fish raised in 20-gal tanks. When the elevated level of subclinical mycobacteriosis in the TU line was recognized, shipments of TU adults to the research community were stopped, and a new strain of TU was imported from the same lab that supplied the original line. We then expanded the surveillance study to compare the prevalence of subclinical mycobacteriosis in the new TU stock with that of other wildtype lines including AB, TAB5 and TAB14 (2 AB × TU hybrid lines), TL, and WIK, all of which were raised in 20-gal tanks. In addition, we compared the prevalence of mycobacteriosis in TU raised in 20-gal and 1-gal tanks. All fish were fixed for histologic sectioning and acid-fast staining, a method that has been validated as a reliable means to diagnose mycobacteriosis.14 Finally, we designed experiments to determine the effects of population density and different procedures for tank cleaning on the prevalence of subclinical mycobacteriosis in TU zebrafish.
Materials and Methods
Zebrafish care and maintenance.
The ZIRC main fish facility uses recirculating water systems with bead and fluidized sand filters. UV lights sterilize postfiltration water. Water temperature is maintained at 28.5 °C and pH between 7.2 and 7.6. Reverse-osmosis–treated city water is added as replacement, and salt is added to a conductivity of 500 μS. TU, AB, TAB5, TAB14, and WIK zebrafish were reared according to standard protocols12 in 20- or 1-gal tanks. Fish were fed Great Salt Lake Artemia nauplii (Artemia International, Fairview, TX) in the morning and commercial flake diet in the afternoon. Juvenile fish received an additional midday flake food meal. Biofilm previously collected from the Artemia cone was negative for mycobacteria.14 The University of Oregon Institutional Animal Care and Use Committee approved all protocols. Fish were euthanized by rapid cooling in ice water.15 Euthanasia of zebrafish reared at 28.5 °C by hypothermal shock was reviewed and approved by the University of Oregon Institutional Animal Care and Use Committee and the NIH Office of Laboratory Animal Welfare.
Tank cleaning and biosecurity.
Only surface-sanitized embryos enter the ZIRC main fish facility. Glass 20-gal tanks are scrubbed with scouring pads (Scotch-Brite General Purpose Scouring Pads, 3M, St Paul, MN), and plastic 1-gal tanks are scrubbed with scrubber pads (Fine Acrylic Scrubber Pads, Lee's Aquarium and Pet Products, San Marco, CA). Tanks are cleaned when algae begin to appear on the front of the tank, obstructing visual monitoring of the population, or when loose debris begins to accumulate on the bottom of the tank. Clean, autoclaved scrubbers and nets are used for each tank and never shared between tanks. Personnel typically wear new shoulder-length disposable plastic gloves (Vet-R-Sem, Jorgensen Laboratories, Loveland, CO) when cleaning each tank. If gloves are not worn, hands and arms (to the level they are immersed in water) are washed with antimicrobial handsoap (BacDown, Fisher Scientific, Pittsburgh, PA) containing 0.5% triclosan before cleaning each tank. Between uses, tanks are scrubbed, soaked in 0.7% bleach solution for at least 1 h, neutralized in 3.175 g/L sodium thiosulfate (Western Chemical, Ferndale, WA) for at least 15 min, rinsed in a tunnel washer (Hobart, Troy, OH) at 82 °C, and air dried.
Histology.
Adult zebrafish were euthanized, fixed in Dietrich fixative, and placed on a laboratory rocker for a minimum of 24 h. Fixed fish were decalcified in 10% trifluoroacetic acid, processed for paraffin sectioning according to standard techniques, and stained with Ziehl–Neelsen acid-fast stain.3
Results
Prevalence of subclinical mycobacteriosis in TU and other wildtype zebrafish.
