Abstract
The brain's default-mode network has been the focus of intense research. This study characterizes the default-mode network activity in late-life depression and the correlation of the default-mode network activity changes with the white-matter hyperintensities burden. We hypothesized that elderly depressed subjects would have altered default-mode network activity, which would correlate with the increased white-matter hyperintensities burden. Twelve depressed subjects (mean Hamilton Depression Rating Scale 19.8±4.1, mean age 70.5±4.9) and 12 non-depressed, comparison subjects (mean age 69±6.5) were included. Functional MRI data were collected while subjects performed a low cognitive load, event-related task. We compared the default-mode network activity in these groups (including depressed subjects pre and post antidepressant treatment). We analyzed the resting connectivity patterns of the posterior cingulate cortex. Deconvolution was used to evaluate the correlation of resting-state connectivity scores with the white-matter hyperintensities burden. Compared with non-depressed elderly, depressed subjects pretreatment had decreased connectivity in the subgenual anterior cingulate cortex and increased connectivity in the dorsomedial prefrontal cortex and the orbitofrontal cortex. The abnormal connectivity was significantly correlated with the white-matter hyperintensities burden. Remitted elderly depressed subjects had improved functional connectivity compared to pretreatment, although alterations persisted in the anterior cingulate and the prefrontal cortex when remitted elderly depressed subjects were compared with non-depressed elderly. Our study provides evidence for altered default-mode network connectivity in late-life depression. The correlation between white-matter hyperintensities burden and default-mode network connectivity emphasizes the role of vascular changes in late-life depression etiopathogenesis.
Keywords: Late-life depression, resting state, connectivity, white matter burden
1. INTRODUCTION
Late-life major depression is associated with emotional suffering, disability, suicide, and poor compliance with medical treatments (Charney et al., 2003). The current understanding of late-life depression's neurobiology is based on findings from structural and functional neuroimaging studies. Structural changes include reduced gray matter volumes and increased white matter hyperintensities burden, which are the anatomical correlates of neurodegeneration and cerebrovascular disease, respectively (Greenwald et al., 1997). Several studies have reported significant bilateral volume reductions of the anterior cingulate, frontal cortex, hippocampus and striatum (Ballmaier et al., 2004, Steffens et al., 2000, Kumar et al., 2000, Greenwald et al., 1997, Andreescu et al., 2008). Increased white-matter hyperintensities burden has been reported in several studies (Greenwald et al., 1997, Butters et al., 2004, Alexopoulos et al., 2002). Some (Alexopoulos et al., 2002), but not all (Salloway et al., 2002) studies found that increased white-matter hyperintensities burden and decreased white matter integrity are associated with poor antidepressant treatment response. The increased white-matter hyperintensities burden is central to the vascular depression hypothesis, which posits that a single vascular lesion or an accumulation of lesions may disrupt prefrontal systems that mediate both mood and executive function (Alexopoulos et al., 1997, Krishnan et al., 1997).
PET studies have described changes of the resting-state cerebral activity in elderly depressed subjects when compared with non-depressed control subjects (Kumar et al., 1993), although these findings have not been replicated (Smith et al., 2009). Recently, fMRI has been adapted to examine the connectivity of the default-mode network, an organized functional network of several brain regions active during resting state and inhibited during the performance of active tasks (Raichle et al., 2001). Analysis of resting state activity may enhance the understanding of the biological underpinning of mental illnesses pathophysiology. Thus, activity in the default-mode network is affected in Alzheimer's disease (Greicius et al., 2004), Major Depressive Disorder (Greicius et al., 2007, Sheline et al., 2009) and anxiety disorders (Zhao et al., 2007). In midlife depression, resting state functional connectivity was significantly increased in depressed subjects compared with healthy control subjects, especially in the sub-network comprising the subgenual cingulate and the thalamus (Greicius et al., 2007). In contrast to the increased default-mode activity observed in midlife depression, decreased functional connectivity in the default network has been described in Alzheimer's disease (Greicius et al., 2004).
To our knowledge, there are no published studies exploring the correlation between default-mode network connectivity and white matter hyperintensities in late-life depression. Compared with mid-life major depression, late-life depression is characterized by a marked heterogeneity in both phenotype (e.g., greater cognitive impairment, greater anxiety) and pathogenesis (e.g., vascular changes in the brain, neurodegeneration, monoamine dysregulation) (Alexopoulos, 2005). FMRI with cognitive tasks showed decreased functional connectivity in late-life depression, thought to reflect vascular and neurodegenerative changes (Aizenstein et al., 2009). Given this multilevel heterogeneity, characterizing the default-mode network activity in late-life depression could help in delineating the functional neuroanatomy of the disorder, especially with regard to the role of indicators of vascular depression phenotype (e.g. WMH). Thus, we in this study we used whole-brain WMH burden as a global biomarker of overall white matter disease.
We hypothesized that subjects with late-life depression would have altered connectivity in the default-mode network when compared with elderly non-depressed subjects. Moreover, given the prominent role of white-matter hyperintensities in the late-life depression vascular hypothesis, we hypothesized that the increased burden of white-matter hyperintensities would be correlated with the altered activity in the default network.
