Abstract
Chronic neuropathic pain is a disabling condition observed in large number of individuals following spinal cord injury (SCI). Recent progress points to an important role of neuroinflammation in the pathogenesis of central neuropathic pain. The focus of the present study is to investigate the role of proinflammatory molecules IL-1β, TNF-α, MCP-1, MMP-9 and TIMP-1 in chronic neuropathic pain in a rodent model of SCI. Rats were subjected to spinal cord contusion using a controlled linear motor device with an injury epicenter at T10. The SCI rats had severe impairment in locomotor function at 7 days post-injury as assessed by the BBB score. The locomotor scores showed significant improvement starting at day 14 and thereafter showed no further improvement. The Hargreaves’ test was used to assess thermal hyperalgesia for hindpaw, forepaw and tail. A significant reduction in withdrawal latency was observed for forepaw and tail of SCI rats at days 21 and 28, indicating the appearance of thermal hyperalgesia. Changes in expression of mRNAs for IL-1β, TNF-α, MCP-1, MMP-9 and TIMP-1 were assessed using real-time polymerase chain reaction in spinal cord including the injury epicenter along with regions above and below the level of lesion at day 28 post-injury. A significant increase was observed in the expression of MCP-1, TNF-α, TIMP-1 and IL-1β in the injury epicenter, whereas only TIMP-1 was upregulated in the area below the injury epicenter. The results of the study suggest that prolonged upregulation of inflammatory mediators might be involved in chronic neuropathic pain in SCI, and that TIMP-1 may play a role in maintenance of chronic below level pain.
Keywords: Spinal cord injury, Neuropathic pain, Inflammation, Cytokines, Chemokines, Matrix metalloproteinases
Introduction
Spinal cord injury (SCI) is a major public health problem leaving the patients with life-long disabilities. The current estimates suggest there are approximately 400,000 individuals in the United States alone with SCI and more than 14,000 new cases occurring every year [1]. Injury to the spinal cord can produce a constellation of problems including chronic pain, autonomic dysreflexia, and motor dysfunction [2]. Epidemiological studies reveal intractable pain develops in 80% of SCI patients, and the common pain medications are ineffective in treating this chronic pain [3, 4]. Central pain commonly occurs above and below the injury level, and is commonly thought to be caused by maladaptive plasticity after injury, resulting in increased excitatory and decreased inhibitory input to dorsal horn neurons [5]. Eliminating chronic pain is a high priority for paraplegics, and 12% rank it as their highest priority, even outranking the prospect of regaining walking, trunk stability, bladder, bowel and sexual function [6]. Currently available drugs for neuropathic pain include antidepressants, anticonvulsants, sodium channel blockers, N-methyl-d-aspartic acid (NMDA) receptor antagonists and opioids [7]. These drugs were designed to treat pain symptoms by blocking neurotransmission, but they provide transient relief in only a fraction of patients, can produce severe CNS side effects, and do not target the underlying cause of central neuropathic pain [8]. Development of new treatment strategies will require improved understanding of the mechanism of induction and maintenance of central neuropathic pain.
Recent progress points to an important role of neuroinflammation in the pathogenesis and maintenance of neuropathic pain [9, 10]. A major contributor to chronic pain associated with spinal cord injury is persistent microglial activation and production of proinflammatory cytokines [11]. Microglia are chronically activated in the spinal cord after clinical [12] and experimental SCI [13, 14]. Microglial activation that contributes to persistent hyperalgesia may last for weeks or months in rodents, but potentially could last for years in people with spinal cord injury. Activated microglia secrete proinflammatory mediators that cause chronic neuronal hyperexcitability [15] resulting in persistent hyperalgesia [16]. These chronically activated microglia release proinflammarory cytokines (IL-1β, TNF-α) and chemokines (MCP-1) that facilitate pain mechanisms [17]. In addition, the increased expression of proinflammatory molecules leads to activation of matrix metalloproteinase-9 (MMP-9) that further contributes to the development of neuropathic pain [18]. A recent study suggested that targeting inflammation in spinal cord injury might improve regeneration and functional recovery and reduce pain [5, 19, 20].