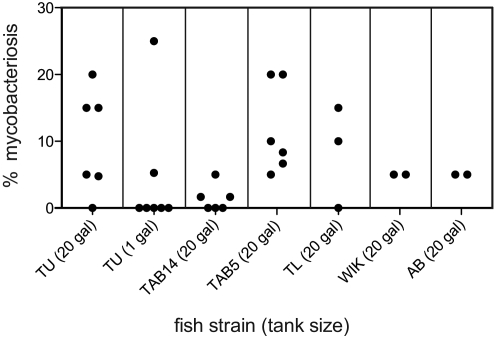
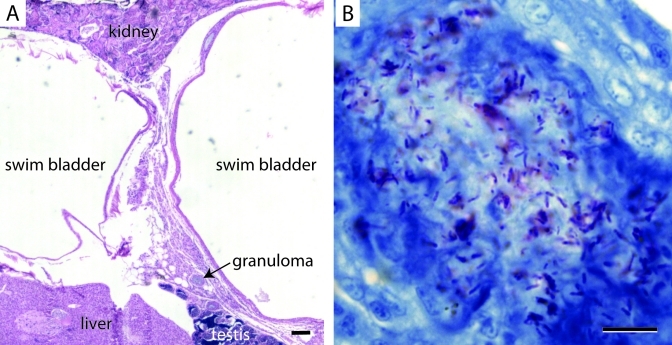
After the new stock of TU zebrafish was imported and raised for approximately 1 y, we tested it and other wildtype stocks for Mycobacterium infections. We sampled TU, TAB14, TAB5, TL, WIK, and AB zebrafish raised in 20-gal tanks for histopathology and acid-fast staining. The age of the fish at sampling ranged from 211 to 694 d, although most fish were 398 d postfertilization or younger. Mycobacteriosis was diagnosed in 10% of TU (n = 121), 2.1% of TAB14 (n = 240), 10% of TAB5 (n = 240), 2.5% of AB (n = 80), 8.3% of TL (n = 60), and 5% of WIK (n = 40; Table 1, Figure 1). The difference in prevalence between TAB14 and TAB5 was significantly different by unpaired Student t test (P = 0.0047). Histologically, these infections were generally characterized by diffuse chronic inflammation surrounding one or both lobes of the swim bladder. In addition, granulomas were present around the swim bladder in many infected fish. Acid-fast–positive bacilli were present within the inflamed tissue and sometimes on the luminal side of the swim bladder wall (Figure 2). Of the 240 sampled TAB5 zebrafish, 4 displayed clinical signs of disease including mild facial hemorrhage, petechiation at the base of the pectoral fins, a small focus of ventral translucence, and a small lateral ulcer. Three of these 4 fish were diagnosed with mycobacteriosis. The remaining TAB5 and all other fish did not display behavioral or clinical signs of disease. Some fish appeared mildly underweight, although this characteristic was not correlated with the diagnosis of mycobacteriosis. Therefore, 1.3% of TAB5 exhibited clinical mycobacteriosis and 8.8% of TAB5 (n = 240) had subclinical disease. All other cases of mycobacteriosis in TU, TAB14, TL, WIK, and AB were subclinical.
Table 1.
Strain-specific predispositions of wildtype populations to subclinical mycobacteriosis
| No. of fish sampled | Fish positive for mycobacteriosis by histopathology |
|||
| Strain | Agea | No. | % | |
| TU | 21 | 369 | 1 | 4.8 |
| TU | 20 | 422 | 3 | 15 |
| TU | 20 | 244 | 4 | 15 |
| TU | 20 | 211 | 0 | 0 |
| TU | 20 | 412 | 1 | 5 |
| TU | 20 | 398 | 3 | 15 |
| Total TU | 121 | 12 | 10 | |
| TUb | 20 | 369 | 0 | 0 |
| TUb | 19 | 369 | 0 | 0 |
| TUb | 20 | 244 | 0 | 0 |
| TUb | 20 | 244 | 0 | 0 |
| TUb | 20 | 251 | 0 | 0 |
| TUb | 19 | 251 | 1 | 5.3 |
| TUb | 20 | 252 | 5 | 25 |
| Total TUb | 138 | 6 | 4.3 | |
| TAB14 | 20 | 694 | 0 | 0 |
| TAB14 | 20 | 497 | 0 | 0 |
| TAB14 | 20 | 288 | 0 | 0 |
| TAB14 | 60 | 392 | 1 | 1.7 |
| TAB14 | 60 | 377 | 3 | 5 |
| TAB14 | 60 | 391 | 1 | 1.7 |
| Total TAB14 | 240 | 5 | 2.1 | |
| TAB5 | 20 | 694 | 4 | 20 |
| TAB5 | 20 | 497 | 4 | 20 |
| TAB5 | 20 | 279 | 1 | 0 |
| TAB5 | 60 | 383 | 6 | 10 |
| TAB5 | 60 | 385 | 4 | 6.7 |
| TAB5 | 60 | 391 | 5 | 8.3 |
| Total TAB5 | 240 | 24 | 10 | |
| AB | 40 | 345 | 1 | 5 |
| AB | 40 | 345 | 1 | 5 |
| Total AB | 80 | 2 | 2.5 | |
| TL | 20 | 342 | 2 | 10 |
| TL | 20 | 330 | 0 | 0 |
| TL | 20 | 273 | 3 | 15 |
| Total TL | 60 | 5 | 8.3 | |
| WIK | 20 | 372 | 1 | 5 |
| WIK | 20 | 344 | 1 | 5 |
| Total WIK | 40 | 2 | 5 | |
Age at sampling in days postfertilization.
