INTRODUCTION
Type 2 diabetes (T2D) is a complex disease that results from the contribution of many genes (1) and environmental factors (2), as well as interactions among genes and the environment. Among the known factors that increase risk are high BMI (3–7) and weight gain (5,8–12), both of which reflect a positive energy balance, and may themselves occur from underlying genetic factors. In contrast, high levels of physical activity, another component of the energy balance equation, is associated with lower incidence of T2D (13–17). A number of specific mechanisms, such as a reduced number of insulin receptors, high fat oxidation, and fat glucose substrate competition in skeletal muscle have been suggested as mechanisms behind the obesity/ T2D association (18). There are also known or hypothesized biological mechanisms by which physical activity may reduce the risk of T2D (18); skeletal muscle is the predominant site for insulin resistance (19), and exercise has been shown to improve insulin sensitivity in these tissues (18).
The peroxisome proliferator-activated receptor delta (PPARD) might be an important candidate gene for T2D and its effect in T2D risk might be modified by physical activity and obesity. Animal studies demonstrate PPARD activation exerts many favorable effects, including reducing weight gain, increasing skeletal muscle metabolic rate and endurance, improving insulin sensitivity (20) and may be involved in the muscle remodeling observed during endurance exercise (21). A role of the PPARD gene variants in mitochondrial function and thus in weight control has been suggested (22). PPARD appears to have a role in the regulation of fatty acid oxidation in several tissues including skeletal muscle and adipose tissue (23). It has been suggested that the mechanisms of action of this gene involve a redistribution of the non-esterified fatty acid (NEFA) flux. The increasing oxidative capability draws the NEFA to the muscle to be preferentially oxidized rather than stored in adipose tissue, thereby leading to a decrease in adipocyte size, enhanced lipolysis, and increased secretion of adiponectin (21). PPARD activation in the liver also appears to decrease hepatic glucose output, thereby contributing to improved glucose tolerance and insulin sensitivity (24). Genetic variation in the PPARD gene might also affect insulin sensitivity by modifying skeletal muscle glucose uptake (25).
The association between PPARD variation and the risk of T2D and related traits has been previously investigated. Although no association between variants of PPARD and T2D was observed in a Korean population, several positive associations between polymorphisms with fasting plasma glucose and BMI were found in non diabetic subjects(26). Other SNPs in the PPARD gene have been associated with insulin (25) and changes in body composition during a lifestyle intervention, including overall adiposity, hepatic fat storage and relative muscle mass (27). In addition, PPARD has been identified in a genome wide linkage analysis as a candidate for pre-diabetes phenotypes in response to exercise training (28). Genetic variation in the PPARD gene is reported to predict the conversion from impared glucose tolerance to T2D in the STOP-NIDDM trial during a 5 year follow up (29). PPARD gene variants had been associated with obesity in some (26,30) but not all studies (31–33).
Data suggests that genetic variation in the PPARD gene increases oxidative metabolism and affects physical endurance and obesity. Based on the proposed functionality of PPARD and their association with T2D-related traits we hypothesize that variation in the PPARD gene is associated with the risk of T2D. In addition we hypothesize that PPARD modified risk is mediated by exercise regimen and BMI. We comprehensively tested this hypothesis using data from a genome-wide association study (GWAS) conducted among middle age women living in Shanghai, China, involving 1,019 T2D cases and 1,709 controls.
Methods
Study Population
The details of the Shanghai Diabetes GWAS has been described elsewhere (34) Briefly, it included 886 incident type 2 diabetes (T2D) cases identified in the Shanghai Women’s Health Study (SWHS), an ongoing population-based cohort study of approximately 75,000 women. (35). Subjects of the SWHS were recruited between 1997 and 2000 and were between 40 and 70 years age at recruitment. In-person interviews, anthropometrics, and blood or buccal cell sample collection were carried out by trained interviewers. Study participants are being followed through biennial in-person surveys to collect information on survival status and occurrence of cancer, diabetes and other chronic diseases. A total of 901 women with self-reported diabetes since study enrollment met the following criteria and were included in the GWAS: 1) age ≤65, 2) on diabetes medication, 3) fasting glucose level >125 mg/dL at least twice and 4) donated a blood sample. After quality checking using the same method described previously for the GWAS of breast cancer, genotyping information was available for 886 subjects.
