Abstract
Administration of endothelial progenitor cells (EPC) is a promising therapy for post-infarction cardiac repair. However, the mechanisms that underlie apparent beneficial effects on myocardial remodeling are unclear. In a porcine model of acute myocardial infarction, we investigated the therapeutic effects of a mixed population of culture modified peripheral blood mononuclear cells (termed hereafter porcine EPC). Porcine EPC were isolated using methods identical to those previously adopted for harvest of EPC in human cell therapy studies. In addition the therapeutic effects of paracrine factors secreted by these cells was evaluated in vitro and in vivo. Intracoronary injection of autologous porcine EPC was associated with increased infarct territory mass and improved regional ventricular systolic function at 2 months compared to control. Treatment with conditioned media derived from autologous EPC was associated with similar improved effects on infarct territory mass and function. Histologic analysis of the infarct territory revealed significantly increased cardiomyocyte size in EPC and conditioned media treated groups, when compared to controls. A paracrine EPC effect was also verified in a pure myocardial preparation in which cardiomyocytes devoid of fibroblast, neuronal and vascular elements directly responded by increasing cell mass when exposed to the same conditioned media. Analysis of conditioned media revealed elevated levels of TGFβ1 (human 267.3±11.8 pg/ml, porcine 57.1±6.1 pg/ml), a recognized mediator of hypertrophic signaling in the heart. Neutralizing antibodies to TGFβ1 attenuated the pro-hypertrophic effect of conditioned media, and use of recombinant TGFβ1 added to fresh media replicated the pro-hypertrophic effects of conditioned media in vitro. These data demonstrate the potential of paracrine factors secreted from endothelial progenitor cells to induce cardiomyocyte hypertrophy contributing to increased infarct territory LV mass, with favorable medium term effects on regional function following myocardial infarction.
Introduction
In recent years, a number of human clinical trials have demonstrated improvement in indices of cardiac function following endothelial progenitor cell therapy in the setting of acute myocardial infarction [1–4]. The mechanism(s) of benefit of such therapy are likely to be complex. It is proposed that progenitor cells may regenerate myocardial mass by differentiating into cardiomyocytes [5,6], may enhance regional function by improving myocardial perfusion through vasculogenesis in the infarct territory [7–9], promote myocardial salvage by reducing apoptosis of injured cardiomyocytes [7,10] or recruit endogenous cardiac progenitor cells [11]. Recent studies have also highlighted a potential role for paracrine factors released by progenitor cells in mediating these beneficial effects [8,10,12]. To date however, no large animal study has examined the paracrine action of progenitor cells with a specific focus on in vivo remodeling following myocardial infarction.
We report here using a porcine model of acute myocardial infarction that therapy with a cell population we have termed porcine endothelial progenitor cells (EPC) resulted in significant infarct territory cardiomyocyte hypertrophy, an effect mediated by factors secreted by injected progenitor cells. Limited porcine CD antibodies and gene sequences (for RNA probes) are available for the analysis of the identity and degree of heterogeneity of cell populations cultured from the pig, but methods for isolating porcine EPC in this study were identical to those employed in previous human cell therapy trials [2].
Materials and Methods
This study was approved by and performed in accordance with guidelines of the Mayo Clinic Institutional Review Board and the Mayo Clinic Institutional Animal Care and Use Committee.
Derivation of endothelial progenitor cells (EPC)
Yorkshire pigs (25 to 35 kg; total of 35 pigs) were used for harvest of EPC, and for the in vivo model of myocardial infarction. EPC were also obtained from normal human volunteers. From both sources, mononuclear cells were harvested from peripheral buffy coat preparations in Ficoll-Paque Plus (15cc). Cells were then washed three times in MCDB 131 supplemented with hydrocortisone, antibiotics, and 10 ng/ml VEGF. Cells were subsequently re-suspended in X vivo-15 medium (BioWhittaker) supplemented with VEGF (1 ng/ml), and seeded on fibronectin-coated plates at a density of 4.9 × 103 cells per mm2 in keeping with previously described methodology [2].
To identify progenitor cells a series of fluorescence stains and antibody detection approaches were used. Briefly, adherent cells were incubated at 37°C with DiI-labeled acetylated low-density lipoprotein (AcLDL) for 1 hour and Alexa-Fluor 488 conjugated isolectin IB4 for 30 min (Molecular Probes). Fluorescent antibody cell sorting (FACS) was also performed to identify cell-surface and intracellular antigens in human and porcine endothelial progenitor cells. Primary antibodies to CD31, CD105, von Willebrand Factor, eNOS, Flk-1, and VE-cadherin (R&D Systems) were used with secondary antibody detection with FITC in each case. Isotype-matched IgG antibodies were used as controls, and the fluorescent intensity of stained cells was gated according to established methods[13]. Conditioned media from both human and pig EPC cultures were obtained after 48 hours of culture of cells in basal serum free media, centrifuged at 1000 rpm for 6 min, followed by passage through a 0.45 μm filter to remove cells and debris.
Induction of myocardial infarction
A closed-chest unconscious porcine model of acute myocardial infarction was utilized [14]. All animal received prophylactic amiodarone (100 mg daily) and aspirin (325 mg daily) beginning two weeks prior to induction of myocardial infarction that was continued throughout the study. Each animal also received lidocaine (60 mg IV bolus and infusion at 1.5 to 3.0 mg/min), unfractionated heparin (10,000 units IV) and benzathine penicillin (300,000 IM) at procedure initiation. Following anesthesia, mechanical ventilation with a Harvard apparatus was maintained on a FiO2 of 40%. Under sterile conditions, a carotid arterial cut down was performed for placement of a 9 Fr arterial sheath. Fifty ml of blood was drawn into EDTA tubes for progenitor cell isolation and culture, as already described. Through an 8 Fr coronary artery guide catheter, a slightly oversized angioplasty balloon catheter (1.1:1 balloon to lumen ratio) was positioned in the middle portion of the left circumflex coronary artery and inflated to produce arterial occlusion for 90 min. Complete arterial occlusion was verified with angiographic contrast every 15 to 30 min. After the procedure the arterial sheath was removed, followed by animal recovery and habitation.
