Abstract
Neural stem cell (NSC) transplantation has been proposed as a future therapy for neurodegenerative disorders. However, NSC transplantation will be hampered by the limited number of brain donors and the toxicity of immunosuppressive regimens that might be needed with allogeneic transplantation. These limitations may be avoided if NSCs can be generated from clinically accessible sources, such as bone marrow (BM) and peripheral blood samples, that are suitable for autologous transplantation. We report here that NSCs can be generated from human BM-derived mesenchymal stem cells (MSCs). When cultured in NSC culture conditions, 8% of MSCs were able to generate neurospheres. These MSC-derived neurospheres expressed characteristic NSC antigens, such as nestin and musashi-1, and were capable of self-renewal and multilineage differentiation into neurons, astrocytes, and oligodendrocytes. Furthermore, when these MSC-derived neurospheres were cocultured with primary astrocytes, they differentiate into neurons that possess both dendritic and axonal processes, form synapses, and are able to fire tetrodotoxin-sensitive action potentials. When these MSC-derived NSCs were switched back to MSC culture conditions, a small fraction of NSCs (averaging 4–5%) adhered to the culture flasks, proliferated, and displayed the morphology of MSCs. Those adherent cells expressed the characteristic MSC antigens and regained the ability to differentiate into multiple mesodermal lineages. Data presented in this study suggest that MSCs contain a small fraction (averaging 4–5%) of a bipotential stem cell population that is able to generate either MSCs or NSCs depending on the culture conditions.
Introduction
Neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and Huntington's disease involve the death and atrophy of neurons in the brain [1]. Currently, there is no cure for neurodegenerative disorders and all available treatment options focus on symptomatic treatment only. Transplantation of stem cells or their derivatives, and mobilization of endogenous stem cells within the adult brain, have been proposed as future therapies for neurodegenerative diseases [2,3]. Although, it may seem unrealistic to induce functional recovery by replacing cells lost through disease, considering the complexity of the human brain structure and function, studies in animal models have demonstrated that neuronal replacement and partial reconstruction of damaged neuronal circuitry is possible [4–6]. Results from clinical trials also suggest that the replacement of cells in the diseased human brain can lead to symptomatic relief [7–11].
Fully differentiated neurons may be the preferred cells for transplants to replace dead neurons in the brains of patients with neurodegenerative disorders. However, terminally differentiated neurons are less likely to survive detachment and subsequent transplant procedures [4,5,12,13]. Neural stem cells (NSCs) can proliferate and subsequently differentiate into all major neural cell lineages of the brain, including neurons, astrocytes, and oligodendrocytes [14,15]. Therefore, NSCs are likely more suitable than fully differentiated neurons for neurodegenerative treatment strategies that use transplantation. Although NSCs can be generated directly from human brain tissue or from human embryonic stem cells [16,17], they are limited by the number of brain donors and available embryonic stem cell lines, and they are not suitable for the autologous transplantation setting. If NSCs can be generated from clinically accessible sources, such as bone marrow (BM) and peripheral blood, then autologous transplantation will be feasible. Mesenchymal stem cells (MSCs) derived from both BM and peripheral blood can be expanded efficiently and can differentiate into many mesodermal tissues, including bone, cartilage, fat, and muscle [18–20]. In addition, it has been reported that a small fraction (usually <5%) of MSCs can differentiate into cells that express neuronal and glial markers, both in vitro [21–23] and in vivo [24–27], suggesting that some MSCs possess neural potential and could be used as therapeutics for neurodegenerative diseases. However, the identity of these cells remains illusive [21–28].