Although the role of microglial activation in initiation of chronic pain has been investigated, which of the many inflammatory mediators are expressed during chronic pain states has received less attention. Furthermore, the role of inflammatory mediators in regions above or below the level of injury of has not been investigated. The goal of the proposed studies is to investigate the potential contribution of proinflammatory cytokines (IL-1β and TNF-α), chemokine (MCP-1), MMP-9 and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in chronic pain following spinal cord injury by examining expression of these mediators using qRT–PCR at the injury epicenter and the areas above and below the level of lesion which may mediate above and below level central neuropathic pain.
Experimental Procedures
Animals
All the experiments were carried out on Sprague-Dawley rats (male, 280–300 g, 3 months) obtained from Charles River, Wilmington, MA, USA. Animals were housed in pathogen free conditions with regulated temperature, humidity, and 12-h light and 12-h dark cycle. Animals had free access to standard rodent chow (8604; Harlan Teklad Laboratories, Madison, WI) and water. All the protocols for live and post-operative animal care of animals were approved by the Institutional Animal Care and Use Committee and were according to the NIH Guide for the Care and Use of Laboratory Animals. A total of 24 rats were used in the study (Sham, n = 10; SCI injured, n = 14).
Spinal Cord Injury
All surgeries were performed under aseptic conditions. The rats were initially anesthetized by a spontaneous inhalation of 4% isoflurane in an induction chamber and then moved to a surgery mat. The anesthesia level was reduced to 2–2.5 % isoflurane delivered in a mixture of 40% oxygen and 58% air through a nose mask. Further adjustments in small increments were made on the percentage of the isoflurane level during the surgery. The linear motor device used to induce SCI is similar to that used for spinal cord injury by Ramu et al. [21]. It allows for manipulation of injury severity by choice of tip diameter, and by precise control of strike velocity, contusion depth, and contusion time [22]. The surgical procedures for inducing SCI involved a midline incision posterior from the thoracic levels T4 to L2, followed by dissection of the bilateral vertebral muscles to expose the dorsal laminae and spinous processes. Laminectomy was performed using rongeurs at T9–T11 to expose the spinal cord while leaving the dura intact. After stabilizing the spinal cord with two hemostatic forceps attached to the rostral and caudal vertebral bodies, the laminectomized section was positioned under the impactor tip of the injury device. SCI was induced between T9 and T11 with the injury epicenter at the T10 level. The contusion injury was produced by using a 2 mm injury tip with velocity of 1.5 m/s and contact duration of 85 ms and deformation depth set to 0.99 mm. These parameters produced a partial injury that provided good prognosis for behavioral improvement. After contusion, the skin was closed with 4–0 sutures and the animal placed in a heated cage to maintain the body temperature during recovery from anesthesia. Rats were given buprenorphine 0.05 mg/kg and a local analgesic, bupivacaine, was applied to the incision site. Bladder expression of SCI rats was performed daily until a reflex bladder was established.
Locomotor Function
Locomotor recovery was assessed using an open-field testing paradigm, the Basso-Beattie-Bresnahan (BBB) Locomotor Rating Scale, which is based on a 21 point scale originally developed in the spinal cord-injured rat [23]. This scale assesses 10 distinct categories that range from limb movement to tail position and involve detailed observations of joint movement, stepping, and coordination. Uninjured animals exhibit a locomotor score of 21, whereas animals that exhibit complete hind limb paralysis are scored as a 0. Each animal was tested in an open field (0.7 × 0.9 m2) for 5 min at 1, 3, 7, 14, 21 and 28 days post-injury.
Thermal Hyperalgesia
Forelimb, hindlimb and tail withdrawal latency (WL) to a heat stimulus was measured prior to injury and days 7, 14, 21, and 28 using methods previously described (Hargreaves et al. 1988; Dirig et al. 1997). Briefly, the rats were placed on a glass plate over a light box, and a radiant heat stimulus (4.7 amps, San Diego Instruments) was applied by aiming a beam of light through a hole in the light box onto the glabrous surface of the paw of each limb though the glass plate. The light beam was turned off automatically by a photocell when the rat lifted the limb, allowing the measurement of time between the start of the light beam and the paw withdrawal. Five minutes were allowed between each trial, and these measurements were averaged for each limb and compared to before injury (baseline) values.
Total RNA Isolation
Spinal cords were harvested at day 28, when the rats demonstrated thermal hyperalgesia. Spinal cord samples 5 mm in length centered at the injury region and 5 mm samples from spinal cord above and below the injury level were stored in RNA Later (Ambion, Austin, TX) for 24 h at 4°C. The injury sample included the T7–T12 region, the above level sample constituted region T2–T6, and the below level sample was from L2–L5. After 24 h, total RNA was extracted using Trizol Reagent (Invitrogen, Life Technologies, Carlsbad, CA) using with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY) according to the manufacturer’s instructions.