Tank size was 1 gal; for all others, tank size was 20 gal.
Figure 1.
Within each wildtype strain, the prevalence of mycobacteriosis in a sample population varied between tanks. The percentage of mycobacteriosis positive fish in a sample population is plotted for each wildtype strain. Each dot represents a sample population from an individual tank.
Figure 2.
A TU wildtype zebrafish that displayed no behavioral or physical abnormalities with histologic evidence of mycobacteriosis. (A) Hematoxylin and eosin staining of chronic inflammation with small granulomas between the cranial and caudal lobes of the swim bladder. (B) Acid-fast staining of chronic granulomatous inflammation at the swim bladder bifurcation. Acid-fast positive bacilli (red) are apparent within a granuloma. Anteriocranial is to the left, and dorsal is up. Scale bar: 100 μm (A), 10 μm (B).
Effect of tank size on prevalence of mycobacteriosis in TU zebrafish.
When the new stock of TU was imported, chlorine-surface–sanitized embryos were brought into the nursery and reared for approximately 4 wk. They then were transferred to either 1- or 20-gal tanks and reared to adulthood. We sampled TU populations from 7 different 1-gal tanks for histologic sectioning and acid-fast staining. Fish age at sampling ranged from 244 to 369 d postfertilization. Of the TU raised in 1-gal tanks, only 4.3% (n = 138) were diagnosed with subclinical mycobacteriosis (Table 1, Figure 1). In addition, the fish positive for mycobacteriosis came from only 2 of the 7 1-gal tanks.
Effects of tank density and cleaning method on disease prevalence.
We hypothesized that the discrepancy between the prevalence of mycobacteriosis in TU raised in 20- and 1-gal tanks may be a result of different cleaning methods. The 20-gal tanks are cleaned with the fish population in the tank. The walls and bottom of the tank are scrubbed, and the debris is either allowed to settle and then is removed by siphon, or the water flow rate is increased to force the loosened debris out the drain. In both cases, debris and liberated biofilm get mixed in the water column, and the fish can be observed swimming through the debris and eating suspended particles. However, when 1-gal tanks are dirty, the fish are transferred into a clean tank, and the fish avoid exposure to loosened biofilm and tank debris. To test our hypothesis, we compared the effects of different methods of tank cleaning on TU raised in 20- and 1-gal tanks. We stocked 6 20-gal tanks with TU juveniles, 3 at high density (368 fish per tank) and 3 at low density (250 fish per tank). For each density, each of the 3 tanks was cleaned by using 1 of the following 3 methods: 1) scrubbing followed by removing debris by siphon; 2) scrubbing followed by increasing the flow of water to push debris out the drain; and 3) cleaning as for a 1-gal tank, that is the entire population was transferred to a new clean tank set-up next to the dirty tank. In addition, we stocked 3 1-gal tanks with 24 or 25 TU juveniles and cleaned these tanks with the 3 methods just described. The fish were raised to approximately 1 y of age, after which 30 fish from each of the 20-gal tanks and all fish from the 1-gal tanks were fixed and processed for histologic sectioning and acid-fast staining. The 20- and 1-gal tanks were cleaned when algae appeared on the front surface of the tank (see Materials and Methods). For tanks that were scrubbed, cleaning usually occurred at 4- to 6-wk intervals. Because tanks sanitized by using bleach, sodium thiosulfate, a 82 °C rinse, and air-dry cycle took longer to become dirty than did tanks that were scrubbed with the fish in the tank, the fish populations that were transferred to a clean tank were moved at 6- to 8-wk intervals.
No Mycobacterium was detected in the TU fish raised in 1-gal tanks regardless of cleaning method (Table 2). Because some fish were inexplicably lost from these tanks (5 from scrub and siphon, 9 from scrub and increase flow, and 2 from tank swap), we cannot rule out the possibility that they might have been infected with Mycobacterium. In the low-density 20-gal tanks, subclinical mycobacteriosis was diagnosed in 1 fish from the scrub and siphon tank (3.3%), 1 fish from the scrub and increased water flow tank (3.3%), and 2 fish from the population that swapped tanks (6.7%). In the high-density 20-gal tanks, subclinical mycobacteriosis was diagnosed in 3 fish from the scrub and siphon tank (10%), 0 fish from the scrub and increased water flow tank, and 1 fish from the population that swapped tanks (3.3%).
