Abstract
Although stress is often hypothesized to contribute to the effects of neighborhoods on health, very few studies have investigated associations of neighborhood characteristics with stress biomarkers. This study helps address the gap in the literature by examining whether neighborhood characteristics are associated with cortisol profiles. Analyses were based on data from the Multi-Ethnic Study of Atherosclerosis Stress study which collected multiple measures of salivary cortisol over three days on a population based sample of approximately 800 adults. Multilevel models with splines were used to examine associations of cortisol levels with neighborhood poverty, violence, disorder, and social cohesion. Neighborhood violence was significantly associated with lower cortisol values at wakeup and with a slower decline in cortisol over the earlier part of the day, after sociodemographic controls. Associations were weaker and less consistent for neighborhood poverty, social cohesion, and disorder. Results revealed suggestive, though limited, evidence linking neighborhood contexts to cortisol circadian rhythms.
Keywords: Neighborhood context, cortisol, biomarker, hierarchical linear modeling
INTRODUCTION
Although a large number of studies have suggested that neighborhood disadvantage is associated with adverse health outcomes (Pickett and Pearl, 2001; Robert, 1999), only recently have researchers begun to examine the possible biological pathways through which neighborhood context may affect the health of residents. Identifying the biological pathways involved is fundamental to increasing our understanding of how neighborhoods are linked to health.
Neighborhoods can influence health through a number of pathways. Two of the most common pathways hypothesized in the literature include the influences of neighborhood physical environments on health behaviors and the influences of neighborhood environments on psychosocial stress (Diez Roux, 2003). A number of studies have focused on aspects of neighborhoods that may constrain healthy behaviors. For example, the built environment of neighborhoods as well as the presence of physical activity and food resources (e.g., public parks, supermarkets) have been linked to the diet and physical activity of residents (Frank et al., 2003; Moore et al., 2009). Although often hypothesized as another important mechanism linking neighborhoods to health, the role of psychosocial stress has been less studied.
Several features of neighborhoods could operate as stressors for residents. Neighborhoods characterized by high deprivation have been theorized to increase residents’ exposure to high crime, violence, physical decay or disorder, and social distrust (Aneshensel and Sucoff, 1996; Schulz et al., 2000a; Schulz et al., 2000b) which may increase stress levels. Stress may be linked to health and cardiovascular outcomes because persons under stress may be more likely to engage in deleterious health behaviors such as smoking, drinking, and drug use as coping strategies (Boardman et al., 2001; Echeverria et al., 2008). In addition, exposure to stressors has a series of direct biological consequences related to the activation of the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis is responsible for mobilizing the body’s resources when an individual encounters psychological or physical stressors (Sapolsky, et al., 1986).Cortisol, a hormone produced by the HPA axis, increases in response to stress and varies according to a circadian rhythm in which levels are typically high upon awakening, increase sharply between 30 to 40 minutes of waking and declines across the remainder of the waking day. In addition to its physiologic role in preparing the body to respond to stressors, cortisol plays a role in several physiologic processes relevant to the development of cardiovascular disease and other health conditions. For example, elevated levels of cortisol are linked to glucose intolerance, abdominal obesity, and hypertension (Bjorntorp and Rosmond, 2000; McEwen and Lasley, 2003; Brindley and Rolland, 1989).
Both acute and sustained exposures to stressors have been linked to alterations of the usual circadian rhythm of cortisol (Adam et al., 2006; McEwen, 1998). While the implications of alterations of the circadian rhythm of cortisol (sometimes referred to as cortisol dysregulation) for health remain to be determined, there is some evidence that various altered patterns are associated with chronic health conditions (Adam and Kumari, 2009; Vreeburg et al., 2009b), including cardiovascular related outcomes such as obesity (Ranjit et al., 2005b) and subclinical atherosclerosis (Dekker et al., 2008; Eller et al., 2001; Matthews et al., 2006).
Although studies have reported alterations in the circadian rhythm of cortisol to be associated with low socioeconomic position and psychosocial characteristics such as hostility and depression (Cohen et al., 2006a; Hajat et al., 2010; Ranjit et al., 2009; Vreeburg et al., 2009a), few have examined whether the exposure to stressors at the neighborhood level alters the daily secretion of cortisol. To our knowledge, only two studies have examined the relationship between cortisol levels and neighborhood context (Chen and Paterson, 2006; Kapuku et al., 2002). Kapuku and colleagues studied cortisol response to a video and physical stressor among twenty-four African American male youths aged 16 to 25. Neighborhood SES was found to be unrelated to baseline cortisol levels. While increased cortisol levels were observed during the stressor protocol, the associations between neighborhood SES and cortisol response to the video and physical stressors were not presented. Chen and Paterson examined the relationship between cortisol and neighborhood SES among 212 adolescents aged 15 to 19 in St. Louis, MO. Baseline salivary cortisol samples were obtained at the end of a rest period before study participants completed a series of psychological measures. Neighborhood SES was found to be positively associated with baseline cortisol levels after adjustment of family SES characteristics. Limitations of these studies include very small sample sizes, lack of generalizability, limited individual-level controls, and reliance on a single cortisol sample. As such, the role of neighborhood context in shaping the daily secretion of cortisol over multiple days has yet to be explored.
This study used data from the MESA Stress study which collected multiple measures of salivary cortisol over three days in a population based sample to examine whether various neighborhood characteristics (poverty, violence, disorder, and poor social cohesion) are associated with various aspects of the cortisol circadian rhythm. Establishing whether neighborhood stressors are associated with daily cortisol profiles would provide evidence to support a specific biologic pathway linking neighborhood to health and lend strength to the importance of place (i.e., neighborhoods) in influencing health.