2. METHOD
2.1. Subjects
The data were collected from depressed participants in the second Maintenance Therapies for Late-Life Depression study (MTLD-II), conducted at the University of Pittsburgh Advanced Center for Intervention and Services Research for Late-Life Mood Disorders (ACISR) between 1999 and 2004. Details of the MTLD-II study protocol are described elsewhere (Reynolds et al., 2006). In brief, depressed participants had a mean age of 70.5, and were diagnosed via Structured Clinical Interview for DSM-IV (SCID) (First et al., 1995) with current non-psychotic, non-bipolar major depressive disorder (single-episode or recurrent), a 17-item Hamilton Depression Rating Scale (HDRS)(Hamilton, 1960) of 15 or higher, and a Mini Mental State Examination (MMSE) (Folstein et al., 1975) score of 17 or higher. Cognitive function was assessed with the Dementia Rating Scale (Mattis, 2004).
During the acute phase of treatment in MTLD-II, depressed subjects were treated openly with paroxetine doses adjusted between 10 and 40 mg/day [mean (SD) final dose: 26 (11) mg/day], combined with weekly interpersonal psychotherapy. Subjects who responded to acute treatment entered a 16-week continuation phase to stabilize their response (Reynolds et al., 2006). Response was defined categorically as a HDRS score of 10 or less (Reynolds et al., 2006). Non-depressed elderly participants were recruited from the community and from the healthy controls registry of the ACISR.
Additional exclusion criteria: clinical diagnosis of Dementia, lifetime history of psychosis or bipolar disorder, history of substance abuse that was not in full remission for at least 3 months prior to enrollment. Patients who did not respond to the acute treatment phase were excluded from the continuation phase of the study.
Twenty-four participants, who consented to the neuroimaging protocol, had intact imaging data and a MMSE of 24 or above were included in this analysis: 12 elderly depressed subjects and 12 elderly comparison subjects. 4/12 comparison subjects and 5/12 depressed subjects received antihypertensive medication. The comparison subjects did not receive psychotropic medications and the elderly depressed subjects were psychotropic-free at baseline.
MRI acquisition
The baseline MR images were obtained at the time of subject enrollment, before initiation of pharmacotherapy. Follow-up scans were obtained after 12 weeks of pharmacotherapy, for 8 depressed subjects who responded to treatment. Post acute-treatment scans were obtained while subjects continued on a maintenance dose of paroxetine.
There are several methods described in the literature for acquiring fMRI data for resting state. Some studies scan subject at rest, while others use the fMRI data from the fixation periods of block-design tasks (Fair et al., 2007), or from simple sensory-motor tasks (e.g. finger tapping). The finger tapping task requires a finger tap every 12 sec. The brain functional activities associated with this simple task do not interfere with the default-mode network activity and has been used in the literature to acquire resting-state data (Greicius et al., 2003, Greicius et al., 2004). In this study, we used finger tapping to acquire fMRI resting-state data.
Imaging data were collected with a 1.5-T Signa scanner (GE Medical Systems). 3D high-resolution anatomical images (SPGRs) were acquired sagittaly using 3D Spoiled Grass (SPGR, TR/TE = 5/25 ms; flip angle = 40°; FOV = 24×24 cm, slice thickness = 1.5 mm, matrix = 256×256 voxels). Fast fluid-attenuated inversion recovery (FLAIR) images (TR/TE = 9002/56 ms Ef; TI = 2200 ms, FOV = 24×24cm, NEX = 1, slice thickness = 5 mm, gap = 1 mm) were also acquired for white-matter hyperintensities volume quantification.
T1-weighted anatomical images were acquired parallel to the plane connecting the anterior and posterior commissures (TR/TE = 500/11 ms, FOV = 24×24cm, slice thickness = 3.8mm, matrix = 256×256). Thirty-six oblique axial slices were acquired with an in-plane resolution of 0.9375 mm × 0.9375 mm. Slice thickness and orientation were chosen to be similar to fMRI data for analysis purposes.
The fMRI data were acquired using a one-shot spiral pulse sequence (TR/TE = 2000/35 ms, FOV = 24×24 cm, slice thickness = 3.8 mm, matrix = 64×64). Twenty-six oblique axial slices were acquired with an in-plane resolution of 3.75 mm × 3.75 mm.
Subjects were instructed to perform a single key-press with both index fingers every time they saw the word “tap” appear on a screen. The stimulus appeared every 12 seconds and remained on the screen for 1 second. In the interim, subjects were instructed to fixate on a white cross-hair in the middle of the screen. There were 24 trials in a 5-minute block. FMRI resting-state data were acquired on all 24 subjects; 8 non-depressed subjects and 11 depressed subjects had FLAIR images available for white-matter hyperintensities assessment.
This study was approved by the University of Pittsburgh Institutional Review Board and we obtained written informed consent from all study participants.
2.2. Data Analysis
2.2.1. White-matter hyperintensities burden
An automated white-matter hyperintensities segmentation and localization method was used to compute the normalized white-matter hyperintensities volumes (Wu et al., 2006b). For each subject, the calculated white-matter hyperintensities volume was normalized for the overall brain volume. A natural logarithm transformation was performed on the normalized white-matter hyperintensities to minimize the skewness.