In vivo EPC infusion
Forty-eight hours after induction of myocardial infarction autologous progenitor cells were harvested from culture. Cells were detached using 0.5 mM EDTA. All cells were washed twice in PBS and resuspended in X vivo-10 basal medium (BioWhittaker) at a final concentration of 3 × 106 viable cells per ml. Animals were anesthetized, and a marginally oversized over-the-wire angioplasty balloon (1.1:1 balloon to lumen ratio) was placed at the same angiographic position used for induction of infarction and inflated. Following confirmation of complete arterial occlusion with angiographic contrast, a 3 to 4 ml aliquot of the autologous EPC cell suspension was infused via the balloon catheter (n = 9 animals). Control animals received same sized aliquots of 0.9% normal saline (n = 9 animals). The balloon was left inflated for 4 min to prevent backflow of cells and to produce stop-flow beyond the site of inflation, and then deflated. This sequence of events was repeated two more times in the same manner with a 4-minute period between occlusions for a total infusion of 10–12 ml of cell suspension or control solution. For animals receiving cell therapy infusions, a total of 3 × 107 EPC were given to each animal.
In vivo conditioned media infusion
Conditioned media was prepared and isolated from porcine EPC cultures. Pigs were anesthetized 48 hours after myocardial infarction. A marginally oversized over-the-wire angioplasty balloon was inflated at the same angiographic location utilized for induction of infarction. Following confirmation of complete arterial occlusion with angiographic contrast, a 4ml aliquot of EPC-derived conditioned media was infused via the balloon catheter (n = 9 animals). Control animals received same sized aliquots of 0.9% saline instead of the conditioned media. The balloon remained inflated for 4 minutes after which it was deflated. This sequence of balloon inflation and infusion of therapy was repeated two more times for a total infusion of approximately 12ml in each case. Subsequent experiments compared the effect of therapy with CM (n = 4) versus basal medium (n = 4) following myocardial infarction, using identical dosing and delivery strategies to those described above.
Magnetic resonance imaging (MRI)
Cardiac imaging with MRI was performed at two time points; forty-eight hours after infarction but prior to therapy, and at 8 weeks post-therapy. A 1.5 Tesla MRI system (Signa Horizon 9.0, GE Medical Systems) with gradient strength of 22mT/m was used, and a phased array surface coil was employed for each study. All images were ECG gated and acquired during suspended respiration. For measurement of ventricular volume and mass, short-axis gradient echo cine images prescribed over the entire ventricle were used with the following imaging parameters: slice thickness = 7 mm, gap = 0 mm, field of view = 20 cm, matrix = 256 × 128, flip angle = 20 degrees, NEX = 1 (FASTCARD, GE Medical Systems,). Echo time (TE) was selected as minimum allowable (range, 6.7 to 7.8 msec). In this pulse sequence, repetition time (TR) ranged from 11.5 to 12.6 msec. The number of cardiac phases acquired per slice was 20. For measurement of infarct size, injection of 0.2 mmol/kg of a gadolinium-based contrast agent (Magnevist, Berlex Laboratories) followed 10 minutes later by an inversion recovery sequence was performed. The imaging plane and scan locations were copied from the short-axis cine prescription to facilitate matching of the delayed enhancement and cine images. Scanning with multiple inversion times was performed with selection of the inversion time that produced best nulling of the myocardial signal. Imaging parameters for the delayed enhancement images were slice thickness = 7 mm, gap = 0 mm, field of view = 20 cm, matrix = 256 × 128, flip angle = 20 degrees, NEX = 1. TE was selected as minimum allowable (6.8 msec). In this pulse sequence, TR was 14.2 for all studies. Two independent observers who were blinded to treatment assignment performed measurements from MRI images for assessment of ventricular volume, mass, and infarct size using a commercially available workstation (Advantage Windows 4.2, GE Medical Systems, Milwaukee, WI) and commercially available software (Mass Analysis Plus, MEDIS Medical Imaging Systems). Regional analysis was performed by division of short-axis segments into 17 segments as recommended by the American Heart Association[15]. The lateral and inferolateral segments were grouped together as the infarct-related territory (IRT) of the left ventricle for regional mass analysis.
Histologic assessment of cardiomyocyte size
Cardiomyocyte size among pigs in each experimental group was evaluated from frozen sections of the infarctrelated territory, using established morphometric methodology[16]. Slides were incubated with anti-laminin antibody (Sigma L8271) and anti-α sarcomeric actin (Sigma A2172) and subsequently labeled with Alexa Fluor 488 (Molecular Probes) and goat anti-mouse IgM Rhodamine-conjugated antibody (Chemicon AP128R). Cell nuclei were stained with DAPI. At least 3 distinct sections from the infarct-related territory in each animal, and at least 5000 cardiomyocytes were studied per animal in each treatment group. Cell size was quantified by measuring cell surface area with laser confocal microscopy (LSM 410 Carl Zeiss) and a 60× (1.3 NA) objective. Cell number per high power field was assessed by counting DAPI stained nuclei per field (at least 100 fields per animal). Two-dimensional confocal images were acquired by scanning 512 × 512 pixels per image and processed with Zeiss KS400 3.0 software [17,18].
Analysis of factors contained within conditioned medium
Conditioned medium from both pig and human EPC cultures was harvested as described above for in vivo CM therapy. Three candidate factors with known cardiotrophic activity were chosen for further analysis by ELIZA: insulinlike growth factor 1 [19] (Quantikine, DG100), TGFβ1 [20,21] (Quantikine, DB100B), and basic fibroblast growth factor [22,23] (Quantikine, DFB50).
