Abstract
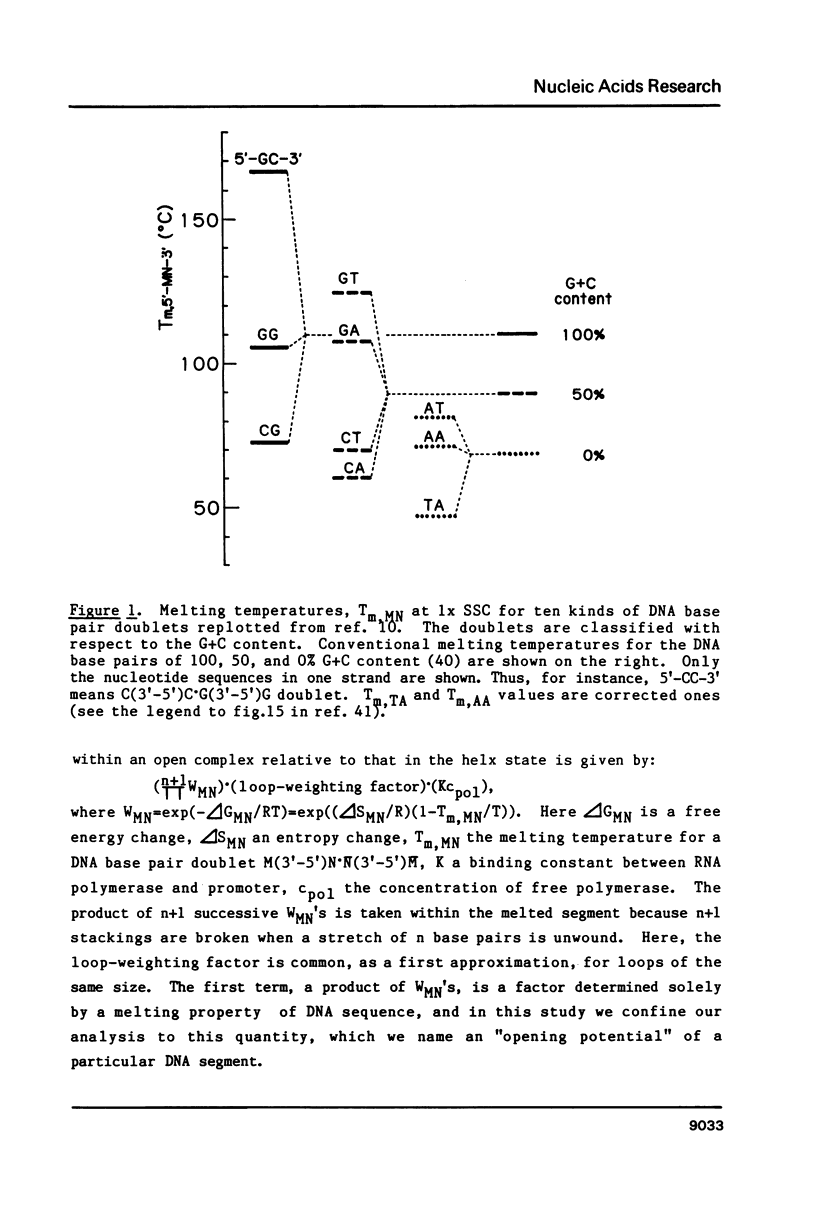
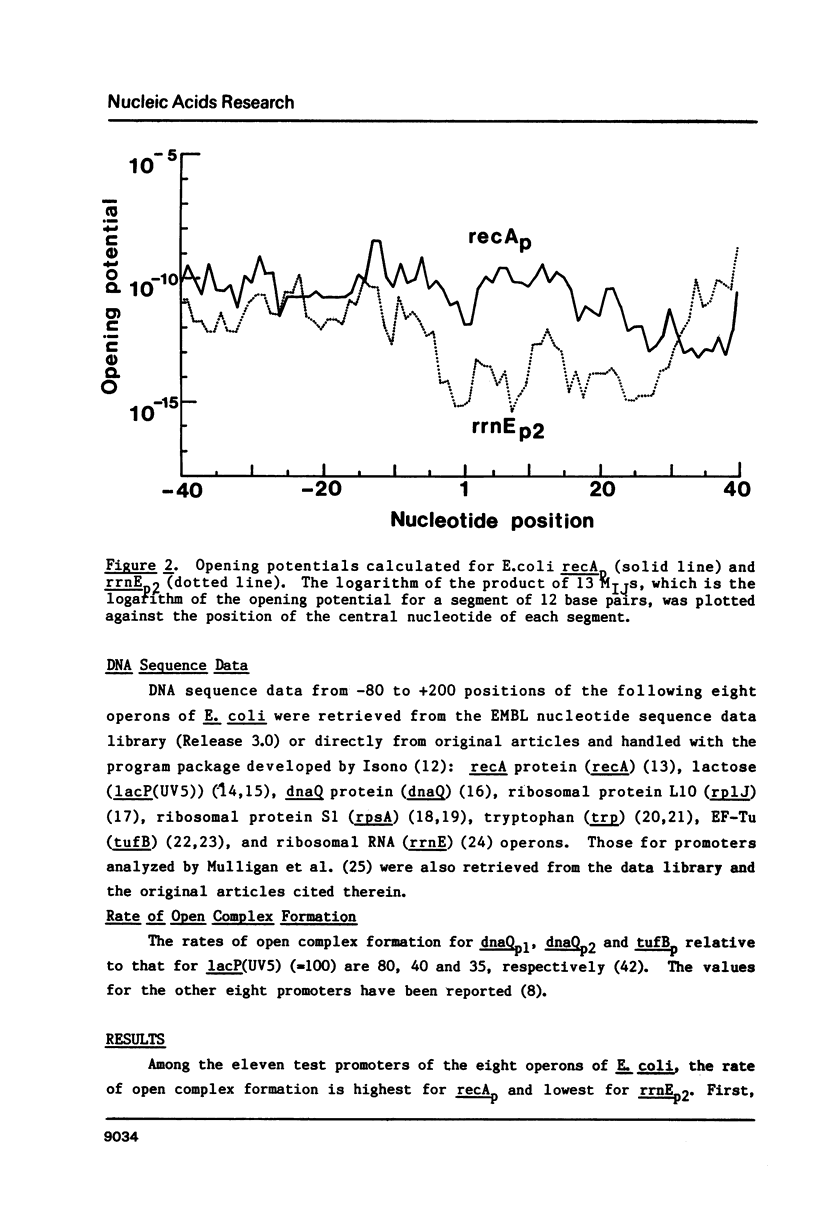
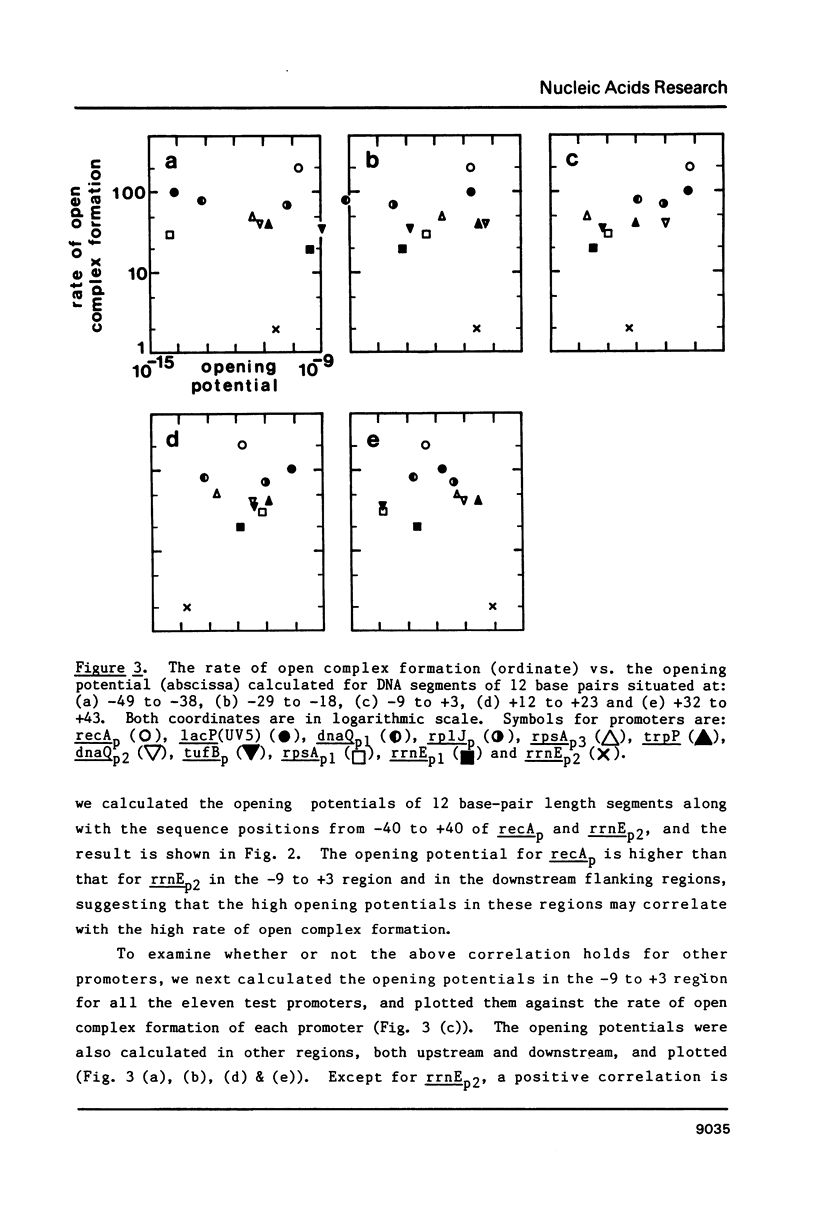
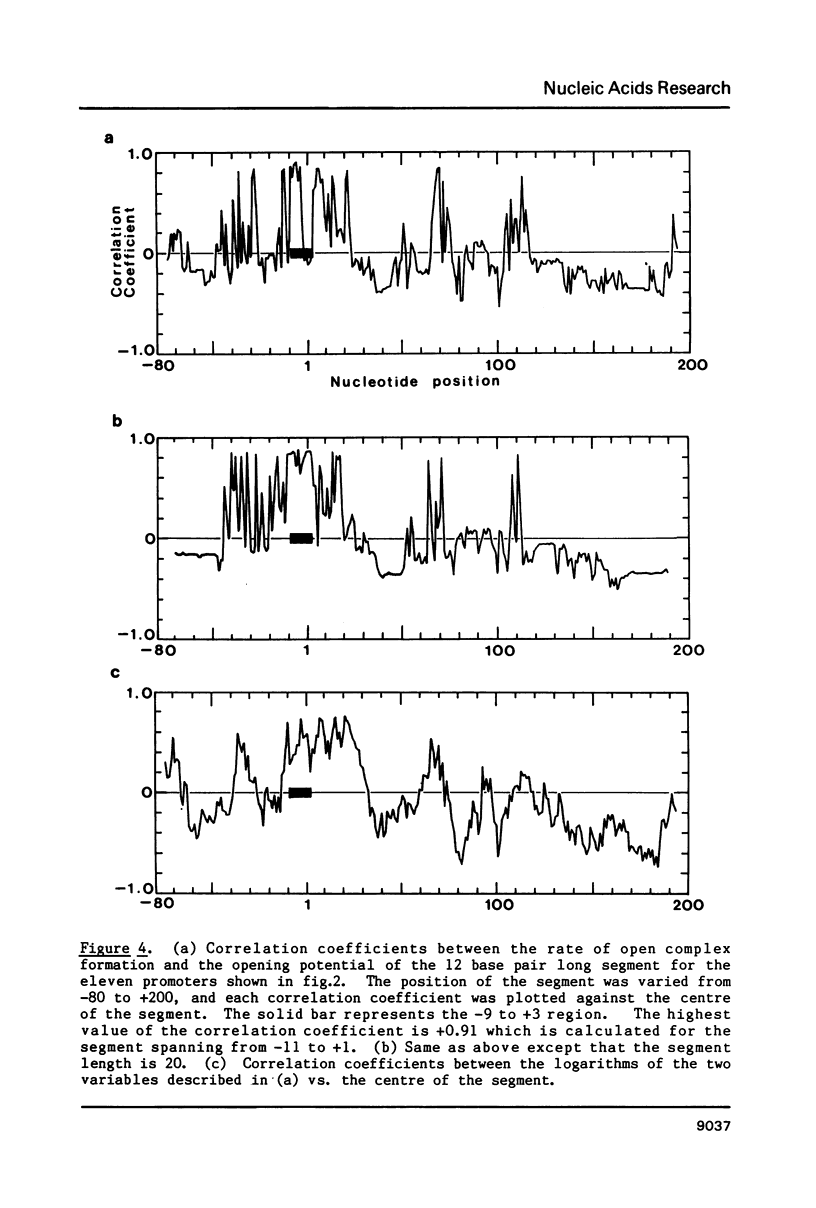
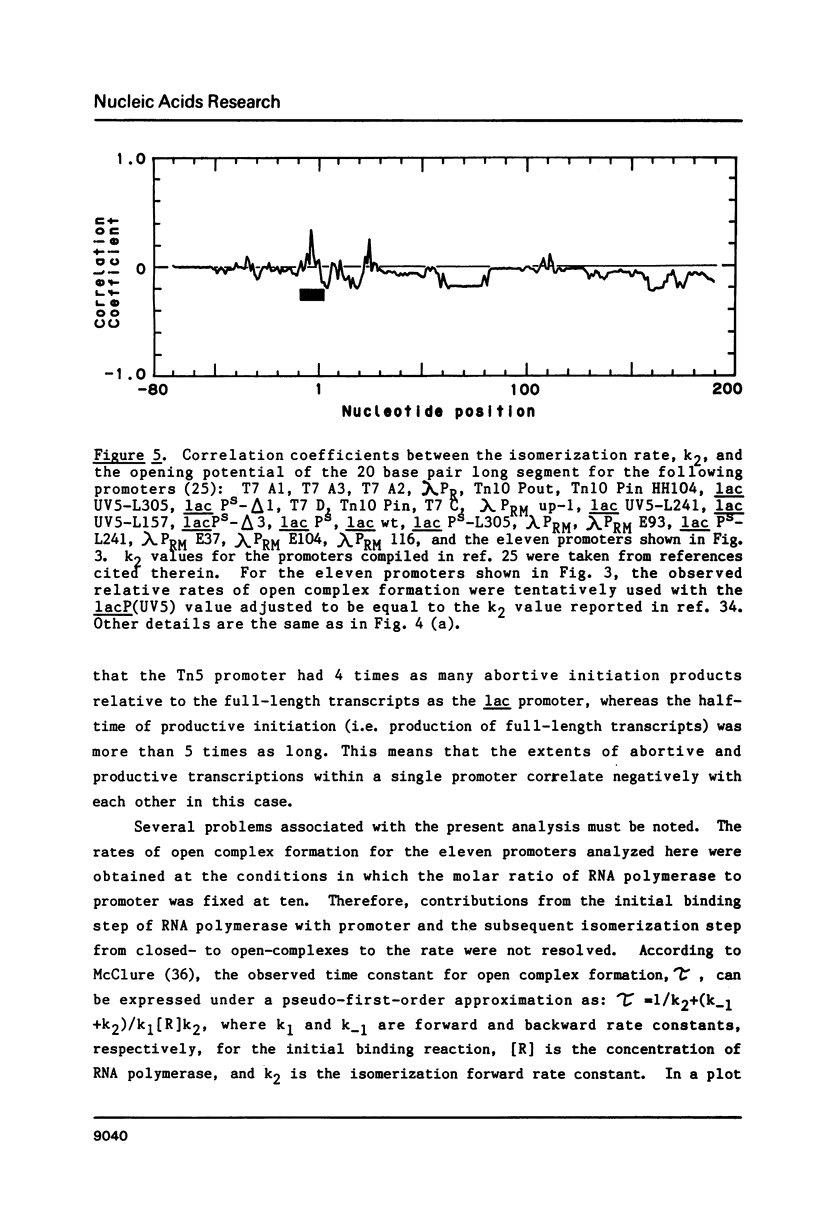
"Opening potential"s of DNA segments of about ten base pairs in length were calculated for eleven promoters of Escherichia coli using the thermodynamic parameter values [Gotoh and Tagashira (1981) Biopolymers 20, 1043-1058] for the stabilities of ten kinds of nearest neighbor base pair doublets in a melting reaction. They were compared with the strength of each promoter determined experimentally with a "mixed transcription" system [Kajitani and Ishihama (1983) Nucleic Acids Res. 11, 3873-3888]. A positive correlation was found between the calculated opening potential in the -9 to +3 region (+1 denotes the nucleotide position at which the transcription starts) and the rate of open complex formation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Bennett G. N., Schweingruber M. E., Brown K. D., Squires C., Yanofsky C. Nucleotide sequence of the promoter--operator region of the tryptophan operon of Escherichia coli. J Mol Biol. 1978 May 15;121(2):113–137. doi: 10.1016/s0022-2836(78)80001-1. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Gamper H. B., Hearst J. E. A topological model for transcription based on unwinding angle analysis of E. coli RNA polymerase binary, initiation and ternary complexes. Cell. 1982 May;29(1):81–90. doi: 10.1016/0092-8674(82)90092-7. [DOI] [PubMed] [Google Scholar]