2.2.2. Resting-state fMRI analyses
Preprocessing
After motion correction, the functional images from each subject were normalized into the standard Montreal Neurological Institute (MNI) template space (Colin27) (Holmes et al., 1998) via the following steps: (i) the functional data were realigned to the first volume using a least square approach and a 6-parameter rigid body transformation to correct for motion correction, (ii) the first volume in the functional data was registered to the subject's high-resolution SPGR image using an affine transformation model, (iii) the subject's SPGR image was then warped to the standard template MNI Colin27 using a deformable model (Wu et al., 2006a), and (iv) the realigned functional images were upsampled and transformed into subject's SPGR space with the affine matrix from step (ii), which were further transformed into the standard space with the computed deformation field from step(iii), (v) the warped functional data were then down-sampled to the original resolution of 3.75 mm × 3.75 mm × 3.75 mm; a Gaussian smoothing filter (6 mm full width at half maximum) was then used on the normalized functional images to reduce spatial noise. The fully deformable registration has previously been shown to increase the effect-size with functional imaging (Wu et al., 2006a), and has shown significant improvement in image registration accuracy in aging brain (Cox et al., In Press).
For each subject, the smoothed, normalized functional images were concatenated to form a 3D+time AFNI dataset. A band-pass temporal filter with the cutoff frequencies of [0.01 to 0.1] Hz was then used to extract the resting-state BOLD signal, which effectively removed the linear trend and high-frequency noise in the data (Lowe et al., 1998).
Connectivity Analysis
Functional connectivity is defined as the temporal correlation of activity between spatially disconnected areas (Greicius et al., 2004). We compared the default-mode network activity among elderly non-depressed subjects and elderly depressed subjects before and after successful antidepressant treatment.
Region of Interest (ROI)
Posterior cingulate cortex (PCC) has been shown to have consistently greater activity during resting state than during cognitive tasks (Mazoyer et al., 2001), and is hypothesized to constitute a core node in the default-mode network (Raichle et al., 2001). Thus, the posterior cingulate has often been used as a seed region to identify the default-mode network. The left and right posterior cingulate from the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002) (1×1×1 mm) in Colin27 space was down sampled to a voxel resolution of 3.75 mm × 3.75 mm × 3.75 mm (left and right PCC combined, 200 voxels). A smaller region-of-interest (ROI) of 39 voxels, centered on the posterior cingulate, was created on template Colin27 by performing erosions (2 iterations, 6 connected, 2.5-dimensional) with a 3×3×3 voxel structuring element (the figures presenting the original and eroded posterior cingulate ROI are posted online as supplemental data).
For each subject, a reference resting-state time-series was extracted by averaging the time-series for all voxels within the posterior cingulate ROI. A correlation coefficient map was calculated on the 3D+time resting state data, with the reference time-series as the regressor of interest using 3dDeconvolve from Analysis of Functional NeuroImages (AFNI) (Cox, 1996). In the map, the correlation coefficient at a given voxel shows the time-series correlation between that voxel and posterior cingulate ROI, which represents the resting state functional connectivity score at the voxel.
To determine the mean resting-state functional connectivity map for each group (depressed and comparison subjects), the correlation coefficient maps were statistically compared to the baseline (=fixation period) using a 1-sample t-test. The resulting t-maps were then thresholded at a corrected p < 0.001 via Monte Carlo simulations [AlphaSim, AFNI (Cox, 1996)], with the whole brain template as mask.
A 2-sample t-test of the correlation coefficient maps was used to identify between-group differences in resting-state functional connectivity (the comparison group versus the depressed group before treatment, the depressed group after treatement versus before treatment, and the the comparison group versus the depressed group after treatment). The resulting t-maps were then thresholded at a corrected p < 0.05 via Monte Carlo simulations [AlphaSim, AFNI (Cox, 1996)] using a small volume correction, correcting for the frontal ROI defined as the area anterior to the anterior commissure.
2.2.3. The correlation of white-matter hyperintensities burden and resting-state connectivity
The voxel-wise correlation between white-matter hyperintensities and the resting-state functional connectivity score was evaluated using 3dDeconvolve (AFNI) (Cox, 1996). The functional connectivity correlation coefficient maps from each group were used as the input data and the corresponding log-transformed white-matter hyperintensities burden from the same group was used as the regressor of interest in 3dDeconvolve. The F-maps were thresholded at a corrected p < 0.05 using Monte Carlo simulations (AlphaSim, AFNI) (Cox, 1996) with the resting-state connectivity map from the related group as ROI mask.
3. RESULTS
The clinical characteristics of the subjects are summarized in Table 1. The difference in Dementia Rating Scale scores between depressed and comparison groups is marginally significant at p < 0.05 but becomes non-significant at p < 0.29 after adjusting for education level. The cardiovascular and endocrine burden, as measured by the Cumulative Illness Rating Scale for Geriatrics, did not differ between the two groups.
Table 1.
Clinical Characteristics of the subjects.