In vitro cardiomyocyte size assay
Hearts were removed from 1 to 2-day old rats (Harlan Sprague-Dawley, Indianapolis, IN), and cardiomyocytes were isolated and cultured as previously described [17]. In line with a pure cardiomyocyte preparation these cells showed no evidence of neural or vascular cell contamination. Cardiomyocytes were incubated for 48 hours at 37°C, 5% CO2 with the following solutions; conditioned media obtained from porcine and human EPC cultures, fresh media (X vivo-15 with 1 ng/ml VEGF) containing TGFβ1 added to achieve the same concentration as that found in conditioned media (Sigma T5050), and conditioned media containing a 100-fold excess of neutralizing antibody to TGFβ1 (Sigma T9429). Cardiomyocytes incubated with fresh media alone served as negative controls. Positive controls were obtained by use of the inductor phenylephrine (100 μM), an α-adrenoreceptor agonist in the presence of 10 μM propanolol, a β-adrenoreceptor antagonist [17,18]. Cardiomyocytes were fixed with 3% paraformaldehyde at 48 hours post treatment. Slides were incubated with α-sarcomeric actin antibody (Sigma A2172) and labeled with Alexa fluor 488 (Molecular Probes), followed by washing with PBS + 0.2% Triton. Cell size was quantified by measuring cell surface area with laser confocal microscopy (LSM 410 Carl Zeiss) and a 60× (1.3 NA) objective. Two-dimensional confocal images were acquired by scanning 512 × 512 pixels per image and processed with Zeiss KS400 3.0 software [17,18].
Data analysis
All data are expressed as mean ± standard error (SE). In all cases a minimum of three independent in vitro experiments were performed. Statistical analysis for paired and unpaired analyses was performed by Student's t-test. Comparison of mean change in endpoints between multiple treatment groups and controls was by ANOVA. Statistical significance was set a priori at p < 0.05.
Results
Endothelial progenitor cell phenotype
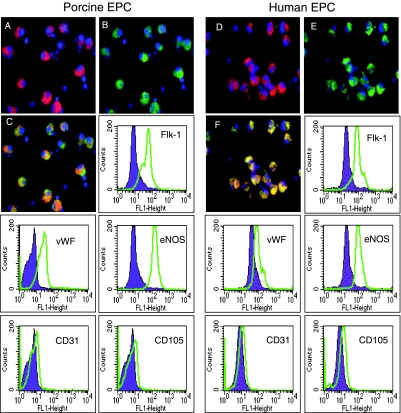
Following 48 hours of culture, both porcine and human EPC demonstrated uptake of both Ac-LDL and isolectin IB4 (Figure 1).These cells also expressed typical endothelial proteins including von Willebrand Factor, eNOS, Flk-1, and VE-cadherin (Figure 1).Autologous porcine EPC were used for in vivo experiments, and both human and porcine EPC were used in subsequent in vitro studies.
FIG. 1.
Immunostaining and fluorescent-activated cell sorting (FACS) analysis of porcine (left) and human (right) endothelial progenitor cells (EPC). A and D, Isolectin IB4 staining, B and E, Dil-labeled acetylated low density lipoprotein staining; C and F, Combined Isolectin IB4 and Dil-labeled acetylated low density lipoprotein immunostaining. FACS analysis shows cell expression of Flk-1, vWF, eNOS, CD31 and CD105 antigens (open histograms) in porcine and human EPC. Filled histograms represent the isotype matched control antibody.
In vivo autologous EPC and conditioned media therapy studies
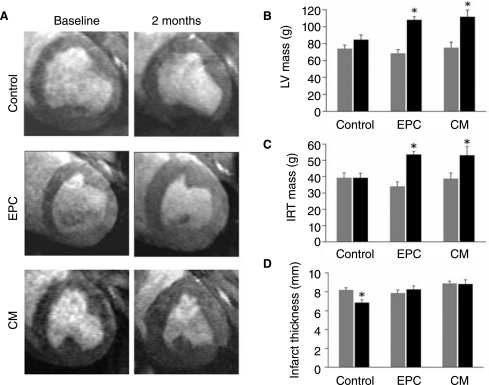
Baseline characteristics of pigs assigned to EPC treatment or control therapy were similar, with no significant differences in baseline body weight, infarct size, ejection fraction, global left ventricular and regional mass, or left ventricular chamber size (ANOVA; p > 0.05 for each comparison) (Table 1).Following therapy the most striking change observed was a significant increase in global and infarct territory LV mass in EPC and CM treated pigs (Figure 2).These changes were significant both within individual EPC and CM groups (comparing baseline to 2 month estimates of LV mass) and when change in LV mass was compared by ANOVA across the 3 experimental groups (Table 2).Improvement in LV ejection fraction was observed within each group when baseline values were compared to 2 month follow up, but comparison of mean change in LV ejection fraction between groups showed no significant difference (Table 2).A similar pattern was observed for cardiac output and stroke volume; significant improvement within each group was observed, but no significant difference was found when this change was compared between groups (Table 2).There was no significant change in end-systolic volume from baseline to 2 months post treatment in individual groups. Control and EPC treated pigs exhibited significant increase in end-diastolic volume (EDV) from baseline to 2 months post treatment but CM treated pigs did not (Table 2).
Table 1.