- Gotoh O. Prediction of melting profiles and local helix stability for sequenced DNA. Adv Biophys. 1983;16:1–52. doi: 10.1016/0065-227x(83)90007-2. [DOI] [PubMed] [Google Scholar]
- Gotoh O., Tagashira Y. Locations of frequently opening regions on natural DNAs and their relation to functional loci. Biopolymers. 1981 May;20(5):1043–1058. doi: 10.1002/bip.1981.360200514. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. In vitro comparison of initiation properties of bacteriophage lambda wild-type PR and x3 mutant promoters. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6381–6385. doi: 10.1073/pnas.77.11.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Mechanism of activation of transcription initiation from the lambda PRM promoter. J Mol Biol. 1982 May 25;157(3):493–525. doi: 10.1016/0022-2836(82)90473-9. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Wang J. C. Physiochemical studies on interactions between DNA and RNA polymerase. Ultraviolet absorption measurements. Nucleic Acids Res. 1978 Sep;5(9):3337–3345. doi: 10.1093/nar/5.9.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono K. A computer program package for storing and retrieving DNA/RNA and protein sequence data. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):101–112. doi: 10.1093/nar/12.1part1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Determination of the promoter strength in the mixed transcription system. II. Promoters of ribosomal RNA, ribosomal protein S1 and recA protein operons from Escherichia coli. Nucleic Acids Res. 1983 Jun 25;11(12):3873–3888. doi: 10.1093/nar/11.12.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Determination of the promoter strength in the mixed transcription system: promoters of lactose, tryptophan and ribosomal protein L10 operons from Escherichia coli. Nucleic Acids Res. 1983 Feb 11;11(3):671–686. doi: 10.1093/nar/11.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Maki H., Horiuchi T., Sekiguchi M. Structure and expression of the dnaQ mutator and the RNase H genes of Escherichia coli: overlap of the promoter regions. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7137–7141. doi: 10.1073/pnas.80.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan T. P., Kolb A., Buc H., McClure W. R. Mechanism of CRP-cAMP activation of lac operon transcription initiation activation of the P1 promoter. J Mol Biol. 1984 Dec 25;180(4):881–909. doi: 10.1016/0022-2836(84)90262-6. [DOI] [PubMed] [Google Scholar]