| Group 1 (Non-Depressed) (N=12) | Group 2 (Depressed before treatment) (N=12) | Group 3 (Depression Remitted) (N=8) | Group Difference t-test (t p) |
||
|---|---|---|---|---|---|
| 1 vs 2 df = 22 |
2 vs 3 df = 6 |
||||
| Subjects (N) | 12 | 12 | ▲8 | ||
| Gender, Female | 4/12 | 7/12 | 3/8 | ||
| Race, Caucasian | ●10/12 | 12/12 | 8/8 | ||
| Handedness, Right | 12/12 | 10/12 | 6/8 | ||
| Age, years (mean, SD) | 69.0±6.5 | 70.5±4.9 | 70.8±5.7 | (0.64, 0.53) | |
| Age of onset, years (mean, SD) | N/A | 67.9±5.7 | 68.7±6.9 | ||
| Number (%) of subjects with recurrent depression (number of episodes of depression >2) | N/A | 7/12 (58%) | 4/8 (50%) | ||
| Education, years (mean, SD) | 16.3±2.7 | 13.6±3.0 | 13.4±3.0 | (−2.26, 0.034) | |
| MMSE (mean, SD) | 28.9±1.0 | 28.0±3.5 | 27.8±2.6 | (−0.88, 0.39) | ◆(0.26, 0.85) |
| DRS (mean, SD) | 140.7±2.8 | 137.0±5.4 | 132.1±9.8 | *(−1.09, 0.29) | △(2.12, 0.087) |
| HDRS-17 (mean, SD) | 1.5±1.6 | 19.8±4.1 | 6.8±4.5 | (13.69, <.0001) | (5.91, <. 001) |
| CIRS-G (mean, SD) | 5.5±4.6 | 6.3±2.6 | (0.48, 0.64) | ||
| CIRS-G cardiovascular burden | 1.6±1.6 | 1.1±1.06 | 0.47 | ||
| CIRS-G endocrine burden | 0.45±0.8 | 0.46±0.87 | 0.9 | ||
Statistical analyses were conducted in SPSS statistical package version 11 for Macintosh.
MMSE: Mini-Mental State Examination; DRS: Dementia Rating Scale; HDRS-17: Hamilton Depression Rating Scale (17 item); CIRS-G: Cumulative Illness Rating Scale for Geriatrics.
1 out of 8 depressed patients (after treatment) did not have the pretreatment data, so 7 patients were used in the two-tail paired t-test for comparison of patients before and after treatment (2 vs 3).
2 out 12 were African Americans.
Controlled for education level, df = 21.
2 out of 7 patients after treatment did not have MMSE measurements, df =4.
1 out of 7 patients did not have DRS measurement, df =5.
3.1. White-matter hyperintensities burden
The automated white-matter hyperintensities segmentation method identified white-matter hyperintensities from the T2-weighted FLAIR images. The white-matter hyperintensities segmentation result for a comparison subject is shown in Figure 1. Normalized white-matter hyperintensities (nWMH) for both depressed and comparison groups (comparison group: nWMH mean = 0.53%, SD = 0.28%; depressed group: nWMH mean = 1.50%, SD = 1.76%) showed that the white-matter hyperintensities burden of the depressed group was significantly higher than for the comparison group (t =1.80, p < 0.05) with a one-tailed unequal variance t-test.
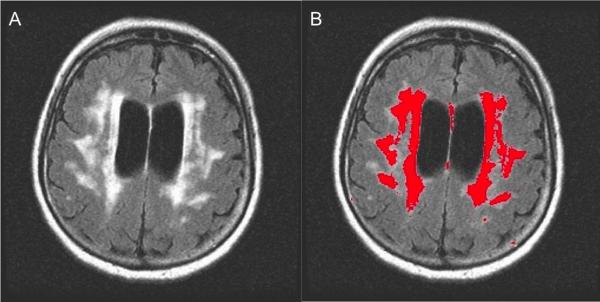
Figure 1.
Automated white-matter hyperintensities segmentation on the flair image of one subject is shown here, (A) A single slice from a subject's FLAIR image (B) white-matter hyperintensities segmentation result with underlying flair image for anatomical reference. The automated white-matter hyperintensities segmentation correlates with semi-quantitative, visual rating methods, such as those used for the Cardiovascular Health Study. In the current study, 37% of the depressed subjects had a moderate-severe burden of white-matter hyperintensities, as quantified by the Cardiovascular Health Study.
3.2. Resting-State Functional Connectivity Maps
Figure 2 presents the default-mode network functional connectivity map from the elderly non-depressed group (Figure 2A), the depressed subject group before treatment (Figure 2B) and after 12 weeks of pharmacotherapy (Figure 2C). The correlation coefficient maps from each group were statistically compared to the baseline 0 using a 1-sample t-test, and the resulting t-map for the default-mode network was then thresholded at a corrected p < 0.001 (a joint threshold of p < 0.01 and 26-voxel cluster size) via Monte Carlo simulations [AlphaSim, AFNI (Cox, 1996)].
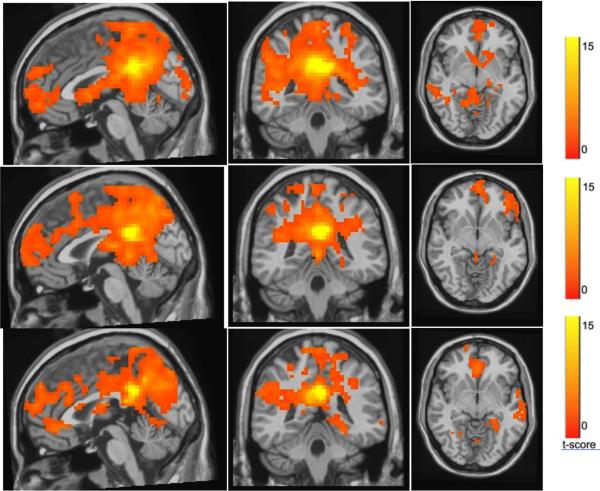
Figure 2.
t-maps of the resting-state connectivity for (A) elderly comparison group, late-life depression group before treatment (B) and after treatment (C). The maps were thresholded at a corrected p < 0.001. Compared with the elderly comparison group (A), the pretreatment late-life depression group (B) had lower default-mode activation in the subgenual anterior cingulate cortex and higher default-mode network activation in the dorso-medial prefrontal cortex and the orbitofrontal cortex.