MATERIALS AND METHODS
Study Population
Data for this study were drawn from a subsample of the Multi-Ethnic Study of Atherosclerosis (MESA). MESA was designed to study the determinants of subclinical cardiovascular disease and included 6,814 men and women aged 44 to 84 years without clinical cardiovascular disease at baseline recruited from six sites using a variety of population-based approaches. The MESA Stress study, an ancillary study to MESA, collected detailed measures of stress hormones, including multiple measures of salivary cortisol over three days on a subsample of 1,002 participants enrolled at the New York and Los Angeles MESA sites between 2004 and 2006. Characteristics of the MESA Stress subsample were similar to those of the eligible sample that was not chosen for the Stress study, with few exceptions; a smaller proportion was in the 75–84 year age range (12.1% compared to 18.2% who were not in the Stress study), a slightly larger fraction were men (47.6% compared to 44.7%) and a higher percentage had some college education (29.7% compared to 23.9%).
Each MESA Stress participant was instructed to collect six salivary samples a day over three typical week days. The participants were instructed to restrict the total duration between the first and last day of sampling to a maximum of one week, but the days of sampling were not required to be consecutive. Samples were returned to the clinic by participants after the three days of collection. The first sample was to be taken immediately after waking (and immediately before getting out of bed), the second sample 30 minutes later, the third sample at around 10:00AM, the fourth sample at around noon (or before lunch if lunch occurred before noon), the fifth sample at around 6:00PM (or before dinner if dinner occurred before 6:00PM), and the sixth sample directly before bed. Detailed instructions and training in sample collection were provided to participants by trained staff. A container with a time tracking device (Track Caps) automatically registered the time at which cotton swabs were extracted to collect each sample. Participants were told of this time tracking device. Prior work has shown that the use of this device increases compliance with the requested timing of samples (Kudielka et al., 2003). Data showed excellent agreement between reported and track caps times with approximately 86% of overall self-recorded times being within 15 minutes of the registered Track-Cap times (Hajat et al., 2010). Collection times from the Track-Cap devices were used in analyses.
Saliva samples were collected using Salivette collection tubes and stored at −20 °C until analysis. Samples were thawed and centrifuged at 3000 rpm for three minutes to obtain clear saliva with low viscosity. Salivary cortisol levels were determined employing a commercially available chemi-luminescence assay (CLIA) with high sensitivity of 0/16 ng/mL (IBL-Hamburg; Germany). Intra- and inter-assay coefficients of variation were below eight percent. Cortisol was measured in nmol per liter.
Of the 1,002 total participants in the MESA Stress Study, 55 participants were excluded from these analyses because they had no track-cap time, insufficient sample for assay, unreliable cortisol values, or had missing values for one of the individual-level covariates (e.g., income, wealth). This resulted in a total of 947 participants over three days, with 15,595 cortisol samples for analysis. An additional 33 participants who were on birth control pills or were taking oral or inhaled steroids were excluded. Due to missing census tract geocode identifiers and missing neighborhood-level data, analyses were further restricted to 892 and 814 participants for models using neighborhood tract data and neighborhood data collected from an independent neighborhood survey (the Community Survey described below), respectively. The final analytical sample contained an average of 17 samples (std=2.48) per person over the three days.
Measures
Individual-level demographic and socioeconomic variables included age (continuous, median=66 years), gender (48% male), self-reported race/ethnicity (19.7% non-Hispanic white (reference); 27.5% non-Hispanic black; 52.8% Hispanic), education (continuous measure of highest educational level completed: 1.2% no schooling; 16.9% grades 1–8; 9.1% grades 9–11; 20.3% high school/GED; 15.8% some college with no degree; 7.4% technical school certificate; 5.4% associate degree; 11.0% bachelor’s degree; 12.9% graduate/professional school), income (continuous measure of family income adjusted for number of dependents, median=$16, 250), and wealth (continuous 5 point summary score, range 0 (lowest wealth) to 4 (highest wealth) indicating whether the person owns a car, owns/pays a mortgage, owns/buying land, or has investments, mean = 1.8).
Other covariates examined as possible confounders and/or mediators included body mass index (23.8 % normal (BMI<25); 39.5% overweight (25≤BMI<30); 36.8% obese (BMI≥30)), type 2 diabetes (defined as fasting plasma glucose≥126 mg/dl (Genuth et al., 2003) or taking medications for diabetes; 17.4%), smoking (53.3% never; 36.0% former; 10.7%current), and physical activity level. Physical activity questions were adapted from the Cross-Cultural Activity Participation Study (Irwin et al., 2000). Scores of intentional exercise, measured in metabolic equivalent (MET)-minutes/week, were categorized into approximate quartiles.
Four neighborhood characteristics that could operate as stressors were selected for investigation a priori: neighborhood poverty, disorder, violence, and social cohesion (Browning and Cagney, 2002; Kawachi et al., 1999; Pickett and Pearl, 2001; Robert, 1999; Ross and Mirowsky, 2001; Wright et al., 2004). Neighborhood poverty, utilized in much of the early neighborhood-health studies as a simple proxy for overall neighborhood socioeconomic conditions, has been consistently found to be negatively associated with health (Pickett and Pearl, 2001; Robert, 1999). Multiple aspects of the deprivation associated with poverty may operate as stressors. Neighborhood disorder and violence may also operate as stressors by creating a sense of unpredictability, threat, or lack of safety (Ross and Mirowsky, 2001; Wright et al., 2004). High neighborhood social cohesion is theorized to support positive collective action as well as feelings of solidarity and trust which have been found to be positively associated with health (Browning and Cagney, 2002; Kawachi et al., 1999). Social cohesion may also help buffer the effects of other sources of stress (Sampson 2003; Sampson et al., 1997). In contrast, the absence of neighborhood social cohesion and associated low solidarity and trust could itself operate as a stressor or enhance the effects of other stressors.