Real-Time PCR Analysis
Gene expression analysis was conducted using quantitative real-time PCR. The following transcripts were analyzed: IL-1β, TNF-α, MCP-1, MMP-9 and TIMP-1. In brief, the RNA samples were treated with DNaseI to remove any contaminating DNA. Complementary DNA (cDNA) was synthesized by using 1 µg total RNA from each sample and random hexamers in a Taqman reverse transcription reaction (Applied Biosystems, Foster City, CA, USA). The expression of mRNAs was analyzed by real-time PCR using SYBR Green dye. Briefly, 10 ng cDNA and gene-specific primers were added to SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaq-DNA polymerase, dNTPs mixture dUTP and optimal buffer components; Applied Biosystems, Foster City, CA, USA) and subjected to PCR amplification (one cycle at 50°C for 2 min, one cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min) in a TaqMan 7500 sequence detection system (Applied Biosystems). PCR reactions were conducted in duplicate. Primer sequences used were custom synthesized (Invitrogen Corporation, Carlsband, CA, USA) based on the published sequences or were designed using Primer Express software (Applied Biosystems Inc. Foster City, CA, USA) and based on GenBank accession numbers (Table 1). The resulting amplicon products were visualized on agarose gel to verify size and specificity of RT–PCR reaction. The relative amounts of amplified transcripts were quantified with the comparative cycle threshold (CT) method using GAPDH as housekeeping gene using the delta-delta Ct method [24].
Table 1.
Primer sequences used for real-time PCR
| Gene | Primer sequence | Position | Size (bp) | |
|---|---|---|---|---|
| IL-1β | Forward | CACCTCTCAAGCAGAGCACAG | 773–793 | 79 |
| (NM 031512) | Reverse | GGGTTCCATGGTGAAGTCAAC | 831–851 | |
| MCP-1 | Forward | AGCACCTTTGAATGTGAACT | 411–430 | 82 |
| (NM 031530) | Reverse | AGAAGTGCTTGAGGTGGTT | 474–492 | |
| TNF-α | Forward | CAAGAGCCCTTGCCCTAA | 669–686 | 181 |
| (NM 012675) | Reverse | CAGAGCAATGACTCCAAAGTA | 829–849 | |
| MMP-9 | Forward | GTGACACCGCTCACCTTCAC | 454–473 | 122 |
| (NM 031055) | Reverse | GCGTGTGCCAGTAGACCATC | 556–575 | |
| TIMP-1 | Forward | GATATGTCCACAAGTCCCAGAACC | 316–339 | 163 |
| (NM 053819) | Reverse | CCACAGCCAGCACTATAGGTCTTT | 455–478 | |
| GAPDH | Forward | ATGACATCAAGAAGGTGGTG | 835–854 | 177 |
| (XR 007913) | Reverse | CATACCAGGAAATGAGCTTG | 992–1011 |
Histology
The histopathology of injured SCs were examined using hematoxylin and eosin (H&E) staining on day 28. Rats were euthanized by intracardiac perfusion with 50 mL of phosphate buffered saline (PBS) solution, followed by 200 ml of 4% formaldehyde PBS solution that were delivered through a 23-gauge needle connected to a perfusion pump. The SC was excised and post fixed in 4% formaldehyde. Segments from the injury epicenter were embedded in paraffin and cut serially in 5 µm sections. Representative samples were stained with standard H&E method.
Statistical Analysis
Significant group differences were determined by using the unpaired Student’s t test. In all cases, a P value of <0.05 was considered significant. Results are expressed as the mean ± standard error.