Figure 4A presents the difference in the functional connectivity activities between the comparison group and the pre-treatment depressed group. Figure 4B presents the difference in the functional connectivity pattern between patients after treatment and before treatment. Figure 4C presents the difference in the functional connectivity pattern between the comparison group and the post-treatment depressed group.
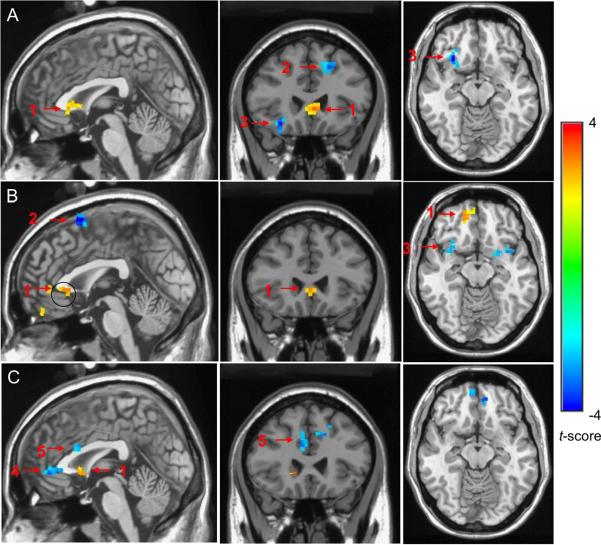
Figure 4.
The group comparisons of the functional connectivity activities with two-sample t-test (a corrected p < 0.05) between (A) the comparison group and late-life depression group before treatment; (B) late-life depression group after treatment and late-life depression group before treatment; (C) the comparison group and the late-life depression group after treatment. In Blue: increased connectivity. In orange/yellow: decreased connectivity. A: When compared with the elderly comparison group, the late-life depression group before treatment had decreased connectivity in the sACC (1) and increased connectivity in the dmPFC (2) and OFC (3). B: Compared to pre-treatment late-life depression group, the post-treatment late-life depression group has improved connectivity in vmPFC/ACC and decreased connectivity in dmPFC. C: Compared with the non-depressed group, the post-treatment late-life depression group has decreased connectivity in sACC (1) and increased connectivity in rostral ACC (4) and dorsal ACC (5).
With above analyses, we found that:
-
(1)
Compared with non-depressed elderly subjects, elderly depressed subjects pretreatment had significantly lower functional connectivity in the BA25 subgenual anterior cingulate cortex (sACC; Talairach coordinates x=81, y=60, z=64; corrected p < 0.05). Compared with non-depressed elderly subjects, elderly depressed subjects pretreatment had significantly higher functional connectivity in the BA 6 dorso-medial prefrontal cortex (dmPFC; Talairach coordinates x=93, y=62, z=106; corrected p < 0.05) and the orbito-frontal cortex (OFC; Talairach coordinates x=52, y=60, z=57; corrected p < 0.05). For all these regions we used a joint threshold of p < 0.05 and 21-voxel cluster size) (see Figure 4A).
-
(2)
Compared with themselves before treatment, depressed subjects who responded to 12 weeks of pharmacotherapy exhibited significant increase in the resting-state connectivity in both subgenual ACC (corrected p < 0.05; joint threshold of p < 0.05 and 24-voxel cluster size) and dorso-medial prefrontal cortex (corrected p < 0.05) (see Figure 4B).
-
(3)
Compared with non-depressed elderly subjects, post-treatment elderly depressed subjects had significantly lower activation in the sACC (corrected p < 0.05; joint threshold of p< 0.05 and 21-voxel cluster size) and a higher activation in the rostral ACC (Talairach coordinates x=79, y=46, z=72; corrected p < 0.05) and dorsal ACC (Talairach coordinates x=79, y=69, z=89; corrected p < 0.05) (see Figure 4C).
3.3. The correlation of white-matter hyperintensities burden and resting-state connectivity
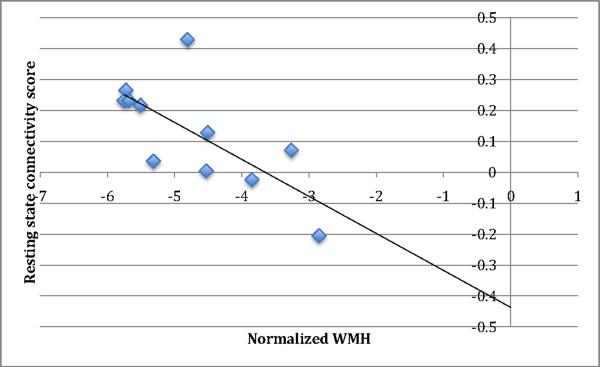
In elderly depressed subjects pretreatment, there was a significant negative correlation between log-transformed, normalized white-matter hyperintensities volume and resting state connectivity (N= 11, averaged r = −0.72, corrected p < 0.05; joint threshold of p < 0.05 and 58-voxel cluster size) in the medial frontal region, i.e., higher white-matter hyperintensities volume was associated with lower resting state connectivity (see Fig 5 and Fig 6).