Table 2.
Percentage of TU zebrafish with mycobacteriosis according to cleaning method, tank size, and population density
| Tank size |
|||
| Cleaning | 20-gal | 20-gal | |
| method | (high density) | (low density) | 1-gal |
| Scrub and siphon | 10% (n = 30) | 3.3% (n = 30) | 0% (n = 19) |
| Scrub and increased flow | 0% (n = 30) | 3.3% (n = 30) | 0% (n = 16) |
| Tank swap | 3.3% (n = 30) | 6.7% (n = 30) | 0% (n = 23) |
Discussion
In a 2005 surveillance study performed at the Zebrafish International Resource Center, subclinical infections with Mycobacterium chelonae were identified in various fish populations.14 The prevalence of infection in the TU population sampled was abnormally high (34%; n = 80), when data from all diagnostic tests were combined. In contrast, the overall prevalence of mycobacteriosis in fish representing the various lines examined, excluding TU, was only 1.25% (n = 160). When the elevated level of mycobacteriosis was recognized, ZIRC stopped shipment of TU adults and replaced the population with a new imported stock, which entered the main fish facility according to standard quarantine and surface-sanitizing protocols.12 When the new TU stock was sampled for histologic sectioning and acid-fast staining, we determined that the prevalence was still increased (10%), although much lower than in the original TU stock. Interestingly, the 2 TU × AB hybrid lines differed significantly in the prevalence of disease. The prevalence of mycobacteriosis in TAB5 was 10% but was only 2.1% in TAB14, a rate similar to the prevalence in AB zebrafish (2.5%). These data illustrate the possibility of a heritable component to Mycobacterium susceptibility and that this trait is shared by TU and TAB5. Alternatively, genes conferring resistance may be present in AB and TAB14. Zebrafish susceptibility to Mycobacterium infection has previously been shown to be influenced by the rag1 gene9 and lta4h locus.10 A genetic component to Mycobacterium susceptibility has been detected in other species as well, including cattle5,7 and humans.2 Given the higher prevalence of mycobacteriosis in TAB5, ZIRC has opted to make TAB14 the only AB × TU hybrid line available for purchase. The 3 generations of TAB5 described here were maintained only for Mycobacterium research.
Although a comparison of Mycobacterium infections across several wildtype lines indicates a genetic component to susceptibility, further analysis of tank cleaning methods suggests that susceptibility can be managed by altering tank husbandry. We found that the prevalence of mycobacteriosis in TU could be lowered to 4.3% by raising the fish in 1-gal tanks. The difference in prevalence between 20- and 1-gal tanks was not significant by unpaired Student t test (P = 0.2665). However, among the 7 1-gal tanks that were sampled, the 25% prevalence diagnosed in a single tank was much greater than the others (5.3% in one tank and 0% in 5 tanks; Figure 1). If this outlier is removed from the data set, the difference in prevalence becomes significant (P = 0.0202). The 25% prevalence diagnosed in one of the 1-gal tanks of TU may be a result of abnormally high exposure to the infectious agent in that tank or may reflect normal variation between tanks. Ignoring the calculations for prevalence, one could look at the 20- and 1-gal tanks as simply being infected or not. In this case, 5 of the 6 20-gal tanks (83%) were positive for mycobacteriosis, whereas 2 of the 7 1-gal tanks (29%) were positive.