Neighborhood poverty levels were derived from the 2000 Decennial Census and reflect the proportion of individuals within the census tract who are below the poverty line. Measures of neighborhood disorder, violence, and cohesion were derived from the Community Survey, a separate population-based telephone survey that asked residents of MESA neighborhoods to provide information on conditions in their neighborhood, defined as the area about one mile around each survey respondent’s residence (Mujahid et al., 2007). Using a combination of random digit dialing and list-assisted sampling, the Community Survey surveyed 5,409 residents who lived within 1 mile of MESA Stress participants. By using a separate study sample to measure community characteristics, we avoid the possibility for same-source bias stemming from the possible correlation between individuals’ perceptions and health outcomes. For example, individuals who are more susceptible to adverse reactions to stressors may tend to evaluate neighborhood conditions as more negative than those who are more able to cope with challenges.
Community Survey participants were asked questions on neighborhood characteristics, including neighborhood disorder (12 items composed of 6 physical and 6 social disorder items, adopted from Ross & Mirowski (Ross and Mirowsky, 1999)), violence (4 items, based on Sampson, Raudenbush, and Earls (Sampson et al., 1997)) and social cohesion (4 items based on Sampson, Raudenbush, and Earls (Sampson et al., 1997)). Participants were asked to specify their level of agreement to each statement on a 5-point Likert scale (1=strongly agree to 5=strongly disagree) for social cohesion and disorder and a 4-point scale (1=often to 4=never) for violence. Cronbach’s alphas were 0.90 for disorder, 0.84 for violence, and 0.75 for cohesion. Mean neighborhood-level scores were derived from averaging responses of all community survey respondents within a 1 mile radius of each MESA participant. The mean number of Community respondents within a mile of each MESA participant was 178 (interquartile range from 38 to 320). Table 1 summarizes the scale items for each neighborhood dimension generated from the Community Survey.
Table 1.
Scale items for the neighborhood dimensions of social cohesion, violence, and disorder from the MESA Community Survey
| Neighborhood Dimension | Scale Items |
|---|---|
| Social Cohesion |
|
| Violence |
|
| Disorder |
|
Statistical analysis
Cortisol levels are typically characterized by high levels in the morning, with increasing levels for approximately 30–45 minutes after awakening followed by a decline over most of the day. We first performed descriptive analyses to ascertain the shape of the cortisol profile over the course of the day using locally estimated scatter plot smoothing (LOESS) curves. Time, as registered by the Track-Caps device, is measured in minutes since wake-up. The LOESS curves indicated a non-linear cortisol response over the day with two inflection points. Accordingly, we estimated a piecewise linear regression with two fixed knots at 30 minutes after wakeup and 120 minutes after wakeup to best capture the non-linearity of cortisol level over the day (Hajat et al., 2010). This strategy allowed us to estimate the initial wakeup values, the initial morning rise (also known as the cortisol awakening response or CAR), the initial decline after the morning rise peak, and then the slope of the decline over the rest of the day.
Due to its skewed distribution, cortisol was natural log transformed for all analyses. In addition, in order to account for the within subject correlation created by the 18 repeated salivary cortisol samples per subject, the piecewise linear regression was estimated by a 2-level hierarchical linear model in which the intercepts and slopes of the early and late decline were allowed to vary randomly by person. Specifically, the slopes of the early and late decline were modeled as random while the slope of the morning was modeled fixed. Alternative specifications for the random components yielded similar results. Day of sampling was accounted for using fixed effects.
In order to capture possible nonlinear associations, each neighborhood characteristic was divided into three categories (i.e., low, medium, high), based on tertiles and investigated separately. We tested for linear trends across tertiles by including tertile levels as a continuous covariate in regression models. All models adjust for individual-level factors including age, gender, self-reported race/ethnicity, education, income, and wealth. As a sensitivity analysis, we also estimated a second set of models that included additional covariates (i.e., BMI, physical activity, smoking, and diabetes) which could confound and/or mediate any neighborhood differences. All covariates were included as main effects and in interactions with slopes.
RESULTS
Selected sample descriptive by neighborhood conditions are shown in Table 2. Each row displays the proportion of residents within each neighborhood tertile that possesses the specified characteristic. Residents of neighborhoods with higher levels of poverty, higher violence, higher disorder, or lower cohesion were more likely to be in the lower income and educational categories, more likely to smoke, be obese, and have diabetes than residents of neighborhoods with less poverty, less violence, less disorder, and more cohesion. More disadvantaged neighborhoods were disproportionately composed of racial/ethnic minorities (though the patterning for Hispanics is less pronounced than for blacks). Lack of physical activity was associated with high neighborhood poverty and with low social cohesion but not with neighborhood violence or disorder. The correlations between neighborhood characteristics were high and absolute values ranged from 0.66 (positive correlation for proportion poor vs. violence) to 0.91 (negative correlation for disorder vs. social cohesion).
Table 2.