Results
Functional Assessment
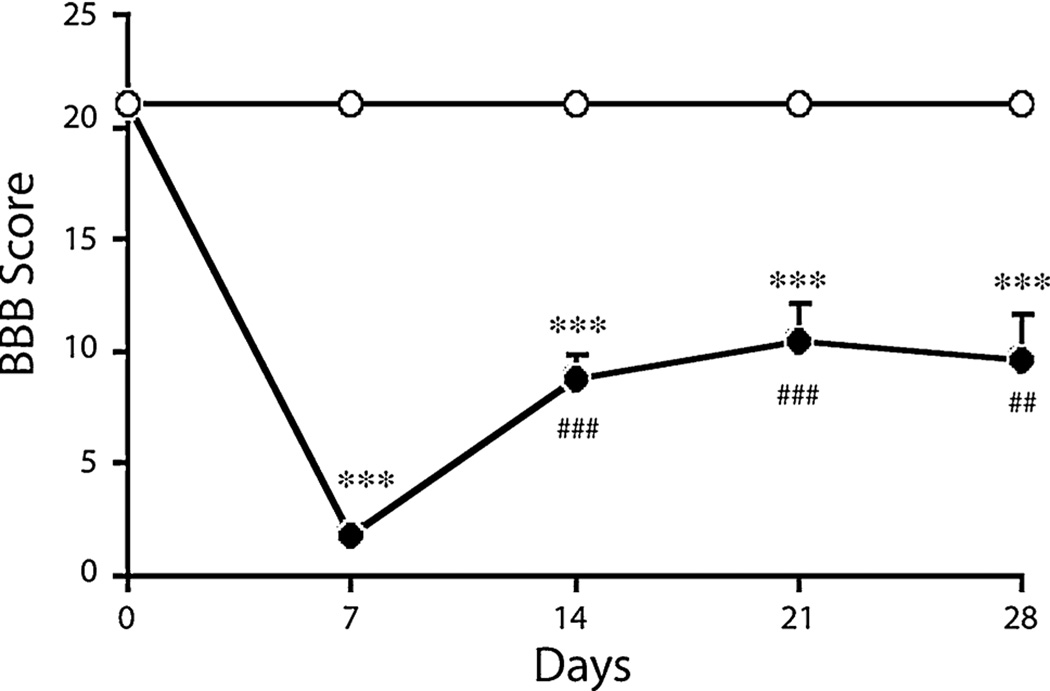
Hematuria and urinary retention were observed in SCI rats within first few days after injury. Immediately after injury complete paraplegia was observed followed by progressive recovery as indicated by higher BBB scores. The BBB scores were less than 6 in all animals on 7 day post contusion (Fig. 1). The SCI injured animals showed significant impairment in locomotor activity starting at day 7 and additional spontaneous recovery (BBB score) by day 14. The scores reached plateau thereafter.
Fig. 1.
BBB scores of Sham (open circle) and SCI (filled circle) rats at day 0, 7, 14, 21 and 28. Values are mean + SEM for Sham (n = 7) and SCI (n = 10) rats. Significantly different from sham animals, ***P < 0.001. Significantly different from SCI (7 day) animals, ###P < 0.001; ##P < 0.01
Thermal Hyperalgesia
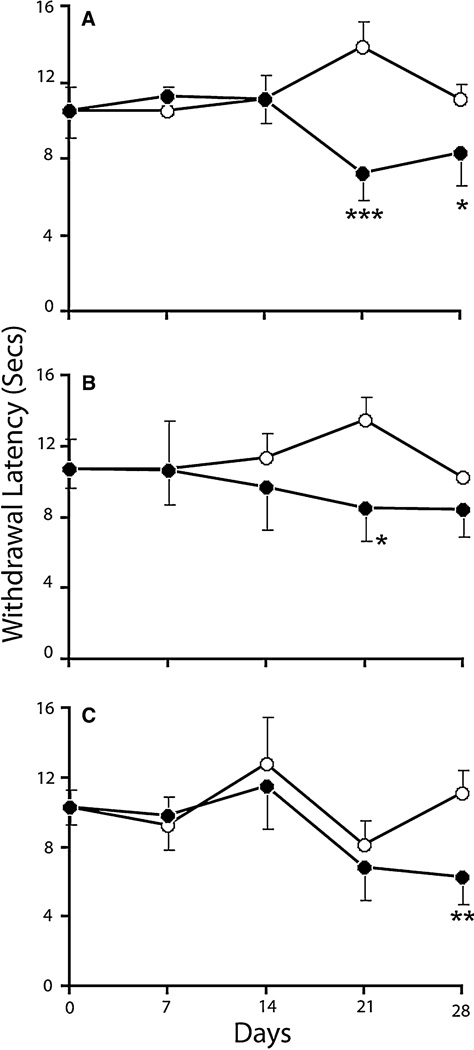
Withdrawal latencies (WL) to a radiant heat source were measured and recorded for fore and hind limbs and tail (Fig. 2). The WL (in seconds) for forelimbs was significantly lower than that of the sham animals at 21 and 28 days post injury. However, WL for hind limbs was lower at 21 day only. The tail WL for the injured animals was significantly lower than that of the shams at 28 days post SCI. The results demonstrate development of thermal hyperalgesia in the injured animals beginning 21 days postinjury.