Structural and Functional Cardiac Indices at Baseline (Post-Infarction and Pretreatment) for All Groups
| Control | EPC | CM | p | |
|---|---|---|---|---|
| Left ventricular mass (g) | 74.1 ±4.2 | 68.5±4.4 | 75.3±6.4 | ns |
| Infarct-related territory mass (g) | 39.4±2.9 | 34.0±3.0 | 38.9±3.5 | ns |
| Ejection fraction (%) | 50.3±5.1 | 49.6±6.4 | 49.7±4.5 | ns |
| Infarct size (g) | 12.5±1.2 | 6.0±1.9 | 13.4±3.0 | ns |
| Infarct size (% of LV mass) | 16.6±1.7 | 8.7±2.7 | 17.6±3.2 | ns |
| End-diastolic volume (ml) | 58.6±3.1 | 55.3±4.3 | 46.1±4.7 | ns |
| End-systolic volume (ml) | 34.4±4.8 | 29.8±5.7 | 23.6±3.6 | ns |
| Cardiac output (l/min) | 2.6±0.3 | 2.3±0.2 | 2.1±0.3 | ns |
| Stroke volume (ml) | 28.7±2.3 | 25.5±2.2 | 22.5±2.6 | ns |
| Infarct wall thickness (mm) | 8.19±0.25 | 7.87±0.32 | 8.90±0.22 | ns |
| Infarct wall thickening (mm) | 39.9±4.1 | 41.9±4.2 | 33.2±3.7 | ns |
| Infarct wall motion (mm) | 4.03±0.34 | 4.52±0.32 | 3.71±0.25 | ns |
| Pig body mass (kg) | 31.2±0.9 | 31.7±0.9 | 28.9±1.4 | ns |
p value denotes comparison of mean baseline values in the control group to mean baseline values in the EPC and CM treated groups using ANOVA. ns = not significant.
FIG. 2.
Changes in myocardial mass before and 2 months post-therapy. A, representative short axis MRI images (end-diastole) pre- and post-therapy in each treatment group. B and C, Changes in global and infarct related territory (IRT) LV mass pre- and post-therapy in each treatment group. D, Changes in infarct thickness pre- and post-therapy in each treatment group. Histograms: Gray bars indicated pre-therapy values, black bars indicate post-therapy values. *represents p < 0.05 for comparison of baseline to 2 month post-therapy values. EPC indicates endothelial progenitor cells and CM conditioned media.
Table 2.
Comparison of Mean Changes in Global Cardiac Size and Function (From Baseline to 2 Months Post Therapy) in all Treatment Groups
| Control Mean ± SE | EPC Mean ± SE | CM Mean ± SE | p value | |
|---|---|---|---|---|
| LV mass (g) | 10.4±5.5 | 39.5±4.6* | 36.4±8.4* | <0.05 |
| IRT mass (g) | 0.0±4.0 | 19.6±3.2* | 14.3±3.8* | <0.05 |
| EF (%) | 7.4±2.9* | 16.9±6.3* | 10.2±4.8* | ns |
| CO (L/min) | 1.2±0.3* | 2.1±0.3* | 0.7±0.3* | ns |
| SV (ml) | 28.7±2.3* | 25.5±2.2* | 22.5±2.6* | ns |
| EDV (ml) | 15.1±5.0* | 18.2±4.8* | 6.4±3.2 | ns |
| ESV (ml) | 2.7±3.2 | −2.5±5.8 | −2.8±3.1 | ns |
p value denotes ANOVA comparison of each treatment group change to control change. *denotes p < 0.05 for students t-test comparison of change from baseline to 2 months post therapy within individual groups.
Regional changes in LV mass, infarct size, wall motion, and wall thickening
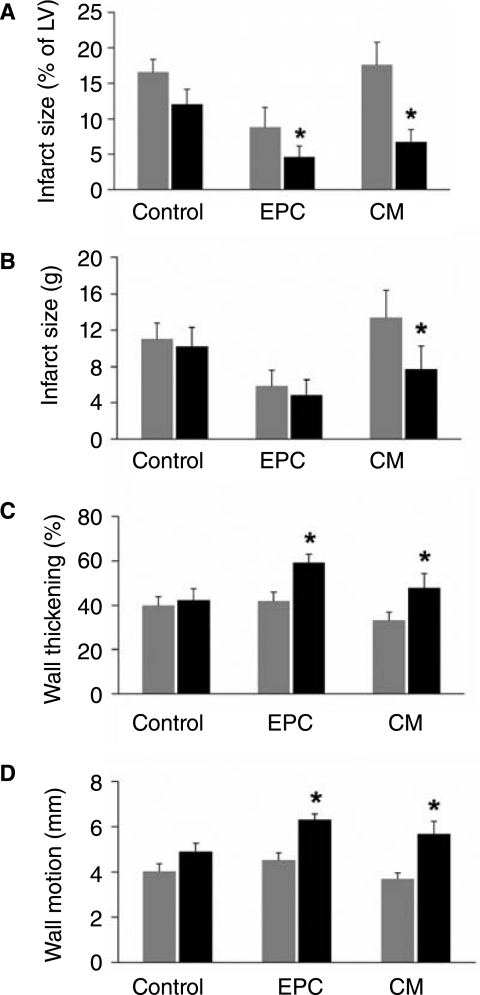
Subsequent analysis of the infarct related territory (IRT) revealed significant increase in mass in both the EPC and CM treated groups (Figure 2)(p < 0.05). Again, these changes were significant when compared by ANOVA to control change (Table 2)(p < 0.05). Control pigs exhibited significant thinning of the infarct wall from baseline to 2 months, but such thinning was not observed in EPC and CM treated pigs (Figure 2)(p < 0.05 for all changes). Infarct size (expressed as a percentage of LV mass) decreased significantly in both EPC and CM treated pigs (Figure 3).When assessed by absolute weight in grams, decrease in infarct size was significant only for CM treated pigs (Figure 3)(p < 0.05). A small but non-significant improvement in infarct wall motion and wall thickening was observed in control pigs, but a greater and statistically significant improvement was observed among EPC and CM treated pigs (Figure 3)(p < 0.05).
FIG. 3.
Changes in infarct-related territory structure and function assessed by MRI in each treatment group from baseline to 2 months post-therapy. A, Change in infarct size expressed as a percentage of LV mass. B, Change in infarct size expressed as mass in grams. C, Change in infarct wall thickening expressed as percentage change from enddiastolic wall thickness to end-systolic wall thickness. D, Change in infarct wall motion during systole expressed in millimeters. Histograms: Gray bars indicate pre-treatment values, black bars indicate post-treatment values. *represents p < 0.05 for comparison of baseline to 2 month values.