- Mangel W. F., Chamberlin M. J. Studies of ribonucleic acid chain initiation by Escherichia coli ribonucleic acid polymerase bound to T7 deoxyribonucleic acid. 3. The effect of temperature on ribonucleic acid chain initiation and on the conformation of binary complexes. J Biol Chem. 1974 May 25;249(10):3007–3013. [PubMed] [Google Scholar]
- McClure W. R. Rate-limiting steps in RNA chain initiation. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5634–5638. doi: 10.1073/pnas.77.10.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova A. F., Beabealashvilli R., Mirzabekov A. D. A study of unwinding of DNA and shielding of the DNA grooves by RNA polymerase by using methylation with dimethylsulphate. Eur J Biochem. 1978 Mar;84(1):301–309. doi: 10.1111/j.1432-1033.1978.tb12169.x. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Shibuya M., Kuchino Y., Kaziro Y. Transcription of the E. coli tufB gene: cotranscription with four tRNA genes and inhibition by guanosine-5'-diphosphate-3'-diphosphate. Mol Gen Genet. 1981;183(1):13–19. doi: 10.1007/BF00270131. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson L. M., Reznikoff W. S. Abortive initiation and long ribonucleic acid synthesis. Biochemistry. 1981 Apr 14;20(8):2081–2085. doi: 10.1021/bi00511a003. [DOI] [PubMed] [Google Scholar]
- Ornstein R. L., Fresco J. R. Correlation of Tm and sequence of DNA duplexes with delta H computed by an improved empirical potential method. Biopolymers. 1983 Aug;22(8):1979–2000. doi: 10.1002/bip.360220811. [DOI] [PubMed] [Google Scholar]