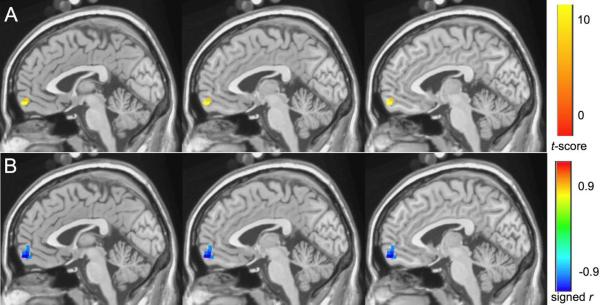
Figure 5.
The white-matter hyperintensities burden and resting state score for the pretreatment late-life depression group shows a significant negative correlation (averaged r =−0.72, corrected p < 0.05) in the medial frontal region: (A) t map, (B) signed r map.
Fig 6.
Correlation plot of resting state connectivity scores and normalized white matter hyperintensities burden (nWMH) in the medial frontal region (r=−0.72) (see also Fig 5).
There was a significant negative correlation between log-transformed, normalized white-matter hyperintensities volume and resting state connectivity in elderly non-depressed comparison subjects in the medial prefrontal region (N = 8; averaged r = −0.80; corrected p < 0.05; joint threshold of p < 0.05 and 58-voxel cluster size). With the assumption that the white-matter hyperintensities do not progress appreciably after 12 weeks of treatment (Maillard et al., 2009), their volume was measured only pretreatment in the depressed group, so we cannot make inferences regarding the correlation of white-matter hyperintensities and resting state scores of post-treatment depressed subjects.
4. DISCUSSION
To our knowledge, this is the first study describing the functional connectivity of the default-mode network in late-life depression. Our results show abnormal connectivity patterns in the prefrontal branch of the default-mode network in acutely depressed elderly subjects. The abnormal functional connectivity is significantly correlated with greater white-matter hyperintensities volume. Moreover, we found some improvement of functional connectivity in the default-mode network following treatment response. We also confirm previous results regarding increased burden of white-matter hyperintensities in elderly depressed subjects when compared with non-depressed individuals.
Previous reports regarding the default-mode network functional connectivity in midlife depression have reported increased subgenual ACC connectivity (Greicius et al., 2007). Our study shows a different functional connectivity pattern of the default-mode network in elderly depressed subjects. The sACC-PCC connectivity was decreased in acutely depressed elderly, and – although it improved after treatment response -- post-treatment subjects continued to present decreased sACC-PCC connectivity when compared with non-depressed control subjects. However, different data collection and data analysis were used in the midlife depression resting-state studies (Greicius et al., 2007); these differences impede the comparison of the resultant patterns of activation in midlife and late-life depression.
Our results emphasize the importance of sACC as an impaired hub in the functional neuroanatomy of major depression (Mayberg et al., 2000, Drevets et al., 1997). Our results also suggest the importance of the cerebrovascular component of late-life depression, represented by the increased white-matter hyperintensities burden.
The correlation between white-matter hyperintensities volume and dysfunctional connectivity in the default-mode network suggests a role of the “vascular depression” hypothesis of late-life depression (Alexopoulos et al., 1997), although the multifactorial etiology of the white-matter hyperintensities (including demelination due to neurodegeneration) does not allow for a definitive conclusion. The post-treatment persistence of sACC-PCC hypo-connectivity suggests a possible persistent feature of late-life depression, associated perhaps with the structural brain changes.
Beyond the novel subject population, our study has several other strengths: (1) a comparison group, which allowed us to compare elderly depressed pre- and post-treatment to non-depressed elders; (2) an automated white-matter hyperintensities segmentation method, which provided a quantitative assessment of white-matter hyperintensities volume; (3) a fully deformable model for inter-subject image normalization, which has previously been shown to significantly improve co-localization of the fMRI signal in aging brains; and (4) acquisition of data pre- and post-treatment, allowing us to make inferences regarding the persistent biological features of late-life depression. Our report also has some limitations: we had a relatively small sample, especially in the post-treatment group and this limited our ability to analyze pre- and post-treatment differences. Our ability to detect white-matter hyperintensities burden differences was hampered by the lack of FLAIR imagines for some of our subjects. Thus, we were not able to further test the behavior of DMN in patients/controls with or without WML in order to verify if the observed connectivity patterns are solely driven by white-matter disease. Also, given our approach to use WMH burden as a global biomarker or overall white matter disease, we only computed the whole brain white-matter hyperintensities burden. Thus, we cannot make more specific inferences regarding the role of region-specific white-matter hyperintensities burden and the decreased functional connectivity in the default-mode network. Future studies focused on DTI-based fractional anisotropy would allow a more precise charting of the disruptions in the DMN Also, with regard to white-matter hyperintensities burden, the significant group difference between elderly depressed and controls was lost when the one-tailed t-test was changed to a two-tailed test. Although none of the participants had evidence of stroke, we did not quantify the presence of lacunae. Overall, depressed subjects had less education than the comparison group; however this had no effect on the pre-post treatment findings in depressed subjects. As there was no significant difference between depressed subjects and comparison subject with regard to both MMSE and Dementia Rating Scale, it is unclear if and how the lower education levels of the depressed subjects interfered with the fMRI findings. Given the design of the parent study, we have no imaging data on the depressed non-responders, as they were excluded from the study. Thus, we cannot make further inferences regarding the correlation between default-mode network changes and treatment response. The between-group comparisons were threshold at a corrected p<0.05; the lower significance threshold allows for a greater risk for type I error. However, we used Monte Carlo simulations (AFNI) to correct for multiple comparisons.