Histologic assessment of cardiomyocyte size
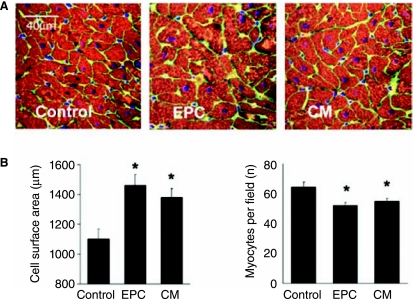
To confirm that cardiomyocyte hypertrophy contributed to the striking changes in IRT cardiac mass in cell and conditioned media treated animals we studied 2 histologic indices of hypertrophy at 2 months post-therapy (Figure 4).Cardiomyocyte size in the IRT was significantly greater in EPC and CM treated pigs versus controls (Figure 4); mean cell surface area in control pigs was 1105 μm2, compared with 1462 μm2 in EPC and 1383 μm2 in CM treated animals (ANOVA; p < 0.01 for each comparison versus control). In addition, cardiomyocyte number per high power field was 64.4 in control pigs compared to 52.1 in EPC and 55.2 in CM treated pigs (ANOVA; p < 0.01 for each comparison versus control). Further in vivo experiments compared the effect of therapy with CM versus basal medium following myocardial infarction (n = 4 for both groups). Cardiomyocyte size in the IRT was significantly greater in the CM group when compared to the basal medium group (relative increase in cell surface area 1.55 ± 0.21, p < 0.001). Of note, PCNA staining of sections from the infarct border zone did not demonstrate evidence of significant cardiomyocyte proliferation at 8 weeks post-therapy in active treatment or control groups (data not shown).
FIG. 4.
Histologic assessment of infarct border zone cardiomyoctye size. A, representative images of border zone sections stained for α-sarcomeric actin (red), nuclei (blue) and laminin (green). B, Quantitative assessment of cardiomyocyte size using cell surface area and cell density (nuclei per high power field). *represents p < 0.05 for comparison to control values.
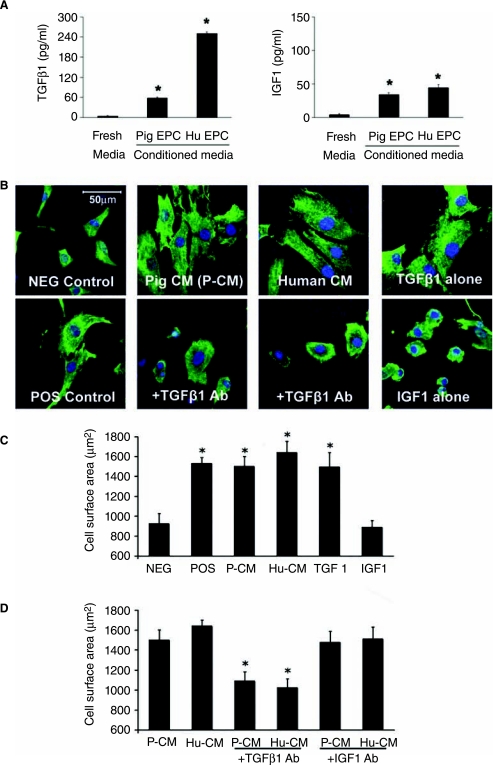
Analysis of conditioned media
Conditioned media from both human and porcine progenitor cell culture contained active TGFβ1 in physiologically relevant concentrations (human 267.3 pg/ml, pig 57.1 pg/ml). Insulin-like growth factor 1 (IGF-1) was also detected in conditioned media (human 44.3 pg/ml, pig 33.9 pg/ml). Fibroblast growth factor (FGF) was not detected in either human or porcine conditioned medium.
In vitro cardiomyocyte size assay
Cardiomyocytes demonstrated marked increases in in vitro cell mass after 48 hours incubation with conditioned media derived from EPC cultures (n = 6 separate experiments for each group) (Figure 5).A significant effect on cardiomyocytes was seen with conditioned medium regardless of human or porcine origin (Figure 5).The relative increase in cell surface area was comparable for all experimental groups with a range in relative increase from 44% to 73% over control studies (p < 0.05 for all experimental vs. control comparisons - Figure 5).The cell mass effect of 1 ml conditioned media (1:2 dilution with basal media) from each cell culture was comparable to that induced by 10 μM phenylephrine, an accepted positive control for induction of in vitro increase in cardiomyocyte cell mass. These changes in cardiomyocyte size were reproduced using recombinant TGFβ1 added to fresh media in similar concentrations to those found in conditioned media (50 and 250 pg/ml). Furthermore, the addition of 100-fold molar excess concentration of neutralizing antibody to conditioned media from both human and porcine EPC attenuated the pro-hypertrophic action, which was previously observed.
FIG. 5.
Analysis of conditioned media and the effects of secreted factors on cardiomyocyte hypertrophy in vitro. A, Quantitative ELISA of TGFβ1 and IGF1 concentration in progenitor cell-derived conditioned media. B, Representative images of cardiomyocytes stained with actin (green) for various control and experimental groups. C, Histogram showing increase in cell surface area for cardiomyocytes exposed to conditioned media (CM) from porcine and human endothelial progenitor cell cultures versus negative and positive controls, and recapitulation of pro-hypertrophic effect by addition of recombinant TGFβ1 to fresh media. There was no such effect mediated by IGF1 treatment. D, Attenuation of the pro-hypertrophic effect of human and porcine CM by neutralizing antibody to TGFβ1 but not by neutralizing antibody to IGF1. *indicates p < 0.05 for experimental versus negative control comparisons.
Discussion
Recently, emerging cell therapy strategies have sought to reverse post-infarction left ventricular dysfunction by direct transplantation of stem or progenitor cells. Enthusiasm for this therapeutic approach has been bolstered by a number of promising early phase human clinical trials [1–3,24,25], but the precise mechanism(s) underlying the apparent beneficial effects have yet to be fully elucidated.