Included in the study are also 133 prevalent T2D cases identified from female controls of the Shanghai Breast Cancer GWAS. The latter study also contributed controls to this GWAS. Details of the Shanghai Breast Cancer GWAS, including subject recruitment, sample collection, processing, laboratory protocols, genotyping, and data cleaning procedures have been described elsewhere (36). Of the 1,938 controls included in the breast cancer GWAS that were genotyped with Affymetrix 6.0, 17 were on diabetes medication and 117 had a blood glucose level >125(mg/dL); these subjects were included as T2D cases in the current study. One of these T2D cases also participated in the SWHS. Thus, a total of 133 independent T2D cases identified from the SBCS controls were included in the case group of this study. After excluding women who had a blood glucose level between 100 and 125 mg/dL and had glycated hemoglobin (HbA1C)>6.1 (n=54) or had no HbA1C data (n=28), women who were younger than age 35 at the time of diabetes diagnosis (n=4), and women with a self-reported history of diabetes but had either no information on diabetes treatment or who had a glucose level <125 mg/dL in the current study (n=8) and one participant from the SBCS control group that developed breast cancer later on, 1,709 women remained as controls for the T2D GWAS.
Genotyping, Quality Control (QC), and Imputation
Genotyping was performed using the Affymetrix 6.0 array that includes 906,602 SNPs. The Birdseed v2 algorithm (http://www.broad.mit.edu/mpg/birdsuite/) was used to call genotypes. QC procedures included removal of SNPs with MAFs<0.01, Hardy-Weinberg P-values less than 0.00001, and samples with more than 5% missing genotypes. Three sets of SNPs on the Affymetrix SNP Array 6.0 were previously genotyped using different platforms including: 1) 669 SNPs genotyped for 1,035 subjects by using Affymetrix Target Genotyping System; 2) 17 SNPs genotyped for 1,091 subjects by Taqman; and 3) 251 SNPs genotyped for 108 subjects by Sequenom. These SNP sets served for cross-platform sample verification. The mean concordance rates were 99.5%, 98.5%, and 98.9% for Affymetrix Target Genotyping, Taqman, and Sequenom, respectively, when compared with the Affymetrix SNP Array 6.0. Additionally, we included one negative control (water) and three positive QC samples (NA15510, NA10851, and NA18505) purchased from the Coriell Cell Repositories (http://ccr.coriell.org/) in each of the 96-well plates genotyped to assess batch-to-batch validation. The average concordance rate between the QC samples was 99.8% with median value of 100%. Of the 26 PPARD gene variants, 18 were monomorphic, leaving 8 SNPs for this study.
Imputation
To provide complete coverage of PPARD we imputed genotypes from the HapMap reference genotypes. The program MACH (http://www.sph.umich.edu/csg/abecasis/MACH/) was used for genotype imputation to determine the probability distribution of missing genotypes conditional on a set of known haplotypes, while simultaneously estimating the fine-scale recombination map. Imputation was based on 570,441 autosomal SNPs genotyped in Stage I that passed the QC procedure, with the phased Asian data from HapMap Phase II (release 22) as the reference. Hapmap Phase II data were used as a reference since it contains a greater selection of SNPs. Only data with high imputation quality (RSQR >0.3 for MACH) were included in the current analysis. A total of 43 SNPs had RSQR>0.3. We excluded all SNPs with MAF<0.05 from the imputed SNPs for analysis. A total of 19 SNPs in PPARD that met these criteria were included in our analyses. After considering the successfully genotyped and high-quality imputed SNPs, we covered 91% of HapMap SNPs with MAF>0.05 in the gene region with an r-squared>=0.8.
Anthropometric measurements
Body weight and height were measured in the SWHS and SBCS using identical protocols. All measurements, including weight, height, and circumference of waist and hips, were taken during in-person interviews according to standard protocol by trained interviewers who were retired medical professionals. From these measurements, the following variables were created: BMI: weight in kg divided by the square of height in meters (kg/m2), WHR: waist circumference divided by hip circumference.
Physical Activity
Physical activity patterns were assessed during the in-person interviews. Regular exercise and sports participation were evaluated for the past 10 years in the SBC and for the past 5 years in the SWHS. For participants from the SWHS the assessment of physical activity was obtained using a validated questionnaire (37).
Data analyses
Demographic and lifestyle parameters were compared between cases controls using Mann Whitney rank sum tests or ANOVA, where appropriate. Chi-squared statistics were used to evaluate differences between cases and controls for categorical variables. Single-marker association analyses were carried out to evaluate their associations with T2D risk. Odds Ratios (ORs) and 95% confidence intervals (CI) were estimated using logistic regression models with adjustment for age and BMI. The association between genotype and T2D risk was evaluated based on an additive genetic model, indexing exposure to the minor allele of each SNP. Haplotypes for the 8 directly genotyped SNPs in PPARD were constructed using Powermarker software version 3.25. We used the sliding window method, using 2–3 SNP sliding windows and haplotype trend analysis (38).
Stratified analyses were performed to investigate any interaction between SNPs in PPARD and exercise participation and BMI categories. Tests for interactions were performed by comparing the model with and without interaction terms with a likelihood ratio test. All analyses were performed using SAS (version 9.1). All P values presented are based on two-tailed tests. P values presented in this paper were not corrected for multiple testing.