- Otsuka J., Kunisawa T. Characteristic base sequence patterns of promoter and terminator sites in phi X174 and fd phage DNAs. J Theor Biol. 1982 Aug 7;97(3):415–436. doi: 10.1016/0022-5193(82)90374-5. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Skouv J., Kajitani M., Ishihama A. Transcriptional organization of the rpsA operon of Escherichia coli. Mol Gen Genet. 1984;196(1):135–140. doi: 10.1007/BF00334105. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisbig R. R., Woody A. Y., Woody R. W. Spectroscopic analysis of the interaction of Escherichia coli DNA-dependent RNA polymerase with T7 DNA and synthetic polynucleotides. J Biol Chem. 1979 Nov 25;254(22):11208–11217. [PubMed] [Google Scholar]
- Roe J. H., Burgess R. R., Record M. T., Jr Kinetics and mechanism of the interaction of Escherichia coli RNA polymerase with the lambda PR promoter. J Mol Biol. 1984 Jul 15;176(4):495–522. doi: 10.1016/0022-2836(84)90174-8. [DOI] [PubMed] [Google Scholar]
- Schnier J., Isono K. The DNA sequence of the gene rpsA of Escherichia coli coding for ribosomal protein S1. Nucleic Acids Res. 1982 Mar 25;10(6):1857–1865. doi: 10.1093/nar/10.