Progenitor cells have been variously hypothesized to regenerate myocardium by transdifferentiation and/or by coaxing non-cardiac or resident progenitor cells to a cardiac muscle fate, thus augmenting recovery of myocardial mass and retarding pathologic LV remodelling [5–7,11,26–28]. Progenitor cells may also harbor beneficial paracrine or neurohormonal activity which thus far has been poorly characterized. In earlier studies, circulating progenitor cells cultured under conditions similar to the present investigation were shown to secrete various cytokines with potential cardiotrophic and neoangiogenic effects, including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), granulocyte colony-stimulating growth factor (G-CSF), granulocyte-macrophage colony-stimulating growth factor (GM-CSF), stromal derived factor 1, and insulin like growth factor 1(IGF-1) [11,29]. Soluble factors released by endothelial progenitor cells are known to augment migration of native endothelial cells and cardiac resident progenitor cells in vitro [11]. Moreover, treatment with conditioned media derived from mesenchymal cells overexpressing the survival gene Akt1 demonstrated reduction in apoptosis of adult ventricular cardiomyocytes exposed to hypoxia and subsequent infarct size in an rodent acute myocardial infarction model [10].
The major findings of the current study were: (1) progenitor cell therapy altered post-infarction remodeling by increasing infarct territory mass, associated with improved regional systolic function (2) therapy with conditioned media alone (derived from progenitor cells) altered infarctrelated remodeling in vivo in a similar fashion to cell therapy (3) significant increases in cardiomyocyte size in vivo were observed in the treated territory in both cell and conditioned media treated animals (4) a cardiotrophic effect of progenitor cell-derived conditioned media was confirmed in vitro, an effect that could in part be attributed to TGFβ1 secreted from both human and porcine CPC studied.
Our studies suggest that induction of cardiac hypertrophy in the infarct border zone may be a heretofore unrecognized mechanism contributing to progenitor cell therapy-mediated cardiac repair. Post-infarction cardiac hypertrophy is initially adaptive and usually develops in myocardial regions remote from the acute infarction. In contrast, we have observed cardiac hypertrophy occurring not only on serial measures of global LV mass but specifically including the infarct border zone. Importantly, these effects occurred as a result of a non-sustained stimulus in the early aftermath of acute infarction. Whether this intriguing pattern of postinfarction hypertrophy that includes the infarct territory is associated with more favorable long term repair is still unclear. It is intriguing to speculate whether early paracrine factor release in the infarct zone kick starts a program of cardiomyocyte hypertrophy.
Over the past decade there has been a growing appreciation of the role of vascular endothelium in regulating cell and organ size [30,31]. Given the capacity for injected progenitor cells to induce significant neovascularisation [27,29,32], it is interesting to consider the possibility that interplay between secreted proangiogenic growth factors (such as VEGF) and cardiotrophic factors (such as TGF 1 and IGF1) may also orchestrate progenitor cell-induced cardiac hypertrophy. It is conceivable that such an interplay may contribute to durable cardiac repair as disruption of coordinated cardiac hypertrophy and angiogenesis has been shown to mediate an eventual transition from compensated hypertrophy to heart failure [33].
TGFβ1 has recently been shown to induce hypertrophic responses in cultured neonatal rat cardiomyocytes via PKC-dependent ATF-2 activation [20]. Upregulation of TGFβ1 occurs in myocardium that has undergone hypertrophy as a result of pressure overload [34–36] and following exposure to a hypertrophic agonist such as angiotensin II [21,36,37]. Furthermore, in a rodent model of myocardial infarction upregulation of TGFβ1, its receptors TβR1 and TβR2, and the downstream signal transducer TGF-β-activated kinase has been detected in remote myocardial territories [38]. Activation of this pathway paralleled the transcriptional upregulation of cardiac markers for ventricular hypertrophy, linking TGFβ1 signaling to the development of compensatory post-infarction myocardial hypertrophy [38]. A multifaceted role for progenitor cell-derived TGFβ1 in cardiac repair might also include cardioprotection during ischemia-reperfusion [39] and prosurvival signaling to endothelial cells during angiogenesis [40].
Finally, a deleterious role for myocardial TGFβ1 signaling during post-infarction ventricular remodeling should also be considered. Using a mouse model of coronary ligation, Okada et al have demonstrated that inhibition of circulating TGFβ1 through adenoviral-mediated overexpression of the soluble TGFβ1 type 2 receptor attenuates post-MI fibrosis and infarct wall thinning, improving post-infarct contractile function and mortality [46, 47]. Timing of therapy to modulate TGFβ1 signaling in this setting may be of critical importance, as previous studies have demonstrated that antagonism of TGFβ1 at the time of infarction may attenuate late remodeling [48], contrasting the findings of Okada et al where treatment was initiated at 3 days post-infarct [46, 47]. Further studies will be required to fully elucidate the complex role of this cytokine in post-MI regeneration and repair.
Limitations
The use of progenitor cell nomenclature is challenging in this porcine model given that most of the accepted human antibodies used to discriminate human EPC populations such as CD133 and CD34 do no work in the pig. However, we have used a cell processing and culture protocol which was identical to that used in the human TOPCARE AMI trial which the current study attempted to model. Moreover the porcine MNC-derived cells in culture shared many antigens found in human EPCs such as Flk1, eNOS, CD105 as well as positivity for AcLDL and Isolectin B4. However, it must also be acknowledged that the progenitor cells used in this study were a heterogenous preparation and therefore the specific lineage of the progenitor cells contributing IGF1 and TGFβ secretion is still unclear.