It has been recently reported by two laboratories, using similar protocols by culturing MSCs in NSC culture conditions, that MSCs can be converted into clonogenic NSCs that grow in neurosphere-like structures [29,30]. In one study working with rat MSCs, Suzuki et al. reported that a considerable proportion [20–60%] of rat MSCs were converted into NSCs [29]. In another study working with human adult MSCs, Hermann et al. reported that >60% of MSCs can be converted into clonogenic NSCs [30]. Both studies suggest that the conversion of MSCs into NSCs could be a transdifferentiation phenomenon [29,30]. In both studies, the MSC-derived NSCs differentiated in vitro into cells with morphological and functional characteristics of neurons, astrocytes, and oligodendrocytes [29,30]. Since these protocols have considerable implications for using autologous NSCs for treating neurodegenerative diseases, we repeated the experimental protocol from Hermann et al. as a prelude to further dissect the underlying mechanisms of how human adult MSCs can be converted to NSCs. We report here that human adult BM-derived MSCs can generate NSCs by using the protocol of Hermann et al. [30]. However, our results demonstrate that only 8% of cells within the MSCs' population were able to generate neurospheres when cultured in NSC culture conditions. These MSC-derived NSCs grow as neurosphere-like structures, express characteristic NSC antigens such as nestin [31] and musashi-1 [32], and are able to self-renew and differentiate into the three main types of neural cells including neurons, astrocytes, and oligodendrocytes. Moreover, when these MSC-derived neurospheres were cocultured with primary astrocytes that were generated from human fetal brain tissues, they differentiate into mature neurons that possess both dendritic and axonal processes, form synapses, and are able to fire tetrodotoxin (TTX)-sensitive action potentials. When switched back to MSC culture condition, a small fraction (4–5%) of MSC-derived NSCs adhered to the flasks, grew as a monolayer, and had the characteristic morphology of MSCs. Those adherent cells also expressed the classical MSC antigens and differentiated into adipocytes, osteoblasts, and chondrocytes. Our data suggest that MSCs are heterogeneous and contain a small population (4–5%) of bipotential stem cells that can generate either MSCs or NSCs depending on the culture conditions. This bipotential stem cell population in the heterogeneous MSCs could provide the basis for the generation of NSCs from MSCs.
Materials and Methods
Generation and characterization of MSCs from human BM samples
Adult BM samples were obtained from healthy donors with approved consent (City of Hope Medical Center, Duarte, CA). Preparation of mononuclear cells (MNCs) from BM samples was performed as previously described [33–35]. MNCs were then resuspended in MSC culture medium containing low glucose Dulbecco modified Eagle medium (DMEM) (Gibco BRL, Rockville, MD), 15% fetal bovine serum (Gemini Bio-Products, Inc., Calabasas, CA), 2 mM l-glutamine, 1 mM sodium pyruvate, and penicillin–streptomycin (Flow Laboratories, Rockville, MD) and plated at an initial density of 1 × 106 cells/cm2. Three days later, the cultures were washed with PBS to remove nonadherent cells, and the remaining monolayers of adherent cells were cultured in fresh medium until they reached 70–85% confluence. The cells were harvested by trypsinization (0.25% trypsin with 0.1% EDTA) and subcultured at densities of 5,000–6,000 cells/cm2. Media were changed every 2–3 days. Cell cultures were passaged again at 70–85% confluence and used in experiments during the 4th–10th passages. Differentiation assays, including those for adipogenic, chondrogenic, and osteogenic lineages, were performed as previously described [36].
Flow cytometry
The antigen profiles of cultured human BM-derived adherent cells in our culture were analyzed by flow cytometry as previously described [33–36]. Fluorescein isothiocyanate (FITC)-labeled and phycoerythrin (PE)-labeled monoclonal antibodies against CD29, CD44, CD73, CD90 (thy-1), CD34, CD45, CD14, and HLA-DR were purchased from Becton Dickinson (San Jose, CA). Antibody against CD105 was purchased from Abcam, Inc. (Cambridge, MA) and antibodies against CD166, CD80, and CD86 were purchased from Research Diagnostic Inc. (Flanders, NJ). Antibody against CD11 was purchased from Beckman Coulter Inc. (Fullerton, CA). Cells were analyzed on a MoFlo fluorescent activated cell sorter (FACS; Cytomation, Inc., Fort Collins, CO). To examine the expression of MSC antigens on MSC-derived neurospheres, neurospheres were collected, trypsinized, and subjected to flow cytometry.