Selected characteristics of MESA stress study participants within each tertile of neighborhood characteristics
| Neighborhood Characteristics | Income <16K (%) | White (%) | Black (%) | Hisp (%) | No High School Education (%) | Diabetes (%) | Currently Smoke (%) | No Physical Activity (%) | Obese (%) |
|---|---|---|---|---|---|---|---|---|---|
| CENSUS BASED | |||||||||
| % Poor | |||||||||
| Tertile 1:Low | 26.53 | 45.9 | 12.6 | 41.5 | 15.3 | 10.2 | 6.1 | 17.9 | 26.9 |
| Tertile 2:Medium | 55.22 | 9.43 | 31.7 | 58.9 | 33.0 | 21.9 | 13.5 | 25.1 | 41.8 |
| Tertile 3:High | 61.79 | 4.32 | 37.9 | 57.8 | 33.2 | 19.9 | 12.6 | 29.8 | 41.5 |
| P for trend | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| COMMUNITY BASED | |||||||||
| Social Cohesion | |||||||||
| Tertile 1:Low | 61.42 | 2.3 | 42.3 | 55.4 | 35.2 | 25.1 | 13.1 | 27.4 | 43.8 |
| Tertile 2:Medium | 49.82 | 15.4 | 30.0 | 54.6 | 28.2 | 16.5 | 11.7 | 27.2 | 38.1 |
| Tertile 3:High | 34.67 | 41.2 | 10.6 | 48.9 | 18.3 | 11.7 | 6.9 | 17.7 | 28.1 |
| P for trend | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 |
| Violence | |||||||||
| Tertile 1:Low | 40.22 | 35.4 | 8.9 | 55.7 | 22.9 | 13.3 | 8.9 | 22.4 | 28.4 |
| Tertile 2:Medium | 50.37 | 21.6 | 25.8 | 52.6 | 31.0 | 19.4 | 7.8 | 25.1 | 41.8 |
| Tertile 3:High | 54.91 | 2.55 | 47.6 | 49.8 | 27.6 | 20.4 | 14.9 | 24.8 | 39.6 |
| P for trend | 0.00 | 0.00 | 0.00 | 0.28 | 0.01 | 0.03 | 0.04 | 0.35 | 0.01 |
| Disorder | |||||||||
| Tertile 1:Low | 33.82 | 43.0 | 10.7 | 46.3 | 17.3 | 11.4 | 7.4 | 18.6 | 27.2 |
| Tertile 2:Medium | 52.06 | 15.0 | 30.3 | 54.7 | 30.7 | 18.4 | 10.9 | 30.1 | 40.1 |
| Tertile 3:High | 59.64 | 1.45 | 44.9 | 53.6 | 31.5 | 22.5 | 14.5 | 24.0 | 40.6 |
| P for trend | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.07 | 0.00 |
Probability value for the trend is based on logistic regression of selected characteristics against neighborhood tertile ranks (i.e., 1, 2, 3) specified as a continuous measure.
Table 3 shows estimates of selected features of the cortisol profile including wake up levels, slopes for the morning rise (from wakeup to 30 minutes post wakeup), initial decline (from 30 to 120 minutes post wakeup), and late decline (after 120 minutes post wakeup) by categories of each neighborhood attribute. These estimates were derived from spline models adjusted for only age and gender. Trends indicated significantly lower levels of cortisol at wakeup and a less steep first decline for adverse neighborhood environments (higher poverty, violence, disorder, and lower social cohesion). There was no strong pattern for morning rise or late decline.
Table 3.
Selected characteristics of daily cortisol values by tertiles of neighborhood characteristics from spline models adjusted for age and gender1
| Neighborhood Characteritstics | Wake up level (nmol/L) | Morning Rise (nmol/L/hr) | First Decline (nmol/L/hr) | Late Decline (nmol/L/hr) |
|---|---|---|---|---|
| CENSUS BASED | ||||
| % Poor | ||||
| Tertile 1:Low | 14.08 | 15.83 | −9.21 | −0.12 |
| Tertile 2:Medium | 13.56 | 15.40 | −8.11 | −0.19 |
| Tertile 3:High | 12.21 | 14.42 | −6.97 | −0.16 |
| P for trend | 0.003 | 0.321 | 0.000 | 0.171 |
| COMMUNITY BASED | ||||
| Social Cohesion | ||||
| Tertile 1:Low | 13.17 | 15.04 | −7.31 | −0.20 |
| Tertile 2:Medium | 12.84 | 15.45 | −7.43 | −0.21 |
| Tertile 3:High | 15.26 | 16.10 | −9.37 | −0.18 |
| P for trend | 0.003 | 0.486 | 0.000 | 0.616 |
| Violence | ||||
| Tertile 1:Low | 15.59 | 15.56 | −9.25 | −0.17 |
| Tertile 2:Medium | 12.89 | 15.55 | −7.31 | −0.21 |
| Tertile 3:High | 12.81 | 14.93 | −6.84 | −0.20 |
| P for trend | 0.000 | 0.699 | 0.000 | 0.509 |
| Disorder | ||||
| Tertile 1:Low | 15.29 | 16.63 | −9.35 | −0.19 |
| Tertile 2:Medium | 13.41 | 15.37 | −7.59 | −0.20 |
| Tertile 3:High | 12.59 | 15.76 | −7.04 | −0.20 |
| P for trend | 0.000 | 0.562 | 0.000 | 0.673 |
Estimates derived from regression models of cortisol with knots at 30 and 120 minutes post wakeup
Probability value for the trend is based on regression models of cortisol level against neighborhood tertile ranks (i.e., 1, 2, 3) specified as a continuous measure.
Standard errors were adjusted for multiple observations of respondents.
Differences in selected aspects of the cortisol curve associated with neighborhood characteristics adjusted for age, gender, race/ethnicity, income, wealth, and education are shown in Table 4. Since cortisol was log-transformed for modeling, associations are presented as percent differences in the cortisol profile feature for each category compared to the reference category (the most advantaged neighborhood tertile). Positive values at wakeup reflect higher cortisol levels. Positive values for the morning rise represent steeper incline while positive values for the two periods of decline represent a more gradual decline.
Table 4.