Fig. 2.
Paw withdrawal frequency in response to thermal stimuli for forelimb (a), hindpaw (b) and tail (c) of sham (open circle) and SCI (filled circle) rats at day 0, 7, 14, 21 and 28. Values are mean + SEM for sham (n = 4) and SCI (n = 7) rats. Significantly different from sham group, *P < 0.05; **P < 0.01; ***P < 0.001
Expression of Inflammatory Markers
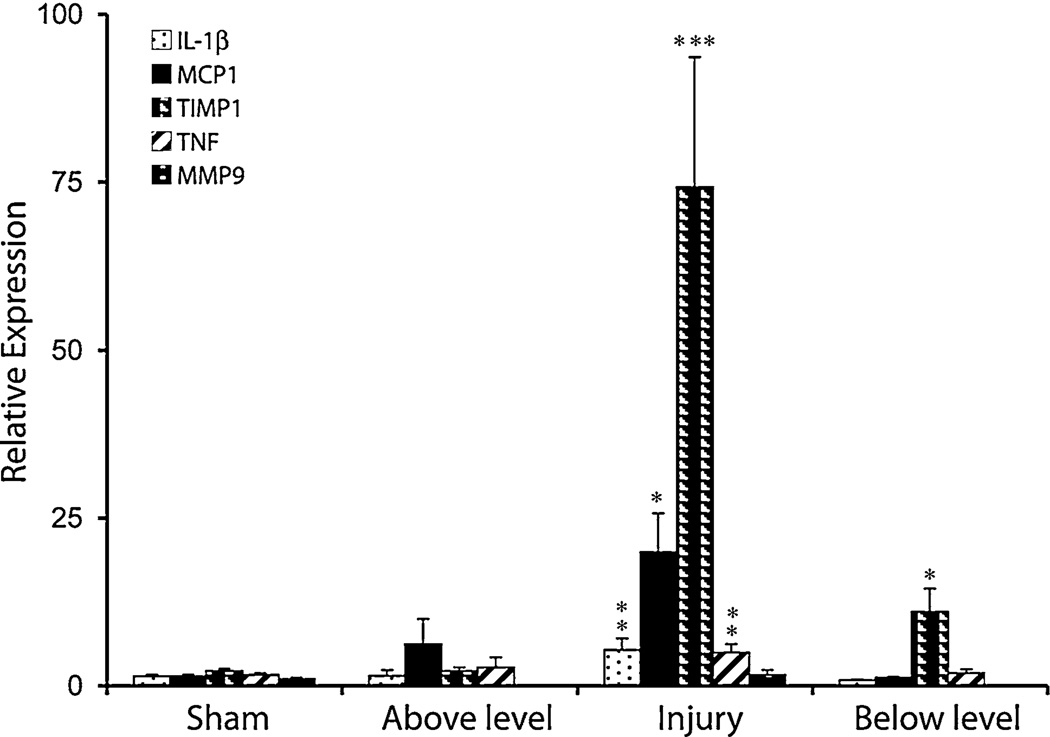
Expression of mRNA for inflammatory markers; cytokines (IL-1β, TNF-α), chemokine (MCP-1), MMP-9 and TIMP-1 were carried in the spinal cord injury, above and below level regions at day 28 post-injury (Fig. 3). The mRNA expression for IL-1β and TNF-α were upregulated to 3.6 and 3.1-fold in the injury region relative to sham levels respectively, whereas no significant upregulation was observed in the above or below level regions. MCP-1 RNA was upregulated by 17.4-fold in the injury area, whereas no significant increase was observed in the above or below level regions. The expression of MMP-9 mRNA was not upregulated in any of the areas of spinal cord 28 days after injury. However, the expression of TIMP-1 was upregulated in the injured and below level spinal regions by 48.7 and 7.5-fold respectively relative to the levels observed in sham-injured spinal cords, and these differences were significant.
Fig. 3.