RESULTS
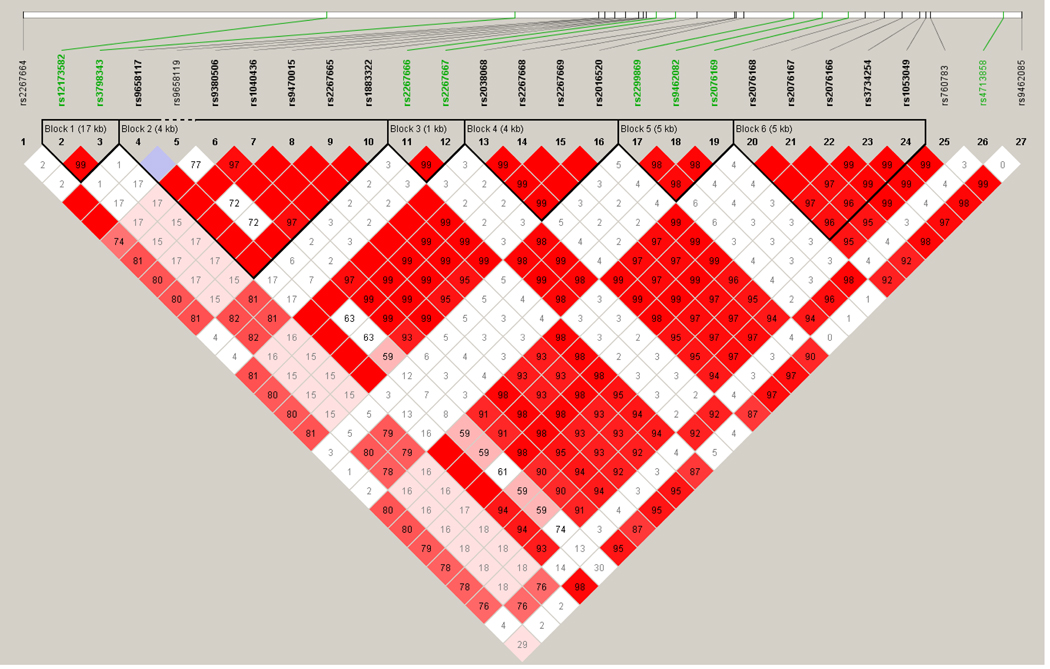
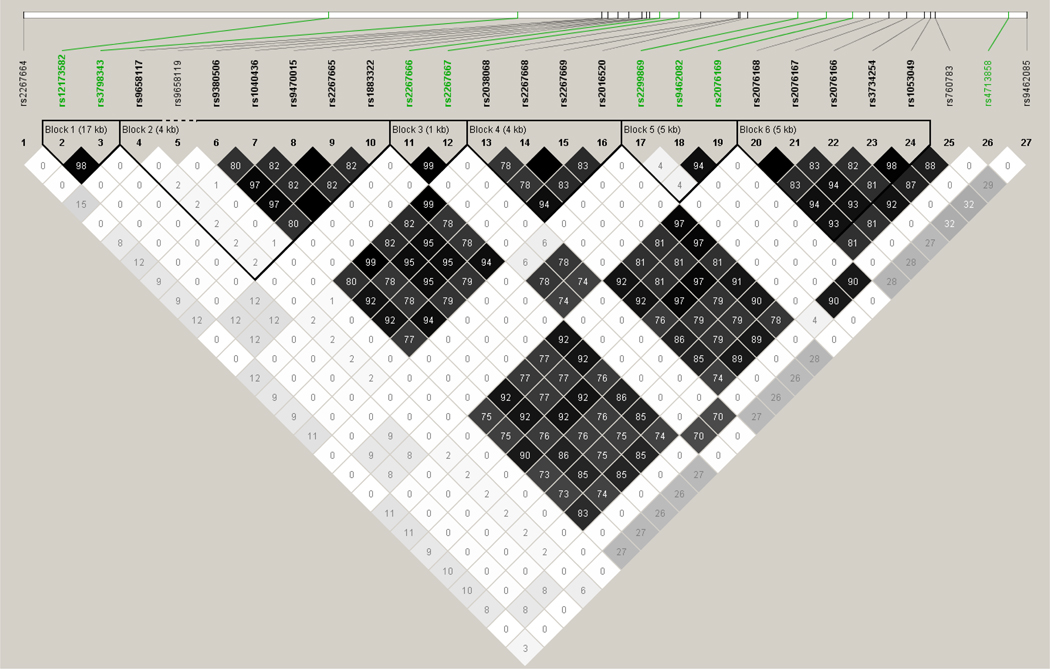
The linkage disequilibrium plots are shown in figures 1 and 2. The general characteristics of the participating study populations are presented in Table 1. Cases were older, had a higher BMI and WHR, were less likely to have consumed alcohol and more likely to exercise than controls. The reason why controls were younger is most likely because the controls come from a breast cancer case control study that had young participants.
Fig 1.