Although we observed a lower prevalence of subclinical mycobacteriosis in TU raised in 1-gal tanks, it is not feasible for ZIRC to raise sufficient numbers of TU for shipping and inhouse stocks in 1-gal tanks. Looking for a way to minimize these infections, we compared 3 different methods of tank cleaning for TU zebrafish raised in 1- and 20-gal tanks at high and low density. The only TU population that experienced the 10% mycobacteriosis rate observed in the initial survey was the high-density, 20-gal tank cleaned by scrub and siphon. Historically, ZIRC staff have had the option to choose whether they clean 20-gal tanks by siphoning or increasing water flow, and only recently have staff members begun using the increased water flow method. Considering these data, we now raise TU zebrafish at low density (250 fish or less) in 20-gal tanks and clean these tanks by scrubbing and increasing water flow to flush debris out the drain. Compared with allowing debris to settle for removal by siphon, the cleaning method using increased water flow may reduce exposure to mycobacterial biofilms or to protozoa living in biofilms, both of which have been shown to increase transmissibility and survivability of Mycobacterium species.1,6,16 The prevalence of subclinical mycobacteriosis detected in the 20-gal tanks cleaned by scrubbing and increasing water flow was lower in the high-density tank (0%) than the low-density tank (3.3%). However, because zebrafish susceptibility to mycobacteriosis is known to be influenced by crowding stress8 and because both tanks experienced less disease than did the high-density tank in which debris was removed by siphoning, we decided to raise TU fish at low density. In addition, we minimize stress on all wildtype lines by keeping stocks used for inhouse work in separate tanks from those used for shipping to the research community. Inhouse stocks are bred and used to generate new stocks for inhouse and shipping. Fish that will be shipped to customers as adults are labeled as ‘for shipping’ in juvenile stages. These fish are not subjected to the stresses associated with movement to crossing tanks and breeding activity. They are removed from the tank for shipment only. After instituting these husbandry protocols, ZIRC resumed shipment of TU adults in 2010. We continue to monitor the wildtype zebrafish populations for clinical and subclinical disease.
Acknowledgments
We thank Dr Michael Kent for reading this manuscript and offering comments and suggestions. The Zebrafish International Resource Center is supported by grant P40 RR012546 from the NIH National Center for Research Resources.
References
- 1.Adekambi T, Ben Salah S, Khlif M, Raoult D, Drancourt M. 2006. Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl Environ Microbiol 72:5974–5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernando SL, Britton WJ. 2006. Genetic susceptibility to mycobacterial disease in humans. Immunol Cell Biol 84:125–137 [DOI] [PubMed] [Google Scholar]
- 3.Humason GL. 1967. Animal tissue techniques. San Francisco (CA): WH Freeman [Google Scholar]
- 4.Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. 2004. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp Biochem Physiol C Toxicol Pharmacol 138:383–390 [DOI] [PubMed] [Google Scholar]
- 5.Koets A, Santema W, Mertens H, Oostenrijk D, Keestra M, Overdijk M, Labouriau R, Franken P, Frijters A, Nielen M, Rutten V. 2010. Susceptibility to paratuberculosis infection in cattle is associated with single-nucleotide polymorphisms in Toll-like receptor 2 which modulate immune responses against Mycobacterium avium subspecies paratuberculosis. Prev Vet Med 93:305–315 [DOI] [PubMed] [Google Scholar]
- 6.Marsollier L, Stinear T, Aubry J, Saint Andre JP, Robert R, Legras P, Manceau AL, Audrain C, Bourdon S, Kouakou H, Carbonnelle B. 2004. Aquatic plants stimulate the growth of and biofilm formation by Mycobacterium ulcerans in axenic culture and harbor these bacteria in the environment. Appl Environ Microbiol 70:1097–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minozzi G, Buggiotti L, Stella A, Strozzi F, Luini M, Williams JL. 2010. Genetic loci involved in antibody response to Mycobacterium avium ssp. paratuberculosis in cattle. PLoS ONE 5:e11117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsay JM, Watral V, Schreck CB, Kent ML. 2009. Husbandry stress exacerbates mycobacterial infections in adult zebrafish, Danio rerio (Hamilton). J Fish Dis 32:931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE, Ramakrishnan L. 2006. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect Immun 74:6108–6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, Moens CB, Ramakrishnan L. 2010. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140:717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watral V, Kent ML. 2007. Pathogenesis of Mycobacterium spp. in zebrafish (Danio rerio) from research facilities. Comp Biochem Physiol C Toxicol Pharmacol 145:55–60 [DOI] [PubMed] [Google Scholar]
- 12.Westerfield M. 1993. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). Eugene (OR): University of Oregon Press [Google Scholar]
- 13.Whipps CM, Dougan ST, Kent ML. 2007. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiol Lett 270:21–26 [DOI] [PubMed] [Google Scholar]
- 14.Whipps CM, Matthews JL, Kent ML. 2008. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish Danio rerio. Dis Aquat Organ 82:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JM, Bunte RM, Carty AJ. 2009. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 48:785–789 [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki Y, Danelishvili L, Wu M, Hidaka E, Katsuyama T, Stang B, Petrofsky M, Bildfell R, Bermudez LE. 2006. The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells. Cell Microbiol 8:806–814 [DOI] [PubMed] [Google Scholar]