Quantitative RT-PCR analysis of IL-1β, TNF-α, MCP-1, MMP-9 and TIMP-1 mRNA in sham and injured spinal cord at lesion site and above and below lesion levels. Values are mean + SEM sham (n = 6) and SCI (n = 7) rats. Significantly different from sham group, *P < 0.05; ***P < 0.001; **P < 0.01
Histology
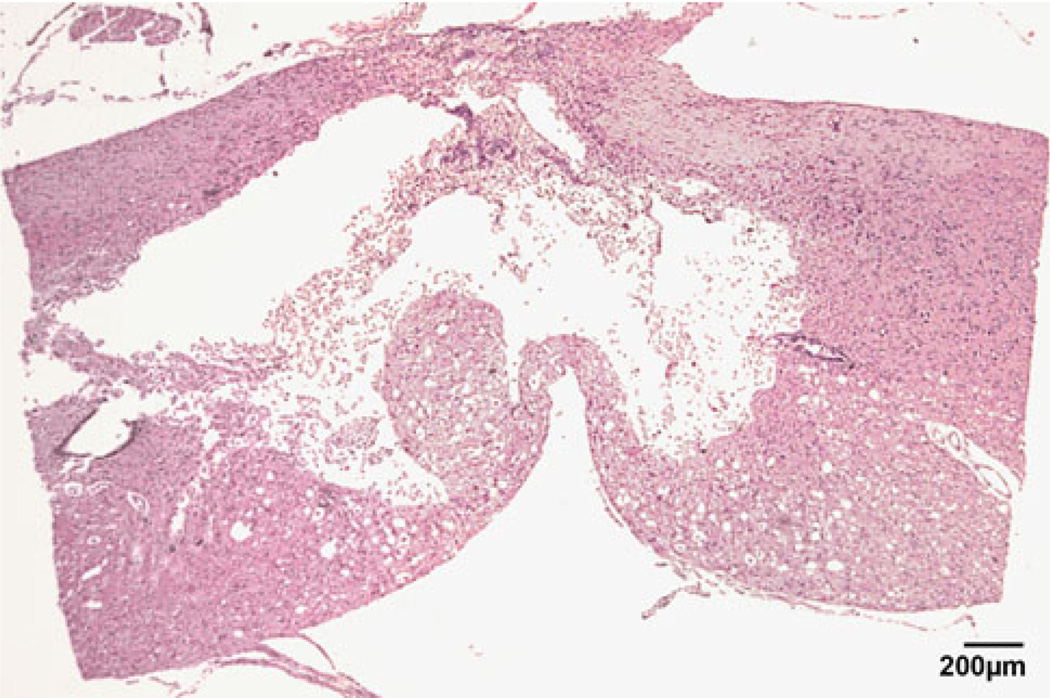
To verify spinal cord injury, histological assessment of the lesion site was done using hematoxylin and eosin staining at day 28 (Fig. 4). It is clear that the injured SC had a significant lesion of the dorsal column system and the cavity, typical of spinal cord injury in the rat, had formed at this time.
Fig. 4.
Histology of spinal cord 28 days post-injury at T10 showing lesion of the dorsal column system (saggital section)
Discussion
BBB scores showed that the animals with spinal cord injury showed significant impairment in locomotor function at the 7 day time point, followed by continued spontaneous recovery at 14 day, which then plateaued. The results clearly suggest that the injury paradigm used in the present study results in paraplegia, a hallmark clinical outcome of SCI [25]. The spinal cord injured animals also showed thermal hyperalgesia as assessed by the Hargreaves test in terms of reduced withdrawal latencies for the forelimb, hindlimb and tail. One exception was that the withdrawal latencies for the hind limbs were not significantly different from sham at the 28 day time point, which might be due residual weakness of the hind limbs. This finding has been observed by other investigators [26]. Thus, the spinal cord injury model used in this study causes severe locomotor dysfunction followed by partial recovery, then paw and tail thermal hyperalgesia.
Expression of proinflammatory cytokines (IL-1β and TNF-α) was upregulated in injured spinal cord 28 days post injury, consistent with a role in development of thermal hyperaglesia. This finding replicates results of Detloff et al. [27], who observed upregulation of TNF-α and IL-1β at day 7 and 21. A significant association has been observed in the timing of upregulation of inflammatory cytokines and development of hyperalgesia after spinal cord injury.