LD Plot for D’ PPARD gene (SNPS in green are the ones that were typed, those in black were imputed SNPs)
Fig 2.
LD Plot for R square PPARD gene (SNPS in green are the ones that were typed, those in black were imputed SNPs)
Table 1.
Characteristics of the study population
| All participants | Controls | Cases | P value | |
|---|---|---|---|---|
| Age (yrs) Median | 50.7 | 47.9 | 55.5 | <0.01 |
| BMI (kg/m2) Mean | 24.0 | 23.1 | 26.5 | <0.01 |
| WHR Mean | 0.82 | 0.80 | 0.84 | <0.01 |
| Current smoking (%) | 2.8 | 2.8 | 2.7 | 0.92 |
| Current drinking (%) | 4.3 | 5.4 | 2.1 | <0.01 |
| Exercise (%) | 31.0 | 29.4 | 33.8 | 0.02 |
| Education (%) | ||||
| None | 7.8 | 8.4 | 6.9 | <0.01 |
| Elementary | 20.9 | 24.4 | 15.2 | |
| High school | 61.1 | 57.0 | 68.1 | |
| Third level | 10.1 | 10.2 | 9.8 |
P-value for comparison of using Wilcoxon Two-sample Tests. For comparison of categorical variables a χ2-square test was used.
A total of eight directly and 31 imputed SNPs in PPARD were included in the analysis. None of the directly genotyped SNPs deviated from Hardy Weinberg equilibrium and none of them showed an association with T2D at P<0.05. Results from haplotype analysis of these SNPs were consistent with results from single SNP analysis; there were no significant associations with T2D risk (data not shown in tables). In analysis of subjects stratified by physical activity, (exercise participation yes/no), and BMI categories we did not observed any effect modification of these two factors on genotypes with T2D risk (Table 3).
Table 3.
Associations of directly typed 8 SNPS with T2D stratified by exercise participation and BMI categories
| SNP number |
Exercise participation | BMI categories | ||||
|---|---|---|---|---|---|---|
| No exercise | Exercise | P interaction | BMI<=25 | BMI>25 | P interaction | |
| rs12173582 | 1.08(0.91–1.29) | 1.18(0.94–1.48) | 0.58 | 1.19(0.99–1.44) | 1.00(0.83–1.21) | 0.85 |
| rs3798343 | 1.07(0.90–1.28) | 1.16(0.93–1.45) | 0.71 | 1.16(0.96–1.40) | 1.01(0.83–122) | 0.78 |
| rs2267666 | 1.01(0.85–1.21) | 0.96(0.75–1.22) | 0.89 | 0.99(0.81–1.21) | 0.98(0.81–1.20) | 0.49 |
| rs2267667 | 1.01(0.84–1.20) | 0.96(0.75–1.23) | 0.93 | 0.98(0.80–1.20) | 0.99(0.82–1.21) | 0.43 |
| rs2299869 | 1.06(0.84–1.33) | 1.19(0.88–1.62) | 0.63 | 1.01(0.78–1.31) | 1.15(0.89–1.49) | 0.17 |
| rs9462082 | 1.01(0.84–1.22) | 0.98(0.75–1.27) | 0.72 | 1.08(0.88–1.33) | 0.93(0.76–1.15) | 0.61 |
| rs2076169 | 1.01 (0.84–1.22) | 1.05(0.81–1.37) | 0.62 | 1.11(0.90–1.36) | 0.98(0.79–1.21) | 0.88 |
| rs4713858 | 1.00(0.83–1.20) | 0.97(0.75–1.26) | 0.82 | 1.07(0.87–1.32) | 0.94(0.77–1.16) | 0.69 |
Analysis stratified by exercise are adjusted for age and BMI, while analyses stratified by BMI are adjusted for age only
When we looked at associations between imputed SNPs and T2D we did not observe any main effects (Table 4). In analysis of subjects stratified by BMI categories we did not observed any effect modification of these two factors on genotypes with T2D risk (Table 4). We did not observe any association between imputed SNPs in analysis stratified by physical activity, (exercise participation yes/no), and/or a gene physical activity interaction (data nota shown in tables).
Table 4.