Adjusted percent differences in wake-up cortisol, morning rise, and early and late decline by tertiles of neighborhood characteristics (updated)
| Percent differences at wakeup | Percent differences in morning rise (wakeup-30 minutes post wakeup) | Percent differences in decline (between 30 to 120 minutes post wakeup) | Percent differences in late decline (120 minutes post wakeup to bedtime) | |||||
|---|---|---|---|---|---|---|---|---|
| Neighborhood Characteristic | Percent diff. | 95% CI | Percent diff. | 95% CI | Percent diff. | 95% CI | Percent diff. | 95% CI |
| Census Based | ||||||||
| Percent Poor | ||||||||
| Tertile 1:Low | ref. | ref. | ref. | Ref. | ||||
| Tertile 2:Medium | 8.65 | [−0.62, 18.78] | −3.71 | [−18.93, 14.36] | 2.41 | [−4.32, 9.62] | 0.03 | [−0.84, 0.90] |
| Tertile 3:High | −3.04 | [−11.91,6.72] | 3.57 | [−12.92, 23.17] | 7.44 | [0.15, 15.26] | 0.49 | [−0.47, 1.46] |
| P for trend | 0.356 | 0.625 | 0.042 | 0.292 | ||||
| Community Survey Based | ||||||||
| Social Cohesion | ||||||||
| Tertile 1:Low | −9.14 | [−18.05, 0.73] | 7.21 | [−11.00, 29.14] | 3.59 | [−4.06,11.84] | 0.98 | [−0.04, 2.01] |
| Tertile 2:Medium | −10.30 | [−18.27, −1.57] | 2.76 | [−12.956, 21.31] | 5.22 | [−1.58, 12.48] | 0.59 | [−0.40,1.60] |
| Tertile 3:High | ref. | ref. | ref. | Ref. | ||||
| P for trend | 0.085 | 0.466 | 0.403 | 0.062 | ||||
| Violence | ||||||||
| Tertile 1:Low | ref | ref | ref | Ref | ||||
| Tertile 2:Medium | −14.50 | [−21.82, −6.52] | 8.77 | [−8.02, 28.62] | 9.16 | [2.09, 16.72] | −0.08 | [−1.02, 0.86] |
| Tertile 3:High | −14.25 | [−22.96, −4.55] | 14.55 | [−6.01, 39.58] | 8.03 | [−0.17, 16.89] | 0.79 | [−0.29, 1.88] |
| P for trend | 0.006 | 0.178 | 0.061 | 0.144 | ||||
| Disorder | ||||||||
| Tertile 1:Low | ref | ref | ref | ref | ||||
| Tertile 2:Medium | −8.27 | [−16.90, 1.25] | 3.88 | [−12.35, 23.02] | 5.62 | [−1.42, 13.17] | 0.02 | [−1.00, 1.05] |
| Tertile 3:High | −10.46 | [−19.20, −0.77] | 14.29 | [−4.59, 37.27] | 3.99 | [−3.92, 12.57] | 0.71 | [−0.31, 1.74] |
| P for trend | 0.040 | 0.141 | 0.378 | 0.152 | ||||
Derived from 2-Level models of log cortisol with random intercept, random early and late decline.
All models adjusted for gender, age, race/ethnicity, (white, black, Hispanic), income, wealth, and education.
Table coefficients, dervived from exponentiated model estimates, represent percent differences in cortisol profile compared to the reference category of neighborhood tertiles (most advantaged tertile). Positive values for the morning rise reflect steeper incline while positive values for the two periods of decline represent a more gradual decline.
Probability value for the trend is based on regression of log cortisol against the neighborhood tertile ranks (i.e., 1, 2, 3) specified as a continuous measure.
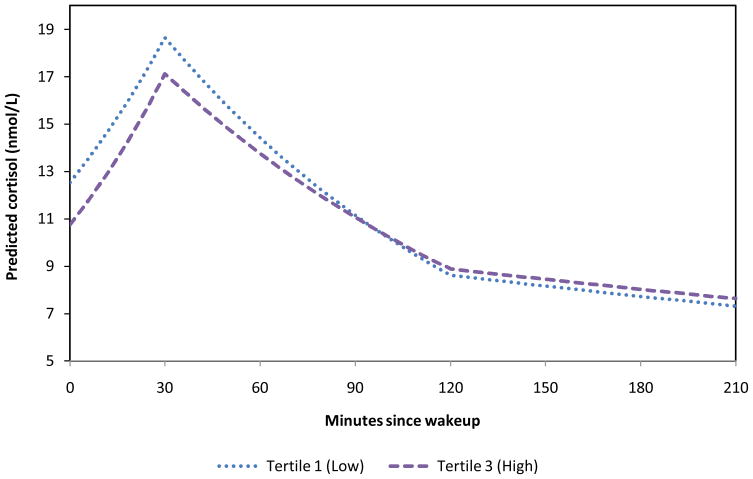
The strongest relationship between neighborhood characteristics and cortisol levels was found for neighborhood violence. Though associations were not always statistically significant, the general pattern was suggestive of lower wakeup values of cortisol, more pronounced increases after wakeup, and a more gradual decline during the rest of the day for neighborhoods with higher violence compared to neighborhoods with lower violence (P-values for trend 0.01, 0.18, 0.06 and 0.14 for wake-up, morning rise, early decline, and late decline, respectively). Individuals residing in higher violence neighborhoods had statistically significant lower levels of cortisol at wakeup (approximately 14% lower) with less pronounced initial declines (approximately 8% to 9% less pronounced) than those residing in low violence neighborhoods. However, a clear dose response was not observed and differences in initial decline were only statistically significant for the middle violence tertile (although the point estimate for the highest tertile showed a similar direction of association). To help illustrate the pattering of cortisol profile by level of neighborhood violence, Figure 1 shows the predicted daily levels of cortisol for low and high tertiles of neighborhood violence.
Figure 1.