Accumulating evidence indicates that the chemokine MCP-1 (CCL2) plays a critical role in chronic pain [28]. We observed upregulation of MCP-1 in injured spinal cord at day 28 post-injury. It has been shown that signaling from MCP-1 is critical to microglial activation and that treatment with MCP-1 neutralizing antibody prevents microglial activation and attenuates neuropathic pain after peripheral nerve injury [29, 30]. In addition, increased MCP-1 has been observed in the serum of spinal cord injury patients and is a predictor of poor prognosis [31]. MCP-1 has been shown to contribute and maintenance of peripheral neuropathic pain [32]. Our study confirms the findings that MCP-1 expression is related to development of neuropathic pain following SCI [33]. Furthermore, expression of MCP-1 was found to correlate with expression of molecules that are involved in nociceptive processing including calcitonin gene-related peptide (CGRP), substance-P, and vanilloid-receptor-1, suggesting that upregulation of MCP-1 may be a primary trigger in development of neuropathic pain [33]. Therefore, inhibition of the MCP-1 pathway may provide a new target for management of neuropathic pain following SCI [34].
MMPs comprise a large family of zinc endopeptidases that have been implicated in the generation of neuroinflammation via cleavage of extracellular matrix proteins and activation of proinflammatory cytokines and chemokines [35]. We did not observe any significant induction in the MMP-9 mRNA expression 28 days following SCI in injured spinal cord or in areas above and below the injury level, suggesting MMP-9 is not upregulated at the time when animals develop hyperalgesia. However, a significant increase in TIMP-1 expression in injured and below level spinal cord was observed 28 days post-injury. It has been shown that after spinal nerve ligation MMP-9 levels increase rapidly in injured primary sensory neurons during the early phase of hyperalgesia [36]. Goussev et al [37] have also observed that MMP-9 is induced early after SCI and returns to baseline by day 28. The role of prolonged upregulation of TIMP-1 in injured spinal cord in the below level region is unclear. A recent study has shown that over expression of TIMP-1 is neuroprotective following acute brain injury [38]. There is an emerging concept is that TIMPs have many MMP-independent biological activities [39, 40]. The role of upregulation of TIMP-1 in the absence of an increase in MMP-9 expression in SCI requires further investigation.
The results from the present study demonstrate that upregulation of proinflammatory cytokines (IL-1 β, TNF-α), chemokine (MCP-1) and TIMP-1 during the development of neuropathic pain. Davies et al. [41] determined elevated levels of circulating cytokines in serum of SCI patients, and the levels were observed to be further elevated in SCI subjects with neuropathic pain clearly confirming the involvement of elevated cytokines in pathogenesis of central neuropathic pain. Further, Nakagawa [42] suggested that in SCI microglia are initially activated and subsequently sustained activation of astrocytes is involved in induction and maintenance of chronic pain. This implies that therapies targeted at expression of these inflammatory mediators by activated astrocytes might be beneficial in ameliorating chronic neuropathic pain observed following SCI.
Acknowledgments
The financial assistance provided by the Steve Palermo Endowment and the National Institute of Health (P30-HD02528) is acknowledged.
Abbreviations
- IL-1β
Interleukin 1 beta
- MCP-1
Monocyte chemotactic protein-1
- MMP-9
Matrix metalloproteinase 9
- TIMP-1
Tissue inhibitor of metalloproteinase 1
- TNF-α
Tumor necrosis factor-alpha
Contributor Information
Rajat Sandhir, Steve Palermo Nerve Regeneration Laboratory, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA; Department of Anatomy and Cell Biology, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA.
Eugene Gregory, Steve Palermo Nerve Regeneration Laboratory, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA; Department of Anatomy and Cell Biology, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA.
Yong-Yue He, Hoglund Brain Imaging Center, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA.
Nancy E. J. Berman, Email: nberman@kumc.edu, Steve Palermo Nerve Regeneration Laboratory, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA; Department of Anatomy and Cell Biology, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA; Department of Neurosurgery, University of Kansas Medical Center, 3901 Rainbow Boulevard, Kansas City, KS 66160, USA.