Association of imputed SNPs in PPARD gene with T2D in all participants and stratified by BMI *
| SNP numbers |
Quality | MAF | All participants | BMI<=25 | BMI>25 | P interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||||
| rs2267664 | 0.9933 | 0.316 | 1.11(0.97–1.28) | 0.13 | 1.18(0.98–1.43) | 0.08 | 1.01(0.83–1.22) | 0.92 | 0.77 |
| rs9658117 | 0.9237 | 0.069 | 0.86(0.62–1.18) | 0.35 | 0.76(0.50–1.16) | 0.20 | 1.04(0.65–1.66) | 0.88 | 0.65 |
| rs9658119 | 0.9991 | 0.06 | 0.68(0.31–1.48) | 0.33 | 1.75(0.34–9.08) | 0.50 | 0.44(0.16–1.21) | 0.11 | 0.72 |
| rs9380506 | 0.9778 | 0.25 | 1.01(0.87–1.17) | 0.94 | 0.92(0.75–1.13) | 0.43 | 1.09(0.89–1.35) | 0.40 | 0.13 |
| rs1040436 | 0.9976 | 0.281 | 0.99(0.86–1.15) | 0.95 | 0.99(0.81–1.21) | 0.94 | 0.99(0.81–1.20) | 0.92 | 0.35 |
| rs9470015 | 0.9971 | 0.245 | 1.01(0.87–1.67) | 0.94 | 1.09(0.89–1.34) | 0.41 | 0.94(0.76–1.16) | 0.55 | 0.13 |
| rs2267665 | 0.9986 | 0.245 | 1.01(0.87–1.17) | 0.94 | 1.09(0.89–1.34) | 0.41 | 0.94(0.76–1.16) | 0.56 | 0.13 |
| rs1883322 | 0.9995 | 0.281 | 0.99(0.86–1.15) | 0.95 | 0.99(0.81–1.21) | 0.94 | 0.99(0.81–1.20) | 0.93 | 0.35 |
| rs2038068 | 0.9993 | 0.281 | 1.00(0.87–1.14) | 0.97 | 0.99(0.81–1.21) | 0.93 | 0.99(0.82–1.21) | 0.96 | 0.37 |
| rs2267668 | 0.9987 | 0.237 | 0.99(0.86–1.16) | 0.95 | 0.92(0.75–1.13) | 0.43 | 1.06(0.86–1.32) | 0.56 | 0.15 |
| rs2267669 | 0.9987 | 0.237 | 0.99(0.86–1.16) | 0.95 | 0.92(0.75–1.13) | 0.43 | 1.06(0.86–1.32) | 0.56 | 0.15 |
| rs2016520 | 0.9976 | 0.271 | 0.98(0.85–1.13) | 0.76 | 0.98(0.80–1.20) | 0.84 | 0.97(0.80–1.19) | 0.79 | 0.47 |
| rs2076168 | 0.9972 | 0.269 | 0.98(0.85–1.13) | 0.79 | 0.98(0.80–1.20) | 0.87 | 0.99(0.81–1.20) | 0.89 | 0.51 |
| rs2076167 | 0.9968 | 0.269 | 0.98(0.85–1.13) | 0.79 | 0.98(0.80–1.20) | 0.87 | 0.99(0.81–1.20) | 0.89 | 0.51 |
| rs2076166 | 0.9921 | 0.235 | 1.01(0.87–1.17) | 0.92 | 1.10(0.89–1.35) | 0.38 | 0.95(0.77–1.17) | 0.63 | 0.14 |
| rs3734254 | 0.9829 | 0.269 | 0.97(0.84–1.13) | 0.73 | 0.99(0.81–1.22) | 0.95 | 0.98(0.80–1.19) | 0.81 | 0.35 |
| rs1053049 | 0.9839 | 0.27 | 0.97(0.84–1.13) | 0.72 | 0.99(0.81–1.22) | 0.96 | 0.97(0.80–1.19) | 0.81 | 0.35 |
| rs760783 | 0.9904 | 0.247 | 1.00(0.86–1.16) | 0.96 | 0.91(0.74–1.12) | 0.38 | 1.06(0.86–1.31) | 0.59 | 0.09 |
| rs9462085 | 0.9643 | 0.476 | 1.03(0.920–1.17) | 0.69 | 0.95(0.79–1.15) | 0.62 | 1.09(0.90–1.30) | 0.38 | 0.37 |
Adjusted for age
DISCUSSION
Common genetic variants in the PPARD gene were evaluated among participants of the Shanghai Diabetes GWAS. We also investigated the interaction between the genetic variants with two modifiable environmental risk factors of T2D (exercise participation and BMI) on T2D risk. We did not find a main gene effect for PPARD with T2D or an interaction between this gene with BMI or exercise participation and the risk of T2D.
There were several reasons why we choose to study associations between this candidate gene with T2D and interactions with BMI and exercise. PPARD appears to have a role in the regulation of fatty acid oxidation in several tissues including skeletal muscle and adipose tissue (23). PPARD activation reduces insulin resistance and adiposity in rodents and primates (39) and is implicated in the adaptative metabolic response of skeletal muscle to endurance exercise by controlling the number of oxidative myofibers (30). In an animal study, adipose tissue-specific overexpression of an activated form of PPARD resulted in a reduction of adipose tissue, an alteration that was shown to be protective against high fat feeding in an rats (40). Expression profiling studies in humans have shown an increase in PPARD expression following endurance exercise (41). PPARD activation in the liver also appears to decrease hepatic glucose output, thereby contributing to improved glucose tolerance and insulin sensitivity (24). Genetic variation in the PPARD gene might also affect insulin sensitivity by modifying skeletal muscle glucose uptake (25). There is also emerging evidence that PPARD plays a prominent role in mitochondrial activity (22).