Generation of neurospheres from MSCs
Generation of neurospheres from MSCs was performed as previously described [30] with slight modifications. MSCs were dissociated with 0.05% trypsin/0.04% EDTA and plated on low-attachment plastic tissue culture flasks (Nalge Nunc International, Rochester, NY, USA) at a concentration of 1–2 × 105 cells/cm2 in NSC culture medium containing the P4-8F medium (AthenaES, Baltimore, MD) supplemented with 20 ng/mL of both epidermal growth factor (EGF; R&D Systems, Inc., Minneapolis, MN) and basic fibroblast growth factor (bFGF; R&D Systems, Inc., Minneapolis, MN). By the following day, cells had started to adhere, forming very loose, sphere-like clusters. On the third day after plating, cell clusters were collected by centrifugation, mechanically dissociated with a fire-polished Pasteur pipette, and replated on low-attachment plastic tissue culture flasks at a concentration of 1–2 × 105 cells/cm2 in the NSC culture medium. Cells did not cluster after this point and neurospheres were apparent within 7–10 days in the culture. To determine the frequency of neurosphere-forming cells within the MSCs, one thousand MSCs were distributed into each well in low-attachment 24-well tissue culture plates (Nalge Nunc International, Rochester, NY, USA) in the NSC culture medium. The number of neurospheres in each well was counted at day 7–10. At this cell density (1,000 cells per well) in the 24-well plates, cells did not cluster together.
Propagation and expansion of MSC-derived NSCs
To determine the self-renewal activity of the neurosphere-forming cells, neurospheres were harvested by centrifugation at 104 g for 5 min, resuspended in 5 mL of NSC culture medium, and mechanically dissociated into single cells by triturating with a fire-polished Pasteur pipette. Cells were plated in 96-well plates in NSC culture medium (500 cells in 200 μL of medium per well). The number of neurospheres in each well was scored 7–10 days after plating. The whole procedure was repeated every 7–10 days until the fifth passage. For expansion of the bulk cultures, neurospheres were harvested by centrifugation at 104 g for 5 min and resuspended in 5 mL of NSC culture medium. The neurospheres were mechanically dissociated into single cells or small clusters by triturating with a fire-polished Pasteur pipette, and replated on low-attachment plastic tissue culture flasks at a concentration of 1–2 × 105 cells/cm2 in the NSC culture medium. The medium was changed once per week and growth factors (EGF and bFGF) were added every 3 days. These neurospheres were expanded for an additional 2–6 passage before characterization and differentiation was started.
Differentiation of MSC-derived NSCs
Differentiation of neurospheres into neural lineages was carried out as previously described [35]. Single, isolated neurospheres were plated on poly-l-ornithine-coated (15 μg/mL) glass coverslips in individual wells of 24-well plates (1.0 mL/well) in DMEM/F-12 medium containing 1% bovine fetal serum (Gemini Bio-Products Inc., Calabasas, CA, USA) and a defined hormone and salt mixture composed of insulin (25 μg/mL), transferrin (100 μg/mL), progesterone (20 nM), putrescine (60 μM), and sodium selenite (30 nM) [35]. The medium was not changed for the duration of the experiment. Coverslips were processed for fluorescence immunocytochemistry 8–10 days after plating [35].
Generation of primary astrocytes from human fetal brain tissues
Human brain tissues were dissected from 15- to 22-week-old fetuses obtained by elective abortion with approved consent (Advanced Bioscience Resources, Alameda, CA, USA). Primary astrocytes were prepared essentially as previously described [37]. Briefly, human fetal brain tissues were digested in Hanks' balanced salt solution (GIBCO/BRL) with a mixture of 0.125% trypsin/0.05% DNase I (Sigma) at 37°C for 20 min. Then, they were washed with PBS and mechanically dissociated. After passing through a 70-μm nylon mesh, the cells were collected, counted and plated onto tissue culture flasks, precoated with 0.1% gelatin (Sigma) at 4°C overnight, at a density of 600,000 cells per cm2 in DMEM plus 10% FBS (Gemini Bio-products, Calabasas, CA) until confluent.
Coculture of MSC-derived NSCs with human fetal brain-derived primary astrocytes
Coculture of MSC-derived NSCs with human fetal brain-derived astrocytes was carried out as previously described with slight modifications [37]. Human fetal brain-derived astrocytes were plated onto 24-well plates, precoated with 0.1% gelatin (Sigma) at 4°C overnight, at a density of 10,000 cells per well in the coculture medium. The coculture medium was composed of DMEM/F-12 (GIBCO/BRL) plus a defined hormone and salt mixture including insulin (25 μg/mL), transferrin (100 μg/mL), progesterone (20 nM), putrescine (60 μM), and sodium selenite (30 nM). Once confluent, glass coverslips, precoated with 500 μg/mL poly-d-lysine overnight at 4°C, were placed on top of the astrocyte layers and MSC-derived NSCs were seeded onto the glass coverslips, either as neurospheres or single cells (trypsinized). Cultures were maintained for 14 days with medium change once at day 7. After 14 days, glass coverslips were processed for immunocytochemistry, electron microscopy (EM), and electrophysiology.