In conclusion, we present novel data highlighting some of the biological basis of late-life depression. Given the high rate of white-matter hyperintensities and concordant cerebrovascular disease, further research might tackle the treatment advantages of using adjuvant treatments such as calcium channel blockers (Taragano et al., 2005), NSAIDs, antihypertensives or lipid lowering drugs. Future research is also necessary to understand the correlation between default-mode network changes and various clinical characteristics of late-life depression, such as severity, duration of current episode, recurrence, comorbidity with anxiety or medical conditions, and treatment response.
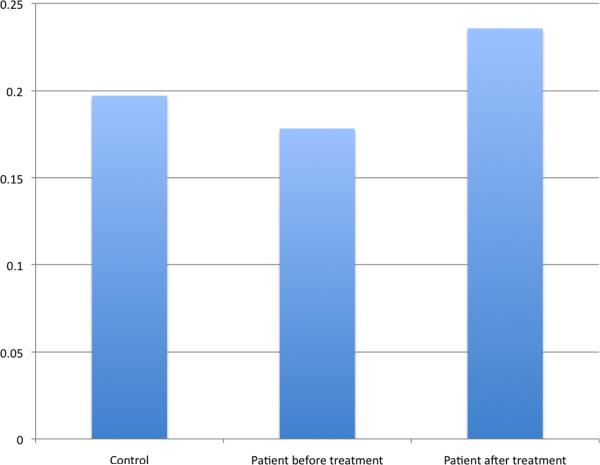
Fig 3.
Resting State connectivity scores (Posterior Cingulate Cortex — Anterior Cingulate Cortex) comparison between nondepressed elderly, late-life depression group before treatment and late-life depression group after treatment.
Acknowledgments
Supported by NIH K23 086686, R01 MH076079, R01MH072947; P30 MH 071944, R37/R01 MH43832, the National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (Dr. Andreescu), the John A. Hartford Foundation Center of Excellence in Geriatric Psychiatry and the University of Pittsburgh Medical Center (UPMC) endowment in Geriatric Psychiatry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest Minjie Wu, Carmen Andreescu, Meryl A. Butters, and Robert Tamburo do not have any potential conflict to acknowledge. Howard Aizenstein has received research support from Novartis Pharmaceuticals. Charles F. Reynolds III has received research support from Pfizer Inc., Eli Lilly and Co., Bristol Meyers Squibb, Forest Pharmaceuticals, and Wyeth Pharmaceuticals.
REFERENCES
- AIZENSTEIN HJ, BUTTERS MA, WU M, MAZURKEWICZ LM, STENGER VA, GIANAROS PJ, BECKER JT, REYNOLDS CF, III, CARTER CS. Altered functioning of the executive control circuit in late-life depression: Episodic and persistent phenomena. American Journal of Geriatric Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXOPOULOS GS. Depression in the elderly. Lancet. 2005;365:1961–70. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, KIOSSES DN, CHOI SJ, MURPHY CF, LIM KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–32. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- ALEXOPOULOS GS, MEYERS BS, YOUNG RC, CAMPBELL S, SILBERSWEIG D, CHARLSON M. 'Vascular depression' hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- ANDREESCU C, BUTTERS MA, BEGLEY A, RAJJI T, WU M, MELTZER CC, REYNOLDS CF, 3RD, AIZENSTEIN H. Gray matter changes in late life depression--a structural MRI analysis. Neuropsychopharmacology. 2008;33:2566–72. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLMAIER M, KUMAR A, THOMPSON PM, NARR KL, LAVRETSKY H, ESTANOL L, DELUCA H, TOGA AW. Localizing gray matter deficits in late-onset depression using computational cortical pattern matching methods. Am J Psychiatry. 2004;161:2091–9. doi: 10.1176/appi.ajp.161.11.2091. [DOI] [PubMed] [Google Scholar]
- BUTTERS MA, WHYTE EM, NEBES RD, BEGLEY AE, DEW MA, MULSANT BH, ZMUDA MD, BHALLA R, MELTZER CC, POLLOCK BG, REYNOLDS CF, 3RD, BECKER JT. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–95. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- CHARNEY DS, REYNOLDS CF, 3RD, LEWIS L, LEBOWITZ BD, SUNDERLAND T, ALEXOPOULOS GS, BLAZER DG, KATZ IR, MEYERS BS, AREAN PA, BORSON S, BROWN C, BRUCE ML, CALLAHAN CM, CHARLSON ME, CONWELL Y, CUTHBERT BN, DEVANAND DP, GIBSON MJ, GOTTLIEB GL, KRISHNAN KR, LADEN SK, LYKETSOS CG, MULSANT BH, NIEDEREHE G, OLIN JT, OSLIN DW, PEARSON J, PERSKY T, POLLOCK BG, RAETZMAN S, REYNOLDS M, SALZMAN C, SCHULZ R, SCHWENK TL, SCOLNICK E, UNUTZER J, WEISSMAN MM, YOUNG RC. Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry. 2003;60:664–72. doi: 10.1001/archpsyc.60.7.664. [DOI] [PubMed] [Google Scholar]
- COX K, AIZENSTEIN HJ, FIEZ J. Striatal outcome processing in healthy aging. CABIN. doi: 10.3758/cabn.8.3.304. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- DREVETS WC, PRICE JL, SIMPSON JR, JR., TODD RD, REICH T, VANNIER M, RAICHLE ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- FAIR DA, SCHLAGGAR BL, COHEN AL, MIEZIN FM, DOSENBACH NU, WENGER KK, FOX MD, SNYDER AZ, RAICHLE ME, PETERSEN SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIRST M, SPITZER RL, GIBBON M, WILLIAMS JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P) Version 2.0 New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- FOLSTEIN MF, FOLSTEIN SE, MCHUGH PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- GREENWALD BS, KRAMER-GINSBERG E, BOGERTS B, ASHTARI M, AUPPERLE P, WU H, ALLEN L, ZEMAN D, PATEL M. Qualitative magnetic resonance imaging findings in geriatric depression. Possible link between later-onset depression and Alzheimer's disease? Psychol Med. 1997;27:421–31. doi: 10.1017/s0033291796004576. [DOI] [PubMed] [Google Scholar]