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M. C., Gussin G. N. Mutations affecting two different steps in transcription initiation at the phage lambda PRM promoter. Proc Natl Acad Sci U S A. 1983 Jan;80(2):496–500. doi: 10.1073/pnas.80.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U. RNA polymerase unwinds an 11-base pair segment of a phage T7 promoter. Nature. 1979 Jun 14;279(5714):651–652. doi: 10.1038/279651a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Stefano J. E., Gralla J. D. Mutation-induced changes in RNA polymerase-lac ps promoter interactions. J Biol Chem. 1982 Dec 10;257(23):13924–13929. [PubMed] [Google Scholar]
- Stefano J. E., Gralla J. D. Spacer mutations in the lac ps promoter. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1069–1072. doi: 10.1073/pnas.79.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana H., Wada A. Ligand-induced melting reaction of specific-sequence DNA molecules. Biopolymers. 1982 Sep;21(9):1873–1885. doi: 10.1002/bip.360210914. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Platt T., Crawford I. P., Nichols B. P., Christie G. E., Horowitz H., VanCleemput M., Wu A. M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981 Dec 21;9(24):6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Gilbert S. F., Nomura M. DNA sequences of promoter regions for rRNA operons rrnE and rrnA in E. coli. Cell. 1979 May;17(1):201–209. doi: 10.1016/0092-8674(79)90308-8. [DOI] [PubMed] [Google Scholar]