Immunocytochemistry
Indirect immunocytochemistry of individual neurospheres and differentiated cells from neurospheres was performed as previously described [35]. Cells were fixed in 4% paraformaldehyde in PBS, then immunocytochemistry was carried out as previously described [35]. Antibodies and dilutions were as follows: β-tubulin III monoclonal, 1:1,000 (Sigma, St Louis, MO); GalC monoclonal, 1:750; glial fibrillary acid protein (GFAP) monoclonal, 1:1,000, and nestin polyclonal, 1:500 (all from Chemicon International, Temecula, CA); musashi-1 monoclonal, 1:300 (R&D Systems Inc., Minneapolis, MN); Synapsin I polyclonal, 1:1,000; MAP-2 monoclonal, 1:1,000; and S-100β polyclonal, 1:100 (all from Abcam, Cambridge, MA). Fluorescence-labeled secondary antibodies were used as F(ab)2 fractions from Jackson Immunoresearch Laboratories (West Grove, PA). After three washes, samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St Louis, MO) and embedded in Vectashield (Vector Laboratories, Burlingame, CA). Samples were visualized with an Olympus BX51 fluorescent microscope, equipped with a Pixera cooled CCD camera. Fluorescent images were collected using appropriate filter sets for each fluorophore. Color composite images were generated using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
EM and electrophysiology
For EM, phase images of cells in selected fields were taken for later identification. The cultures were processed for EM preparation as previously described [37,38]. Serial thin sections (60 nm) with previously selected fields were examined in a FEI Company™ Tecnai™ Twin 12 electron microscope at 80 kV.
Standard whole-cell patch-clamp recording methodologies were used to examine the physiological properties of neuron-like cell generated from MSC-derived NSCs. Whole-cell patch-clamp recordings were carried out with patch-clamp amplifiers (Axopatch 200A, Axon Instruments, Foster City, CA) as previously described [39,40]. Electrophysiological recordings were carried out at room temperature in the external recording solution containing: NaCl 145 mM, KCl 3 mM, HEPES 10 mM, CaCl2 3 mM, glucose 8 mM, and MgCl2 2 mM (pH 7.30). The micropipettes (2–6 MΩ) were filled with internal recording solution containing: potassium gluconate 136.5 mM, KCl 17.5 mM, NaCl 9 mM, MgCl2 1 mM, HEPES 10 mM, and EGTA 0.2 mM (pH 7.30). Signals were filtered at 5 kHz using a 4-pole low pass Bessel filter. Pulse protocols were generated and current responses recorded online using a Compaq 386 microcomputer and the pClamp program interfaced to the Axopatch 200A via an Axon Instruments TL-1 interface board. To establish the parameters for performing the whole-cell patch-clamp recording, we have included the following controls: (1) positive controls, neurons generated from four different sources of NSCs that were derived from two independent human fetal brain tissues and two human adult brain tissues after 14 days in coculture with human fetal brain tissue-derived astrocytes; (2) negative controls, undifferentiated MSCs derived from two human fetal and two adult BM samples. To examine the excitability of neurons, transient membrane currents from neurons in 14d cultures induced by stepping holding membrane potential from–60 mV to 0 mV (50 ms) were recorded. Then the cell was switched to current-clamp and currents (0.5–1 nA, 20 ms) were injected through the patch pipette to examine whether action potential could be induced. TTX (0.5 μM; Sigma, St. Louis, MO) was used to block voltage-dependent Na+ channels. A minimum of 10 cells from coculture were recorded.
Generation of MSCs from MSC-derived NSCs
Neurospheres were harvested from the NSC culture medium, and switched to the MSC culture medium, either as spheres or single cells (trypsinized). To determine the frequency of cells within neurospheres that were capable of differentiating into MSCs, neurospheres were harvested from the NSC culture medium, trypsinized and resuspended in MSC culture medium. Limiting dilutions of cells (900, 300, and 100 cells per well in 24-well plates) were grown in MSC culture condition, and the number of adherent cell clusters (>50 cells) was scored 7–10 days after plating.