Our results are consistent with a previous study of Koreans that sequenced the PPARD gene and found no association between variants and T2D (26) and another study of 7495 middle-age white people (32). However in the Korean study, PPARD variation was associated with fasting glucose in non diabetic subjects (26). PPARD variation has also been related with conversion from impaired fasting glucose to T2D in an intervention trial (the STOP-NIDDM) (29). Similarly PPARD variation has been associated with insulin resistance in the Korean study (26) but not in another study (32). PPARD gene variants have also been associated with obesity in some (26,30) but not all studies(31–33).
One polymorphism in PPARD, rs2016520, is believed to be functional. The minor allele of this SNPs was associated to higher fasting glucose in non diabetic Korean subjects (26). In a small study of 663 subjects conducted in Shanghai this polymorphism was associated with fasting glucose and insulin resistance in both normoglucose tolerant and diabetic Chinese subjects (42). However, no association between this polymorphism and T2D has been observed in a cross sectional study of 402 cases and 436 controls, similar to our results (31). An association between rs2016520 and BMI (43) has also been reported. Although this SNP was not directly genotyped in the present study, it was imputed with high accuracy (RSQR=0.995).
Three SNPs in the PPARD gene (rs6902123, rs1053049 and rs2076167) were significantly associated with whole body insulin sensitivity assessed by the euglycemic hyperinsulinemic clamp (25). In our population, rs1053049 and rs2076167 were not associated with T2D. Unfortunately, we could not assess the association between rs6902123 and T2D in our populations as this SNPs had a MAF<0.05 in this study and thus we could not include them in the analysis. Two of these SNPs, rs6902123, rs1053049, also influenced changes in body composition during a lifestyle intervention, including overall adiposity, hepatic fat storage and relative muscle mass (27). One of these SNPs, rs1053049, was associated with fasting glucose in the Korean study (26). In another study of 769 middle-age people the carriers of the rs6902123 variant were at an increase risk of conversion from IGT to T2D during a 5 year follow up (29).
Strengths of the current study include a relatively large study, and a good coverage of the genetic variation of PPARD. Furthermore, we had detailed information about exercise participation on these subjects, which allowed us to pursue investigations of gene-exercise interactions. A limitation of our study is that we performed a relatively large number of tests which may have increased the risk for Type 1 error and no replication has been done. However, the study is hypothesis driven and is based on results from other studies. Another possibility is that the study might not have had enough power to test interactions between PPARD variants and the environmental factors. We only had enough power to detect moderated sized interactions (for effect size of 1.5 or greater we had at least 80% power).
In summary, we did not find a main gene effect for PPARD with T2D or an interaction between this gene with BMI or exercise participation and the risk of T2D among middle age Chinese women.
Table 2.
Association of directly typed 8 SNPS with T2D as outcome *
| rs# | Test Allele |
Reference Allele |
Freq test allele |
Freq Ref allele |
OR(95%CI, Heterozygote) |
OR(95% CI, Homozygote) |
OR (95%CI, Additive model) |
P trend |
|---|---|---|---|---|---|---|---|---|
| rs12173582 | T | C | 0.316 | 0.684 | 1.17(0.97–1.42) | 1.15(0.84–1.58) | 1.11(0.97–1.27) | 0.13 |
| rs3798343 | C | C | 0.316 | 0.684 | 1.14(0.95–1.39) | 1.14(0.84–1.56) | 1.10(0.96–1.26) | 0.19 |
| rs2267666 | T | T | 0.281 | 0.719 | 1.01(0.83–1.22) | 0.96(0.69–1.36) | 0.99(0.86–1.14) | 0.91 |
| rs2267667 | G | C | 0.283 | 0.717 | 1.00(0.83–1.22) | 0.96(0.57–2.14) | 0.99(0.85–1.14) | 0.88 |
| rs2299869 | C | G | 0.142 | 0.858 | 1.13(0.91–1.39) | 1.11(0.59–2.10) | 1.11(0.92–1.33) | 0.28 |
| rs9462082 | G | A | 0.237 | 0.763 | 1.04(0.86–1.26) | 0.91(0.60–1.37) | 1.00(0.86–1.16) | 0.99 |
| rs2076169 | A | G | 0.231 | 0.769 | 1.05(0.87–1.27) | 1.00(0.66–1.51) | 1.03(0.88–1.20) | 0.72 |
| rs4713858 | G | A | 0.245 | 0.755 | 1.04(0.86–1.26) | 0.87(0.58–1.31) | 0.99(0.85–1.15) | 0.88 |
Adjusted for age and BMI
ACKNOWLEDGEMENTS