References
- 1.Sekhon LHS, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26(24S):S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 2.Deumens R, Joosten EA, Waxman SG, Hains BC. Locomotor dysfunction and pain: the scylla and charybdis of fiber sprouting after spinal cord injury. Mol Neurobiol. 2008;37:52–63. doi: 10.1007/s12035-008-8016-1. [DOI] [PubMed] [Google Scholar]
- 3.Judith AT, Diana DC, Catherine AW, Catherine BM. Chronic pain associated with spinal cord injuries: a community survey. Arch Phys Med Rehabil. 2001;82:501–509. doi: 10.1053/apmr.2001.21855. [DOI] [PubMed] [Google Scholar]
- 4.Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal Cord. 2001;39:256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- 5.Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 8.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 9.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang HT. Subacute human spinal cord contusion: few lymphocytes and many macrophages. Spinal Cord. 2007;45:174–182. doi: 10.1038/sj.sc.3101910. [DOI] [PubMed] [Google Scholar]
- 13.Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKay SM, Brooks DJ, Hu P, McLachlan EM. Distinct types of microglial activation in white and grey matter of rat lumbosacral cord after mid-thoracic spinal transection. J Neuropathol Exp Neurol. 2007;66:698–710. doi: 10.1097/nen.0b013e3181256b32. [DOI] [PubMed] [Google Scholar]
- 15.Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci. 2007;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mika J. Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness. Pharmacol Rep. 2008;60:297–307. [PubMed] [Google Scholar]
- 18.Chattopadhyay S, Myers RR, Janes J, Shubayev V. Cytokine regulation of MMP-9 in peripheral glia: implications for pathological processes and pain in injured nerve. Brain, Behavior, Immun. 2007;21:561–568. doi: 10.1016/j.bbi.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- 20.Tan AM, Zhao P, Waxman SG, Hains BC. Early microglial inhibition preemptively mitigates chronic pain development after experimental spinal cord injury. J Rehabil Res Dev. 2009;46:123–133. [PubMed] [Google Scholar]
- 21.Ramu J, Bockhorst KH, Mogatadakala KV, Narayana PA. Functional magnetic resonance imaging in rodents: methodology and application to spinal cord injury. J Neurosci Res. 2006;84:1235–1244. doi: 10.1002/jnr.21030. [DOI] [PubMed] [Google Scholar]
- 22.Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19:219–226. doi: 10.1177/1545968305278635. [DOI] [PubMed] [Google Scholar]
- 23.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamotova A, Sramkova T, Rokyta R. Intensity of pain and biochemical changes in blood plasma in spinal cord trauma. Spinal Cord. 2010;48:21–26. doi: 10.1038/sc.2009.71. [DOI] [PubMed] [Google Scholar]
- 26.Gwak YS, Hains BC, Johnson KM, Hulsebosch CE. Locomotor recovery and mechanical hyperalgesia following spinal cord injury depend on age at time of injury in rat. Neurosci Lett. 2004;362:232–235. doi: 10.1016/j.neulet.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and proinflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, et al. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu SQ, Ma YG, Peng H, Fan L. Monocyte chemoattractant protein-1 level in serum of patients with acute spinal cord injury. Chin J Traumatol. 2005;8:216–219. [PubMed] [Google Scholar]
- 32.Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, et al. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knerlich-Lukoschus F, Juraschek M, Blomer U, Lucius R, Mehdorn HM, Held-Feindt J. Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J Neurotrauma. 2008;25:427–448. doi: 10.1089/neu.2007.0431. [DOI] [PubMed] [Google Scholar]
- 34.White FA, Feldman P, Miller RJ. Chemokine signaling and the management of neuropathic pain. Mol Interv. 2009;9:188–195. doi: 10.1124/mi.9.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji R-R, Xu Z-Z, Wang X, Lo EH. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci. 2009;30:336–340. doi: 10.1016/j.tips.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goussev S, Hsu JY, Lin Y, Tjoa T, Maida N, Werb Z, et al. Differential temporal expression of matrix metalloproteinases after spinal cord injury: relationship to revascularization and wound healing. J Neurosurg. 2003;99:188–197. doi: 10.3171/spi.2003.99.2.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tejima E, Guo S, Murata Y, Arai K, Lok J, van Leyen K, et al. Neuroprotective effects of over-expressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma. 2009;26:1935–1941. doi: 10.1089/neu.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 41.Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1384–1393. doi: 10.1016/j.apmr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa T. Spinal astrocytes as therapeutic targets for pathological pain. J Pharmacol Sci. 2010;114:347–353. doi: 10.1254/jphs.10r04cp. [DOI] [PubMed] [Google Scholar]