Results
Characterization and verification of human BM-derived MSCs
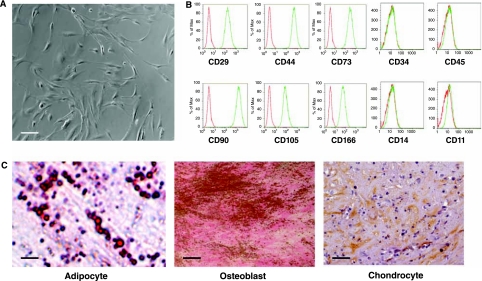
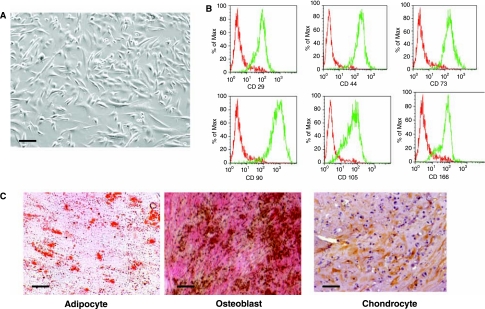
MSCs were derived from three human adult BM samples obtained from 22-, 34- and 52-year-old healthy donors. These MSCs were propagated for 4–10 passages to generate sufficient numbers of cells for experiments. Regardless of donor or passage, these MSCs had the typical morphology of MSCs; we observed two morphologically distinct groups of cells: spindle-shaped cells and large cuboidal or flattened cells (Fig. 1A). These cells expressed the characteristic antigens of MSCs, including CD29, CD44, CD73, CD90, CD105, and CD166 (Fig. 1B), and did not express hematopoietic markers CD45, CD34, CD14, or CD11 (Fig. 1B). These cells also did not express the costimulatory molecules CD80 and CD86, and human leukocyte antigen (HLA) class II HLA-DR antigens (data not shown). Furthermore, these cells differentiated into the three mesodermal lineages including adipocytes, osteoblasts, and chondrocytes (Fig. 1C). Taken together, these results demonstrate that MSCs from all three different donors, regardless of the number of passages, displayed typical MSC morphology, expressed antigens that are characteristic of MSCs, and were able to differentiate into adipocytes, osteoblasts, and chondrocytes.
FIG. 1.
Derivation and characterization of human adult bone-marrow-derived mesenchymal stem cells (MSCs). (A) Morphology of the MSCs derived from human adult BM samples. (B) Flow cytometric analysis for the expression of characteristic MSC antigens including CD29, CD44, CD73, CD90, CD105, CD166, CD34, CD45, CD14, and CD11. Red line represents the isotype control and green line represents the staining against each specified antibodies. (C) Differentiation of MSCs into three mesodermal lineages: adipocytes (oil red O staining), chondrocytes (collagen II antibody staining), and osteoblasts (von Kossa staining for calcium deposits). Scale bars: (A) 100 μm; (C) adipocyte 10 μm, osteoblast 8 μm, and chondrocyte 50 μm.
Derivation of NSCs from MSCs
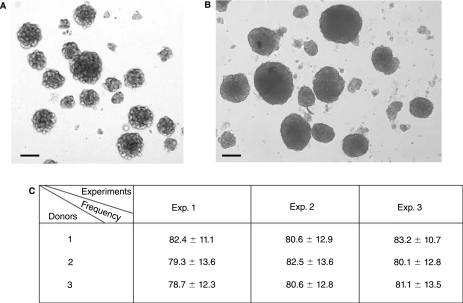
To convert MSCs into cells with characteristics of NSCs, we followed the protocol of Hermann et al. [30]. After 4–10 passages, MSCs were trypsinized, collected, and cultured in NSC culture medium containing the P4-8F medium supplemented with 20 ng/mL of both EGF and bFGF. We observed that under these culture conditions cells clumped together and formed loose spheres after 1 day of culture (Fig. 2A). We have analyzed those loose spheres and our results demonstrate that the loose spheres were not neurospheres (data not shown). On day 3 after plating, all cells, including the loose spheres, were collected, mechanically dissociated with a fire-polished Pasteur pipette, and replated in the NSC culture medium. After this passage, the cells stopped clumping together and neurospheres were apparent within 7–10 days (Fig. 2B). To determine the frequency of neurosphere-forming cells within the MSC population, 1,000 MSCs were distributed into each well of 24-well plates, and the number of neurospheres that formed in each well was scored after 7–10 days. We observed that on average 8% of the MSCs generated neurospheres when cultured in NSC culture conditions and there was minimal variation among these MSCs derived from the three donors (Fig. 2C).