Predicted daily values of cortisol by tertiles of neighborhood violence
Neighborhood poverty, social cohesion, and disorder were less consistently associated with cortisol. Being in the highest tertile of neighborhood poverty was associated with a 7% less steep initial decline compare to the lowest poverty tertile and the trend across poverty tertiles in the initial decline was statistically significant. Lower social cohesion and higher disorder were associated with lower cortisol levels at wake with differences in the order of 8–10%. A statistically significant trend in wake up values across tertiles was observed for disorder but not for social cohesion (only the middle tertile differed significantly from the highest social cohesion tertile although the direction of the association was similar for the lowest tertile).
We also estimated models that additionally adjusted for BMI, diabetes, smoking, and physical activity as previously defined (results not shown). The point estimates remained relatively unchanged, indicating that the behavioral and health variables included did not significantly confound or mediate the relationship between neighborhood and cortisol patterns.
In addition, we examined the overall model fit with and without the neighborhood predictors. The likelihood ratio tests revealed that the inclusion of neighborhood poverty or violence significantly improved the model fit (P-values=0.009, 0.002, respectively), compared to the base model which did not include any neighborhood parameters. The inclusion of neighborhood social cohesion or disorder, however, did not result in a significantly improved model (P-values=0.11, 0.13, respectively).
DISCUSSION
While the link between neighborhoods and health has been well established, few studies have explored the possible biological mechanisms through which neighborhood context affects health. This study used a population-based sample to examine the association between various aspects of the cortisol circadian rhythm and neighborhood context. Overall, we found suggestive, albeit limited, evidence linking neighborhood conditions to cortisol. Greater neighborhood violence (often hypothesized to be an important neighborhood stressor) tended to be associated with lower cortisol values at wakeup and a slower decline in cortisol over the earlier part of the day. Although some similar patterns (in terms of associations with wake up levels and early decline) were observed for the other neighborhood measures (neighborhood poverty, social cohesion, and disorder) associations were weaker, less consistent and less often statistically significant.
In contrast to the paucity of studies investigating neighborhood factors in relation to cortisol profiles, several studies have investigated the relationship between individual socioeconomic status and cortisol. Although results have not always been consistent, one finding that appears to be emerging in studies that model different features of the diurnal cortisol profile using multiple repeated measures is a flatter decline over the day in the lower compared to the highest SES groups (Cohen et al., 2006a; Ranjit et al., 2005a). Other studies have also found lower wake-up values and sharper increases in the cortisol awakening response in lower compared to higher SES groups (Kunz-Ebrecht et al., 2004; Steptoe et al., 2003; Wright and Steptoe, 2005) but this has not been replicated in all studies (Cohen et al., 2006b; Steptoe et al., 2003; Lupien et al., 2001). Stress challenge studies have also found that lower education is associated with a stronger cortisol response to a social stressor (Fiocco et al., 2007). Consistent with our results for neighborhood violence, prior analyses of MESA data have also observed lower levels of wake-up cortisol and less steep decline during the early part of the day in lower socioeconomic groups (Hajat et al., 2010). In addition, Hajat and colleagues (Hajat et al., 2010) also found that these patterns remained after adjustment for health behaviors.
Because of residential segregation by individual SES, the observed patterns in cortisol profile by neighborhood characteristics may be attributable to compositional differences. Indeed, our analyses showed that associations of neighborhood characteristics with cortisol were substantially reduced after addition of race/ethnicity and socioeconomic controls (as illustrated by the comparisons of Tables 3 and 4). However, we found that the general pattern of lower wake up, and slower decline associated with exposure to higher levels of neighborhood violence was present even after adjusting for individual-level SES including education, income, and wealth. The magnitudes of these associations were comparable to those found for individual SES. For example, Hajat and colleagues (2010) found that those in the lowest SES category had 16% to 18% lower levels of cortisol at wakeup and 11% to 12% less steep early decline, compared to those in the highest SES category. Dowd et al. (In press) found lower education (<12 years of school) to be associated with approximately 29% lower levels of cortisol at wakeup, compared to higher education (12+ years of school). In other MESA analyses, higher levels of hostility were also found to be associated with a less steep initial decline (Ranjit et al., 2009). Replication of these results in other samples is needed before any conclusions can be drawn, but the similar patterns observed for potential individual and neighborhood psychosocial stressors suggest that this pattern may represent a response to a variety of different kinds of stressors.
Although exposure to violence is often hypothesized to operate as a neighborhood stressor, empirical examinations of this relationship are rare. One study recently reported alterations in cortisol rhythms in a small sample of children who developed post traumatic stress disorder as a consequence of exposure to community violence (Suglia et al., 2010). To our knowledge, no large population study has investigated the association of community violence with cortisol levels of residents. Our relatively stronger findings for neighborhood violence compared to other neighborhood characteristics support the proposition that contexts which are uncertain, unpredictable, or threatening are the most likely to elicit a cortisol response (Mason 1968; Dickerson and Kemeny 2004). The more general measures of higher disorder and neighborhood poverty may not be tapping into these particular domains. More specific measurements of relevant neighborhood stressors may be necessary to detect neighborhood effects on stress pathways. Social cohesion may also not be tapping into stress-eliciting features of neighborhoods. Social cohesion represents more a positive dimension of neighborhood context rather than a negative stressor, per se. The absence of positive social and emotive determinants may not the same as the presence of negative and potentially harmful stimuli. The null findings for social cohesion are consistent with results from previous research that found negative experiences were more strongly linked to cortisol than positive states (Adam et al., 2006). Studies with larger sample sizes are needed to test whether social cohesion buffers the cortisol effects of other stressors.