We thank the participants and research staff of the Shanghai Women’s Health Study and Shanghai Breast Cancer Study for their contributions to the study. This research was supported in part by the United States National Institutes of Health (NIH) grants KO1 DK082639, R01CA124558, R01CA64277, R37CA70867, R01CA90899 and R01CA100374, as well as Ingram professorship funds and research award funds to WZ, R01 CA118229, R01CA92585 from the NIH and a research grant from Allen Foundation Fund to XOS, the Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources (NCRR)/NIH to JL, R01CA122756 and Department of Defense Idea Award BC050791 to QC, and DK58845 and HG004399 to FBH. Sample preparation, SBCS/SWHS GWAS scanning, and SWHS/SMHS targeted genotyping (Replication II) were conducted at the Survey and Biospecimen Shared Resources and Vanderbilt Microarray Shared Resources that are supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA68485). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reference List
- 1.Hansen L, Pedersen O. Genetics of type 2 diabetes mellitus: status and perspectives. Diabetes Obes Metab. 2005 Mar;7(2):122–135. doi: 10.1111/j.1463-1326.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Schulze MB, Hu FB. Primary prevention of diabetes: what can be done and how much can be prevented? Annu Rev Public Health. 2005;26:445–467. doi: 10.1146/annurev.publhealth.26.021304.144532. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 4.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132(3):501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 6.Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34(10):1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 7.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol. 1997;145(7):614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 8.Wannamethee SG, Shaper AG. Weight change and duration of overweight and obesity in the incidence of type 2 diabetes. Diabetes Care. 1999;22(8):1266–1272. doi: 10.2337/diacare.22.8.1266. [DOI] [PubMed] [Google Scholar]
- 9.Oguma Y, Sesso HD, Paffenbarger RS, Jr., Lee IM. Weight change and risk of developing type 2 diabetes. Obes Res. 2005 May;13(5):945–951. doi: 10.1038/oby.2005.109. [DOI] [PubMed] [Google Scholar]
- 10.Holbrook TL, Barrett-Connor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes. 1989;13(5):723–729. [PubMed] [Google Scholar]
- 11.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997 Aug 1;146(3):214–222. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 12.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 14.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 15.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS., Jr Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325(3):147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 16.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130(2):89–96. doi: 10.7326/0003-4819-130-2-199901190-00002. [DOI] [PubMed] [Google Scholar]
- 17.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27(10):2518–2539. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 18.Spelberg A MJE. Towards prevention of non-insulin dependent diabetes mellitus. In: RDG L, editor. Causes of diabetes. Chichester: Jonh Wiley & Sons; 1993. pp. 319–345. [Google Scholar]
- 19.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 20.Reilly SM, Lee CH. PPAR delta as a therapeutic target in metabolic disease. FEBS Lett. 2008 Jan 9;582(1):26–31. doi: 10.1016/j.febslet.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredenrich A, Grimaldi PA. PPAR delta: an uncompletely known nuclear receptor. Diabetes Metab. 2005 Feb;31(1):23–27. doi: 10.1016/s1262-3636(07)70162-3. [DOI] [PubMed] [Google Scholar]
- 22.Stefan N, Thamer C, Staiger H, Machicao F, Machann J, Schick F, et al. Genetic variations in PPARD and PPARGC1A determine mitochondrial function and change in aerobic physical fitness and insulin sensitivity during lifestyle intervention. J Clin Endocrinol Metab. 2007 May;92(5):1827–1833. doi: 10.1210/jc.2006-1785. [DOI] [PubMed] [Google Scholar]
- 23.Fredenrich A, Grimaldi PA. Roles of peroxisome proliferator-activated receptor delta in skeletal muscle function and adaptation. Curr Opin Clin Nutr Metab Care. 2004 Jul;7(4):377–381. doi: 10.1097/01.mco.0000134370.93686.0a. [DOI] [PubMed] [Google Scholar]
- 24.Seedorf U, Aberle J. Emerging roles of PPARdelta in metabolism. Biochim Biophys Acta. 2007 Sep;1771(9):1125–1131. doi: 10.1016/j.bbalip.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Vanttinen M, Nuutila P, Kuulasmaa T, Pihlajamaki J, Hallsten K, Virtanen KA, et al. Single nucleotide polymorphisms in the peroxisome proliferator-activated receptor delta gene are associated with skeletal muscle glucose uptake. Diabetes. 2005 Dec;54(12):3587–3591. doi: 10.2337/diabetes.54.12.3587. [DOI] [PubMed] [Google Scholar]