FIG. 2.
Generation of neural stem cells (NSCs) from mesenchymal stem cells (MSCs). (A) Loose clusters of MSCs in NSC culture medium within 24 h after plating. (B) Neurospheres generated from MSCs at Day 8 after plating in NSC culture conditions. (C) Number of neurosphere-forming cells per 1,000 MSCs; 1,000 MSCs per well were plated in 24-well plates and the number of neurospheres in each well was scored 7 days later. A total of 24 wells were analyzed for each donor. Data for the number of neurospheres per well are presented as the mean ± SD in each well. Experiments were performed in triplicate using the MSCs from the same donor to ensure the reproducibility of the results. These data are compiled from three different donor samples. Scale bars: (A and B) 100 μm.
Characterization and verification of MSC-derived NSCs
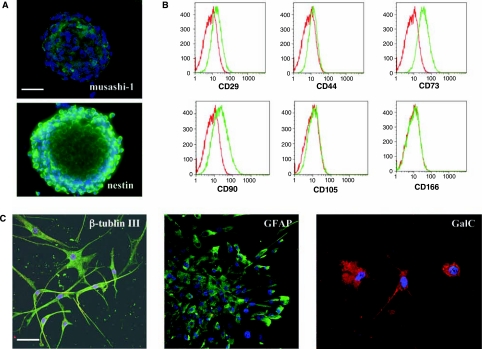
To determine if the cultured spheres derived from MSCs resembled neurospheres, we determined whether these spheres expressed antigens that were characteristic of NSCs, could differentiate into neural cells of multiple lineages, and had self-renewal potential. First, we investigated whether these spheres expressed two characteristic antigens for NSCs, nestin [31] and musashi-1 [32]. Immunocytochemistry revealed that most of the cells (>85%) within a single sphere express nestin and musashi-1 (Fig. 3A). In addition, we investigated whether these spheres continued expressing antigens that are characteristic of MSCs. FACS analysis showed that these MSC-derived spheres did not express CD44, CD105, and CD166, but did weakly express CD29, CD73, and CD90 (Fig. 3B). We next examined whether these MSC-derived spheres were capable of multilineage differentiation into neurons, astrocytes, and oligodendrocytes. Single spheres were plated onto poly-L-ornithine-coated glass coverslips in 24-well culture plates in the absence of EGF and bFGF and in the presence of 1% bovine fetal serum, and cultured for 8–10 days [35]. Fluorescence immunocytochemistry for neuronal (β-tubulin III) and glial cell antigens (GFAP; galactosylceramidase, GalC), in combination with examination of cell morphology, was used to identify the phenotype of the cells that comprised the differentiated spheres. Cells within a single sphere were able to differentiate into GFAP-positive cells with astrocyte morphology, β-Tubulin III-positive cells with neuronal morphology, and GalC-positive cells with oligodendrocyte morphology (Fig. 3C). When analyzed for their potential to differentiate into cells of a mesodermal lineage [36], our results demonstrate that these MSC-derived NSCs have lost their capacity to differentiate into adipocytes, osteoblasts, or chondrocytes (data not shown).
FIG. 3.
Characterization of mesenchymal stem cell (MSC)-derived neural stem cells (NSCs). (A) Immunocytochemical analysis for the expression of nestin and musashi-1 on MSC-derived NSCs. FITC-labeled secondary antibody was used for both nestin and musashi-1. DAPI was used for nuclear staining. (B) Flow cytometric analysis for the expression characteristic MSC antigens on MSC-derived NSCs. Antigens include CD29, CD44, CD73, CD90, CD105, and CD166. Red line represents the isotype control and green line represents the staining against each specified antibodies. (C) Differentiation of MSC-derived NSCs into three neural lineages including neurons (β-tubulin III; FITC-labeled secondary antibody), astrocytes (glial fibrillary acid protein; FITC-labeled secondary antibody), and oligodendrocytes (galactosylceramidase; Texas Red-labeled secondary antibody). DAPI was used for nuclear staining. Scale bars: (A) 50 μm; and (C) 50 μm.