Progenitor cell therapy in this animal model recapitulates the findings of previous human studies demonstrating preservation of infarct thickness, reduction in infarct size and improved regional systolic function following cell therapy [3,24,41]. There are also a number of differences between this model and human cell therapy that are worthy of mention. First, progenitor cell function may be altered or impaired in patients receiving cell therapy, particularly the elderly and those with multiple risk factors for vascular disease [32], whereas the animals studied here were all healthy prior to induction of myocardial infarction. Second, adjunctive post-infarction pharmacotherapy may interfere with the signalling pathways of the candidate paracrine factors which we have identified, modulating this component of the reparative response to cell therapy in humans. Specifically, angiotensin II blockade [42], statins [43,44] and β-blockers [45] may affect growth factor expression and/or signaling in the heart. Interplay of this kind between cell and pharmacologic therapy for acute myocardial infarction has not yet been examined in human or experimental studies, but certainly merits greater attention as this treatment approach evolves. Finally the focus of this study was the cardiomyocyte size and LV mass changes after cell therapy (with particular emphasis on changes in the infarct and border zone) and not traditionally studied parameters such as angiogenesis and scar formation.
In conclusion, this study shows circulating progenitor cells exert a potent paracrine effect impacting cardiomyocyte size with direct consequences for infarct-related remodeling post myocardial infarction. These data strongly support a robust contribution of progenitor cells to regional post infarct repair with mechanistic and therapeutic implications for future human studies in the cell therapy field.
Acknowledgments
This work was supported in part by funding from the National Institutes of Health (HL66958 to NMC), a Principal Investigator Grant from Science Foundation Ireland (NMC) and the Irish Heart Foundation/Bristol Myers Squibb (traveling fellowship award to BD), and funding from the Irish Heart Foundation (BH).
References
- 1.Strauer BE. Brehm M. Zeus T. Kostering M. Hernandez A. Sorg RV. Kogler G. Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–8. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 2.Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Dobert N. Grunwald F. Aicher A. Urbich C. Martin H. Hoelzer D. Dimmeler S. Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 3.Wollert KC. Meyer GP. Lotz J. Ringes-Lichtenberg S. Lippolt P. Breidenbach C. Fichtner S. Korte T. Hornig B. Messinger D. Arseniev L. Hertenstein B. Ganser A. Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Aviles F. San Roman JA. Garcia-Frade J. Fernandez ME. Penarrubia MJ. de la Fuente L. Gomez-Bueno M. Cantalapiedra A. Fernandez J. Gutierrez O. Sanchez PL. Hernandez C. Sanz R. Garcia-Sancho J. Sanchez A. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–8. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 5.Badorff C. Brandes RP. Popp R. Rupp S. Urbich C. Aicher A. Fleming I. Busse R. Zeiher AM. Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 6.Orlic D. Kajstura J. Chimenti S. Jakoniuk I. Anderson SM. Li B. Pickel J. McKay R. Nadal-Ginard B. Bodine DM. Leri A. Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 7.Kocher AA. Schuster MD. Szabolcs MJ. Takuma S. Burkhoff D. Wang J. Homma S. wards NM. Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 8.Kinnaird T. Stabile E. Burnett MS. Lee CW. Barr S. Fuchs S. Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs S. Baffour R. Zhou YF. Shou M. Pierre A. Tio FO. Weissman NJ. Leon MB. Epstein SE. Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–32. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 10.Gnecchi M. He H. Liang OD. Melo LG. Morello F. Mu H. Noiseux N. Zhang L. Pratt RE. Ingwall JS. Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 11.Urbich C. Aicher A. Heeschen C. Dernbach E. Hofmann WK. Zeiher AM. Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Ziegelhoeffer T. Fernandez B. Kostin S. Heil M. Voswinckel R. Helisch A. Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 13.Schmid I. Uittenbogaart CH. Giorgi JV. A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry. 1991;12:279–85. doi: 10.1002/cyto.990120312. [DOI] [PubMed] [Google Scholar]
- 14.Klein HH. Schubothe M. Nebendahl K. Kreuzer H. Temporal and spatial development of infarcts in porcine hearts. Basic Res Cardiol. 1984;79:440–7. doi: 10.1007/BF01908144. [DOI] [PubMed] [Google Scholar]
- 15.Cerqueira MD. Weissman NJ. Dilsizian V. Jacobs AK. Kaul S. Laskey WK. Pennell DJ. Rumberger JA. Ryan T. Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002;18:539–42. [PubMed] [Google Scholar]
- 16.Senthil V. Chen SN. Tsybouleva N. Halder T. Nagueh SF. Willerson JT. Roberts R. Marian AJ. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res. 2005;97:285–92. doi: 10.1161/01.RES.0000177090.07296.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Terzic C. Gacy AM. Bortolon R. Dzeja PP. Puceat M. Jaconi M. Prendergast FG. Terzic A. Structural plasticity of the cardiac nuclear pore complex in response to regulators of nuclear import. Circ Res. 1999;84:1292–301. doi: 10.1161/01.res.84.11.1292. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Terzic C. Gacy AM. Bortolon R. Dzeja PP. Puceat M. Jaconi M. Prendergast FG. Terzic A. Directed inhibition of nuclear import in cellular hypertrophy. J Biol Chem. 2001;276:20566–71. doi: 10.1074/jbc.M101950200. [DOI] [PubMed] [Google Scholar]
- 19.Schaub MC. Hefti MA. Harder BA. Eppenberger HM. Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes. J Mol Med. 1997;75:901–20. doi: 10.1007/s001090050182. [DOI] [PubMed] [Google Scholar]
- 20.Lim JY. Park SJ. Hwang HY. Park EJ. Nam JH. Kim J. Park SI. TGF-beta1 induces cardiac hypertrophic responses via PKC-dependent ATF-2 activation. J Mol Cell Cardiol. 2005;39:627–36. doi: 10.1016/j.yjmcc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Schultz Jel J. Witt SA. Glascock BJ. Nieman ML. Reiser PJ. Nix SL. Kimball TR. Doetschman T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109:787–96. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corda S. Mebazaa A. Gandolfini MP. Fitting C. Marotte F. Peynet J. Charlemagne D. Cavaillon JM. Payen D. Rappaport L. Samuel JL. Trophic effect of human pericardial fluid on adult cardiac myocytes. Differential role of fibroblast growth factor-2 and factors related to ventricular hypertrophy. Circ Res. 1997;81:679–87. doi: 10.1161/01.res.81.5.679. [DOI] [PubMed] [Google Scholar]