- 26.Shin HD, Park BL, Kim LH, Jung HS, Cho YM, Moon MK, et al. Genetic polymorphisms in peroxisome proliferator-activated receptor delta associated with obesity. Diabetes. 2004 Mar;53(3):847–851. doi: 10.2337/diabetes.53.3.847. [DOI] [PubMed] [Google Scholar]
- 27.Thamer C, Machann J, Stefan N, Schafer SA, Machicao F, Staiger H, et al. Variations in PPARD determine the change in body composition during lifestyle intervention: a whole-body magnetic resonance study. J Clin Endocrinol Metab. 2008 Apr;93(4):1497–1500. doi: 10.1210/jc.2007-1209. [DOI] [PubMed] [Google Scholar]
- 28.An P, Teran-Garcia M, Rice T, Rankinen T, Weisnagel SJ, Bergman RN, et al. Genome-wide linkage scans for prediabetes phenotypes in response to 20 weeks of endurance exercise training in non-diabetic whites and blacks: the HERITAGE Family Study. Diabetologia. 2005 Jun;48(6):1142–1149. doi: 10.1007/s00125-005-1769-4. [DOI] [PubMed] [Google Scholar]
- 29.Andrulionyte L, Peltola P, Chiasson JL, Laakso M. Single nucleotide polymorphisms of PPARD in combination with the Gly482Ser substitution of PGC-1A and the Pro12Ala substitution of PPARG2 predict the conversion from impaired glucose tolerance to type 2 diabetes: the STOP-NIDDM trial. Diabetes. 2006 Jul;55(7):2148–2152. doi: 10.2337/db05-1629. [DOI] [PubMed] [Google Scholar]
- 30.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004 Oct;2(10):e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouni-Berthold I, Giannakidou E, Faust M, Berthold HK, Krone W. The peroxisome proliferator-activated receptor delta +294T/C polymorphism in relation to lipoprotein metabolism in patients with diabetes mellitus type 2 and in non-diabetic controls. Atherosclerosis. 2005 Dec;183(2):336–341. doi: 10.1016/j.atherosclerosis.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Grarup N, Albrechtsen A, Ek J, Borch-Johnsen K, Jorgensen T, Schmitz O, et al. Variation in the peroxisome proliferator-activated receptor delta gene in relation to common metabolic traits in 7,495 middle-aged white people. Diabetologia. 2007 Jun;50(6):1201–1208. doi: 10.1007/s00125-007-0668-2. [DOI] [PubMed] [Google Scholar]
- 33.Robitaille J, Gaudet D, Perusse L, Vohl MC. Features of the metabolic syndrome are modulated by an interaction between the peroxisome proliferator-activated receptor-delta - 87T>C polymorphism and dietary fat in French-Canadians. Int J Obes (Lond) 2006 Sep 5; doi: 10.1038/sj.ijo.0803450. [DOI] [PubMed] [Google Scholar]
- 34.Shu XO, Long J, Cai Q, Qi L, Xiang YB, Cho YS, et al. Identification of new genetic risk variants for type 2 diabetes. PLoS Genet. 2010;6(9) doi: 10.1371/journal.pgen.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, et al. The Shanghai Women's Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005 Dec 1;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 36.Zheng W, Long J, Gao YT, Li C, Zheng Y, Xiang YB, et al. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet. 2009 Mar;41(3):324–328. doi: 10.1038/ng.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthews CE, Shu XO, Yang G, Jin F, Ainsworth BE, Liu D, et al. Reproducibility and validity of the Shanghai Women's Health Study physical activity questionnaire. Am J Epidemiol. 2003;158(11):1114–1122. doi: 10.1093/aje/kwg255. [DOI] [PubMed] [Google Scholar]
- 38.Velez DR, Fortunato SJ, Williams SM, Menon R. Interleukin-6 (IL-6) and receptor (IL6-R) gene haplotypes associate with amniotic fluid protein concentrations in preterm birth. Hum Mol Genet. 2008 Jun 1;17(11):1619–1630. doi: 10.1093/hmg/ddn049. [DOI] [PubMed] [Google Scholar]
- 39.Grimaldi PA. Regulatory role of peroxisome proliferator-activated receptor delta (PPAR delta) in muscle metabolism. A new target for metabolic syndrome treatment? Biochimie. 2005 Jan;87(1):5–8. doi: 10.1016/j.biochi.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, et al. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003 Apr 18;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 41.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005 Sep;19(11):1498–1500. doi: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- 42.Hu C, Jia W, Fang Q, Zhang R, Wang C, Lu J, et al. Peroxisome proliferator-activated receptor (PPAR) delta genetic polymorphism and its association with insulin resistance index and fasting plasma glucose concentrations in Chinese subjects. Diabet Med. 2006 Dec;23(12):1307–1312. doi: 10.1111/j.1464-5491.2006.02001.x. [DOI] [PubMed] [Google Scholar]
- 43.Aberle J, Hopfer I, Beil FU, Seedorf U. Association of peroxisome proliferator-activated receptor delta +294T/C with body mass index and interaction with peroxisome proliferator-activated receptor alpha L162V. Int J Obes (Lond) 2006 Dec;30(12):1709–1713. doi: 10.1038/sj.ijo.0803345. [DOI] [PubMed] [Google Scholar]