To determine if MSC-derived NSCs had self-renewal potential, MSC-derived neurospheres were harvested and mechanically dissociated into single cells. Five hundred cells were plated into each well of 96-well plates in NSC culture medium. Seven days after plating, the number of neurospheres in each well was scored. This whole process was repeated four more times (through the 5th passage). For donor 1, the average number of spheres generated in each well (n = 60) from the first to the 5th passage ranged from 99 to 104 neurospheres showing that the percentage of neurosphere-forming cells in each passage is very constant averaging 20% (Table 1).On the basis of the results from donor 1, we calculated that there is an average 40-fold expansion of NSCs in each passage (Table 1).The data from the other two donors were almost identical to the data from donor 1 (Table 1), suggesting that there was no significant donor effect on the self-proliferation and expansion of MSC-derived NSCs. Taken together, these results demonstrate that MSC-derived NSCs possess the characteristics of NSCs.
Table 1.
Number of NSCs in Subsequent Cultures of MSC-Derived NSCs
| Passage | 1st | 2nd | 3rd | 4th | 5th |
|---|---|---|---|---|---|
| Donor | |||||
| 1 | 102.8 ± 11.5 (20.4) | 99.2 ± 9.4 (19.8) | 101.6 ± 13.3 (20.2) | 104.5 ± 13.3 (20.8) | 99.6 ± 9.8 (19.9) |
| 2 | 101.4 ± 10.3 | 101.6 ± 11.2 | 99.6 ± 11.6 | 99.2 ± 11.6 | 101.2 ± 11.8 |
| 3 | 103.4 ± 12.5 | 102.1 ± 11.3 | 104.2 ± 13.9 | 99.4 ± 10.8 | 101.6 ± 11.6 |
Abbreviations: MSC, mesenchymal stem cell; NSC, neural stem cell.
Cells were plated in 96-well plates as 500 cells/200 μL per well for each passage. These wells were scored 7 days later for the presence of spheres. The total number of wells analyzed for each passage is 60. The number of spheres per well is presented as the mean ± SD in each passage. The numbers in parenthesis represent the frequency of NSCs.
These data are compiled from three independent experiments using MSCs derived from different donor samples. Each neurosphere averages 200 cells, so each well contains an average of 20,000 cells (100 neurospheres × 200 cells/ neurosphere). Since the percentage for neurosphere-forming cells in each passage remains constant (averaging 20%), the estimate for the magnitude of expansion in each passage is 40-fold (20,000 cells/500 cells).
Generation of functional neurons from MSC-derived NSCs in vitro
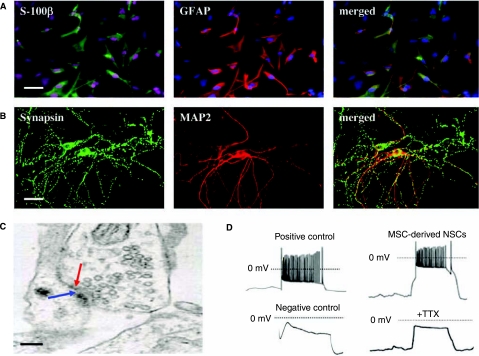
One of the hallmarks of mature CNS neurons is their ability to form synapses [41]. Astrocytes produce a variety of soluble and membrane-associated factors that influence the development of the CNS [42]. It has been previously demonstrated that primary astrocytes derived from both neonatal and adult rat hippocampus provide an excellent environment to guide the NSCs from adult rat hippocampus to develop essential properties of functional CNS neurons [37]. To further examine the functional aspects of MSC-derived NSCs, we generated primary astrocytes from human fetal brain tissues and used to co-culture with those MSC-derived NSCs. Using a previously reported protocol [37], primary astrocytes were generated from three different human fetal brain tissues. Consistent with the previous report [37], majority of cells in these cultures were astrocytes, which were GFAP+ and/or S-100β+ (Fig. 4A). Importantly, these astrocytes did not give rise to any detectable neurons (MAP-2+) under coculture condition (data not shown). To investigate if these human fetal brain-derived primary astrocytes can support the differentiation of mature neurons from NSCs, MSC-derived NSCs, either as neurospheres or single cells (trypsinized), were cocultured with these human fetal brain-derived primary astrocytes. In coculture, synapse formation between neurons derived from MSC-derived NSCs occurred within 2 weeks, as indicated by punctuate staining of synapsin I, a synaptic vesicle protein concentrated at synapses in mature neurons (Fig. 4B). We further analyzed the synaptic boutons by EM and found