- 23.Schultz JE. Witt SA. Nieman ML. Reiser PJ. Engle SJ. Zhou M. Pawlowski SA. Lorenz JN. Kimball TR. Doetschman T. Fibroblast growth factor-2 mediates pressure-induced hypertrophic response. J Clin Invest. 1999;104:709–19. doi: 10.1172/JCI7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Britten MB. Abolmaali ND. Assmus B. Lehmann R. Honold J. Schmitt J. Vogl TJ. Martin H. Schachinger V. Dimmeler S. Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–8. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 25.Schachinger V. Assmus B. Britten MB. Honold J. Lehmann R. Teupe C. Abolmaali ND. Vogl TJ. Hofmann WK. Martin H. Dimmeler S. Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Yeh ET. Zhang S. Wu HD. Korbling M. Willerson JT. Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003;108:2070–3. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto A. Gwon HC. Iwaguro H. Yamaguchi JI. Uchida S. Masuda H. Silver M. Ma H. Kearney M. Isner JM. Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 28.Kawamoto A. Tkebuchava T. Yamaguchi J. Nishimura H. Yoon YS. Milliken C. Uchida S. Masuo O. Iwaguro H. Ma H. Hanley A. Silver M. Kearney M. Losordo DW. Isner JM. Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–8. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 29.Rehman J. Li J. Orschell CM. March KL. Peripheral blood “endothelial progenitor cells”. are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 30.MacLellan WR. Brand T. Schneider MD. Transforming growth factor-beta in cardiac ontogeny and adaptation. Circ Res. 1993;73:783–91. doi: 10.1161/01.res.73.5.783. [DOI] [PubMed] [Google Scholar]
- 31.Li RK. Li G. Mickle DA. Weisel RD. Merante F. Luss H. Rao V. Christakis GT. Williams WG. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 1997;96:874–81. doi: 10.1161/01.cir.96.3.874. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi N. Calderone A. Izzo NJ., Jr. Maki TM. Marsh JD. Colucci WS. Hypertrophic stimuli induce transforming growth factor-beta 1 expression in rat ventricular myocytes. J Clin Invest. 1994;94:1470–6. doi: 10.1172/JCI117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray MO. Long CS. Kalinyak JE. Li HT. Karliner JS. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–63. doi: 10.1016/s0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto-Ida M. Takimoto Y. Aoyama T. Akao M. Takeda T. Kita T. Activation of TGF-beta1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2006;290:H709–15. doi: 10.1152/ajpheart.00186.2005. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Is tissue mass regulated by vascular endothelial cells?. Prostate as the first evidence. Endocrinology. 1998;139:441–2. doi: 10.1210/endo.139.2.5858. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto K. Yoshitomi H. Rossant J. Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–63. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 37.Caplice NM. Doyle B. Vascular progenitor cells: origin and mechanisms of mobilization, differentiation, integration, and vasculogenesis. Stem Cells Dev. 2005;14:122–39. doi: 10.1089/scd.2005.14.122. [DOI] [PubMed] [Google Scholar]
- 38.Shiojima I. Sato K. Izumiya Y. Schiekofer S. Ito M. Liao R. Colucci WS. Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta JL. Chen HJ. Li DY. Protection of myocytes from hypoxiareoxygenation injury by nitric oxide is mediated by modulation of transforming growth factor-beta1. Circulation. 2002;105:2206–11. doi: 10.1161/01.cir.0000015602.94990.3d. [DOI] [PubMed] [Google Scholar]
- 40.Vinals F. Pouyssegur J. Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling. Mol Cell Biol. 2001;21:7218–30. doi: 10.1128/MCB.21.21.7218-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssens S. Dubois C. Bogaert J. Theunissen K. Deroose C. Desmet W. Kalantzi M. Herbots L. Sinnaeve P. Dens J. Maertens J. Rademakers F. Dymarkowski S. Gheysens O. Van Cleemput J. Bormans G. Nuyts J. Belmans A. Mortelmans L. Boogaerts M. Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 42.Lim DS. Lutucuta S. Bachireddy P. Youker K. Evans A. Entman M. Roberts R. Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–91. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park HJ. Galper JB. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors up-regulate transforming growth factor-beta signaling in cultured heart cells via inhibition of geranylgeranylation of RhoA GTPase. Proc Natl Acad Sci U S A. 1999;96:11525–30. doi: 10.1073/pnas.96.20.11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Y. Ohmori K. Chen Y. Sato C. Kiyomoto H. Shinomiya K. Takeuchi H. Mizushige K. Kohno M. Effects of pravastatin on progression of glucose intolerance and cardiovascular remodeling in a type II diabetes model. J Am Coll Cardiol. 2004;44:904–13. doi: 10.1016/j.jacc.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 45.Shigeyama J. Yasumura Y. Sakamoto A. Ishida Y. Fukutomi T. Itoh M. Miyatake K. Kitakaze M. Increased gene expression of collagen Types I and III is inhibited by beta-receptor blockade in patients with dilated cardiomyopathy. Eur Heart J. 2005;26:2698–705. doi: 10.1093/eurheartj/ehi492. [DOI] [PubMed] [Google Scholar]
- 46.Okada H. Takemura G. Kosai K. Li Y. Takahashi T. Esaki M. Yuge K. Miyata S. Maruyama R. Mikami A. Minatoguchi S. Fujiwara T. Fujiwara H. Post-infarction gene therapy against transforming growth factor -beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–2437. doi: 10.1161/01.CIR.0000165066.71481.8E. [DOI] [PubMed] [Google Scholar]
- 47.Liao R. Yin and yang of myocardial transforming growth factor-β1; timing is everything. Circulation. 2005;111:2416–2417. doi: 10.1161/01.CIR.0000167557.59069.D9. [DOI] [PubMed] [Google Scholar]
- 48.Li T-S. hayashi M. Ito H. Furutani A. Murata T. Matsuzaki M. Hamano K. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–2445. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]