An important strength of our study is the direct assessment of potentially stressful neighborhood conditions such as violence, disorder and cohesion, using standardized instruments and aggregation across multiple respondents. This allowed us to characterize these dimensions for buffers of approximately one mile around each participant’s home. Although this is an improvement over the use of crude census proxies for arbitrary census areas, it still has important limitations. For example, there is undoubtedly measurement error in informant reports (although this is somewhat reduced through the averaging process). In addition, the relevant geographic area is unknown and may be misspecified in our analyses. Both of these factors could result in biases towards the null.
To our knowledge, this is the first observational study that has used a population-based sample of adults with multiple repeat measures of cortisol over several days to examine the relationship between cortisol level and neighborhood characteristics. However, despite its comparably large sample size, the data is limited for subgroup analyses (e.g., race stratified models) and identification of complex patterns; capturing complex cortisol response patterns may require a larger sample and/or more repeat measures. For example, denser sampling later in the day may be necessary to fully capture differences in the late decline. Moreover, although few if any studies have data over a three day period, even three days may be insufficient to capture cortisol patterns due to chronic exposures. These measurement limitations may have resulted in less precise point estimates. In addition, our neighborhood measures were assessed at a single point-in-time, and therefore may not adequately capture long-term neighborhood context, resulting in attenuation of associations of neighborhood characteristics with health (Do, 2009).
We found that the magnitude and direction of the associations generally remained unchanged after adjustment for body mass index, diabetes, exercise levels and smoking (factors previously found to be associated with cortisol) (Clow et al., 2004; Hansen et al., 2008; Oltmanns et al., 2006) which suggests that these factors do not significantly confound or mediate the relationship between neighborhood stressors and cortisol. However, our ability to investigate this fully was limited by sample size and by the measures available. In addition to the known difficulties in measuring health behaviors, our measurements of physical activity and smoking reflect habitual activities rather than daily activities on the days in which samples were collected. Consequently, our adjustments did not account for the timing of these activities which have been found to affect cortisol levels when executed within an hour of sampling.
A major challenge in research on the health consequences of alterations in the circadian rhythm of cortisol is identifying which alterations are the most relevant to health. It is plausible that various types of alterations involving both elevated and reduced levels and various patterns reflecting altered responsivity are etiologically important. For example, both a very small and a very large awakening response have been linked to adverse health outcomes (Adam and Kumari, 2009; Chida and Steptoe, 2009). A slower rate of decline in cortisol across the day has been found to be associated with chronic and acute stress (Adam et al., 2006), with hostility (Ranjit et al., 2009) with coronary calcification (Matthews et al., 2006), with obesity (Ranjit et al., 2005b) and with increased mortality from breast cancer (Sephton et al., 2000). However, due in part to differences in modeling approaches used, not all studies linking cortisol levels to health have investigated the same parameters or have identified consistent patterns (Saxbe, 2009), and the implications of different patterns for health have yet to be determined.
Our results provide suggestive, albeit only weak evidence congruent with the hypothesis that neighborhood stressors may affect the pattern of cortisol secretion over the course of the day, specifically with lower wake up values and a less pronounced decline during the rest of the day. Identifying associations of distal neighborhood factors with stress biomarkers is rendered difficult not only due to measurement issues (for both exposures and outcomes) but also because of the many intermediaries involved. Although inconclusive, our analyses suggest that further investigation of the effects of neighborhood contexts on stress biomarkers is warranted.
Acknowledgments
Financial Support: This research was supported in part by the Michigan Center for Integrative Approaches to Health Disparities (P60MD002249) funded by the National Center on Minority Health and Health Disparities and by R01 HL76831. Additional support for D. Phuong Do was provided by the Kellogg Health Scholars Program (grant P0117943). MESA was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI). NHLBI had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Author contributions: DPD developed the research questions, drafted the paper, and conducted the analyses; ADR assisted with developing the research questions and writing the paper and supervised the data collection and analysis; AH assisted with analyses and paper writing; other coauthors provided comments on successive drafts and are listed in alphabetical order.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience--cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Science USA. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Aneshensel CS, Sucoff CA. The neighborhood context of adolescent mental health. J Journal of Health and Social Behavior. 1996;37:293–310. [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Boardman JD, Finch BK, Ellison CG, Williams DR, Jackson JS. Neighborhood disadvantage, stress, and drug use among adults. Journal of Health and Social Behavior. 2001;42:151–165. [PubMed] [Google Scholar]
- Brindley DN, Rolland Y. Possible connections between stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clinical Science (Lond) 1989;77:453–461. doi: 10.1042/cs0770453. [DOI] [PubMed] [Google Scholar]
- Browning CR, Cagney KA. Neighborhood structural disadvantage, collective efficacy, and self-rated physical health in an urban setting. Journal of Health and Social Behavior. 2002;43:383–399. [PubMed] [Google Scholar]
- Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Journal of Health Psychology. 2006;25:704–714. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosomatic medicine. 2006a;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic medicine. 2006b;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Dekker MJ, Koper JW, van Aken MO, Pols HA, Hofman A, de Jong FH, Kirschbaum C, Witteman JC, Lamberts SW, Tiemeier H. Salivary cortisol is related to atherosclerosis of carotid arteries. Journal of Clinical Endocrinology & Metabolism. 2008;93:3741–3747. doi: 10.1210/jc.2008-0496. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV. Residential environments and cardiovascular risk. Journal of Urban Health. 2003;80:569–589. doi: 10.1093/jurban/jtg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do DP. The dynamics of income and neighborhood context for population health: do long-term measures of socioeconomic status explain more of the black/white health disparity than single-point-in-time measures? Social Science & Medicine. 2009;68:1368–1375. doi: 10.1016/j.socscimed.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Nalini R, Do DP, Young EA, House JS, Kaplan GA. Education and Levels of Salivary Cortisol Over the Day. Annals of Behavioral Medicine. doi: 10.1007/s12160-010-9224-2. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria S, Diez-Roux AV, Shea S, Borrell LN, Jackson S. Associations of neighborhood problems and neighborhood social cohesion with mental health and health behaviors: the Multi-Ethnic Study of Atherosclerosis. Health Place. 2008;14:853–865. doi: 10.1016/j.healthplace.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Eller N, Netterstrom B, Hansen AM. Cortisol in urine and saliva: relations to the intima media thickness, IMT. Atherosclerosis. 2001;159:175–185. doi: 10.1016/s0021-9150(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Fiocco AJ, Joober R, Lupien SJ. Education modulates cortisol reactivity to the Trier Social Stress Test in middle-aged adults. Psychoneuroendocrinology. 2007;32:1158–63. doi: 10.1016/j.psyneuen.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Frank L, Engelke P, Schmid T. Health and community design: the impact of the built environment on physicial activity. Island Press; Washington, DC: 2003. [Google Scholar]
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- Hajat A, Roux AD, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: A review. Scandinavian Journal of Clinical and Laboratory Investigation. 2008;68:448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Mayer-Davis EJ, Addy CL, Pate RR, Durstine JL, Stolarczyk LM, Ainsworth BE. Moderate-intensity physical activity and fasting insulin levels in women: the Cross-Cultural Activity Participation Study. Diabetes Care. 2000;23:449–454. doi: 10.2337/diacare.23.4.449. [DOI] [PubMed] [Google Scholar]
- Kapuku GL, Treiber FA, Davis HC. Relationships among socioeconomic status, stress induced changes in cortisol, and blood pressure in African American males. Annals of Behavioral Medicine. 2002;24:320–325. doi: 10.1207/S15324796ABM2404_08. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Kennedy BP, Glass R. Social capital and self-rated health: a contextual analysis. American Journal of Public Health. 1999;89:1187–1193. doi: 10.2105/ajph.89.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Steptoe A. Work stress, socioeconomic status and neuroendocrine activation over the working day. Social Science & Medicine. 2004;58:1523–1530. doi: 10.1016/S0277-9536(03)00347-2. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Mason JW. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosomatic Medicine. 1968;30:567–607. [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosomatic medicine. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- McEwen B, Lasley EN. Allostatic load: when protection gives way to damage. Advances in Mind-Body Medicine. 2003;19:28–33. [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Moore LV, Diez Roux AV, Nettleton JA, Jacobs DR, Franco M. Fast-food consumption, diet quality, and neighborhood exposure to fast food: the multi-ethnic study of atherosclerosis. American Journal of Epidemiology. 2009;170:29–36. doi: 10.1093/aje/kwp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. American Journal of Epidemiology. 2007;165:858–867. doi: 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Dodt B, Schultes B, Raspe HH, Schweiger U, Born J, Fehm HL, Peters A. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. European Journal of Endocrinology. 2006;154:325–331. doi: 10.1530/eje.1.02074. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. Journal of Epidemiology and Community Health. 2001;55:111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Diez Roux AV, Sanchez B, Seeman T, Shea S, Shrager S, Watson K. Association of Salivary Cortisol Circadian Pattern with Cynical Hostility: The Multi-Ethnic Study of Atherosclerosis. Psychosomatic medicine. 2009;71:748–755. doi: 10.1097/PSY.0b013e3181ad23e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Kaplan GA. Material hardship alters the diurnal rhythm of salivary cortisol. International Journal of Epidemiology. 2005a;34:1138–1143. doi: 10.1093/ije/dyi120. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005b;30:615–624. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Robert S. Socioeconomic position and health: the independent contribution of community socioeconomic context 1. Annual Review of Sociology. 1999;25:489–516. [Google Scholar]
- Ross C, Mirowsky J. Disorder and decay: The concept and measurement of perceived neighborhood disorder. Urban Affairs Review. 1999;34:412. [Google Scholar]
- Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. Journal of Health and Social Behavior. 2001;42:258–276. [PubMed] [Google Scholar]
- Samspon RJ. The neighborhood context of well-being. Perspecitves in Biology and Medicine. 2003;46:S53–S64. [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Saxbe D. A field (researcher’s) guide to cortisol: tracking HPA axis functioning in everyday life. Health Psychology Review. 2009;2:163–190. [Google Scholar]
- Schulz A, Israel B, Williams D, Parker E, Becker A, James S. Social inequalities, stressors and self reported health status among African American and white women in the Detroit metropolitan area. Social Science & Medicine. 2000a;51:1639–1653. doi: 10.1016/s0277-9536(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Schulz A, Williams D, Israel B, Becker A, Parker E, James SA, Jackson J. Unfair treatment, neighborhood effects, and mental health in the Detroit metropolitan area. Journal of Health and Social Behavior. 2000b;41:314–332. [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, Marmot M. Socioeconomic status and stress-related biological responses over the working day. Psychosomatic medicine. 2003;65:461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Staudenmayer J, Cohen S, Wright RJ. Posttraumatic Stress Symptoms Related to Community Violence and Children’s Diurnal Cortisol Response in an Urban Community-Dwelling Sample. International Journal of Behavioral Medicine. 2010;17:43–50. doi: 10.1007/s12529-009-9044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Archives of General Psychiatry. 2009a;66:617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Kruijtzer BP, van Pelt J, van Dyck R, DeRijk RH, Hoogendijk WJ, Smit JH, Zitman FG, Penninx BW. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology. 2009b;34:1109–1120. doi: 10.1016/j.psyneuen.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–590. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evans R, Gold DR. Community violence and asthma morbidity: the Inner-City Asthma Study. American Journal of Public Health. 2004;94:625–632. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]