- GREICIUS MD, FLORES BH, MENON V, GLOVER GH, SOLVASON HB, KENNA H, REISS AL, SCHATZBERG AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREICIUS MD, KRASNOW B, REISS AL, MENON V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREICIUS MD, SRIVASTAVA G, REISS AL, MENON V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES CJ, HOGE R, COLLINS L, WOODS R, TOGA AW, EVANS AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- KRISHNAN KR, HAYS JC, BLAZER DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- KUMAR A, BILKER W, LAVRETSKY H, GOTTLIEB G. Volumetric asymmetries in late-onset mood disorders: an attenuation of frontal asymmetry with depression severity. Psychiatry Res. 2000;100:41–7. doi: 10.1016/s0925-4927(00)00067-6. [DOI] [PubMed] [Google Scholar]
- KUMAR A, NEWBERG A, ALAVI A, BERLIN J, SMITH R, REIVICH M. Regional cerebral glucose metabolism in late-life depression and Alzheimer disease: a preliminary positron emission tomography study. Proc Natl Acad Sci U S A. 1993;90:7019–23. doi: 10.1073/pnas.90.15.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE MJ, MOCK BJ, SORENSON JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- MAILLARD P, CRIVELLO F, DUFOUIL C, TZOURIO-MAZOYER N, TZOURIO C, MAZOYER B. Longitudinal follow-up of individual white matter hyperintensities in a large cohort of elderly. Neuroradiology. 2009;51:209–20. doi: 10.1007/s00234-008-0489-0. [DOI] [PubMed] [Google Scholar]
- MATTIS S. Dementia Rating Scale-2: Professional Manual. Psychological Assessment Resources; Odessa, FL: 2004. [Google Scholar]
- MAYBERG HS, BRANNAN SK, TEKELL JL, SILVA JA, MAHURIN RK, MCGINNIS S, JERABEK PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- MAZOYER B, ZAGO L, MELLET E, BRICOGNE S, ETARD O, HOUDE O, CRIVELLO F, JOLIOT M, PETIT L, TZOURIO-MAZOYER N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–98. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- RAICHLE ME, MACLEOD AM, SNYDER AZ, POWERS WJ, GUSNARD DA, SHULMAN GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS CF, 3RD, DEW MA, POLLOCK BG, MULSANT BH, FRANK E, MILLER MD, HOUCK PR, MAZUMDAR S, BUTTERS MA, STACK JA, SCHLERNITZAUER MA, WHYTE EM, GILDENGERS A, KARP J, LENZE E, SZANTO K, BENSASI S, KUPFER DJ. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354:1130–8. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- SALLOWAY S, BOYLE PA, CORREIA S, MALLOY PF, CAHN-WEINER DA, SCHNEIDER L, KRISHNAN KR, NAKRA R. The relationship of MRI subcortical hyperintensities to treatment response in a trial of sertraline in geriatric depressed outpatients. Am J Geriatr Psychiatry. 2002;10:107–11. [PubMed] [Google Scholar]
- SHELINE YI, BARCH DM, PRICE JL, RUNDLE MM, VAISHNAVI SN, SNYDER AZ, MINTUN MA, WANG S, COALSON RS, RAICHLE ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH GS, KRAMER E, MA Y, KINGSLEY P, DHAWAN V, CHALY T, EIDELBERG D. The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry. 2009;24:798–808. doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEFFENS DC, BYRUM CE, MCQUOID DR, GREENBERG DL, PAYNE ME, BLITCHINGTON TF, MACFALL JR, KRISHNAN KR. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–9. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- TARAGANO FE, BAGNATTI P, ALLEGRI RF. A double-blind, randomized clinical trial to assess the augmentation with nimodipine of antidepressant therapy in the treatment of “vascular depression”. Int Psychogeriatr. 2005;17:487–98. doi: 10.1017/s1041610205001493. [DOI] [PubMed] [Google Scholar]
- TZOURIO-MAZOYER N, LANDEAU B, PAPATHANASSIOU D, CRIVELLO F, ETARD O, DELCROIX N, MAZOYER B, JOLIOT M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- WU M, CARMICHAEL O, LOPEZ-GARCIA P, CARTER CS, AIZENSTEIN HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp. 2006a;27:747–54. doi: 10.1002/hbm.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU M, ROSANO C, BUTTERS M, WHYTE E, NABLE M, CROOKS R, MELTZER CC, REYNOLDS CF, 3RD, AIZENSTEIN HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006b;148:133–42. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO XH, WANG PJ, LI CB, HU ZH, XI Q, WU WY, TANG XW. Altered default mode network activity in patient with anxiety disorders: an fMRI study. Eur J Radiol. 2007;63:373–8. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]