that typical synaptical structures were associated with the dendrites, spines, and cell bodies of neurons derived from MSC-derived NSCs (Fig. 4C). The whole-cell patch-clamp recordings were performed to further determine whether neurons derived from MSC-derived NSCs are electrically active and whether their morphologically defined synapses are functional. Neurons generated from four different sources of NSCs that were derived from two independent human fetal brain tissues and two human adult brain tissues after 14 days in coculture with human fetal brain tissue-derived astrocytes were used as positive controls. Undifferentiated MSCs derived from two human fetal and two adult BM samples were used as negative controls. Injection of depolarizing currents (0.5–1 nA) induced repetitive action potentials on the neurons in the positive controls (Fig. 4D) and not on the undifferentiated MSCs in the negative controls (Fig. 4D). Injection of depolarizing currents (0.5–1 nA) on the neurons derived from MSC-derived NSCs after 14 days in coculture with human fetal brain tissue-derived astrocytes induced repetitive action potentials that were abolished in the presence of neurotoxin TTX (Fig. 4D), suggesting that neurons generated from MSC-derived NSCs are electrically active, similar to those generated from NSCs that were derived from fetal and adult brain tissues. Taken together, these results show that MSC-derived NSCs, like those derived from fetal or adult brain tissues, possess the potential to differentiate into neurons with essential properties of mature CNS neurons.
FIG. 4.
Coculture of mesenchymal stem cell (MSC)-derived neural stem cells (NSCs) with primary astrocytes derived from human fetal brain tissues. (A) Astrocytes derived from human fetal brain tissues. Cells were stained to detect two astrocyte markers, s-100β (green) and GFAP (red), as well as DAPI to detect nuclei. Scale bar, 50 μm. (B) Synapse formation between neural progeny of MSC-derived NSCs in coculture with human fetal brain-derived astrocytes. Cells (14d) were stained for MAP-2 and synapsin. Scale bar, 50 μm. (C) Ultrastructure of synapses between neural progenies of MSC-derived NSCs. Red and blue arrows point to presynaptic vesicles and postsynaptic membranes, respectively. Scale bar, 450 nm. (D) Firing action potentials. Positive control shows a typical firing of repetitive action potentials by the neurons generated from human brain tissue-derived NSCs in the positive control group in response to pulses of current, and negative control displays that pulses of current do not induce the undifferentiated MSCs in the negative control group to fire repetitive action potentials. Injection of depolarizing currents (0.5–1 nA) on the neurons derived from MSC-derived NSCs induces the firing of repetitive action potentials that is abolished in the presence of neurotoxin TTX (0.5 μM). Scale bars, 30 mV and 50 ms.
Derivation of MSCs from MSC-derived NSCs
To further understand the possible underlying mechanisms of how human adult BM-derived MSCs can be converted into NSCs, we investigated if these MSC-derived NSCs still retain the potential to differentiate into MSCs even though they had lost their MSC characteristics. To investigate whether these MSC-derived NSCs could generate MSCs, MSC-derived neurospheres were harvested from the NSC culture medium and switched to MSC culture conditions, either as spheres or as single cells. In MSC culture conditions, the MSC-derived NSCs, regardless of whether they were plated as spheres or single cells, adhered to the culture flask and showed the morphology similar to that of MSCs (Fig. 5A). Furthermore, these adherent cells expressed the characteristic antigens of MSCs (Fig. 5B) and can be induced to differentiate into adipocytes, osteoblasts, and chondrocytes (Fig. 5C). These results demonstrate that MSC-derived NSCs, which had lost their MSC characteristics, maintained the ability to generate MSCs when the culture conditions changed from NSC culture conditions to MSC culture conditions.
FIG. 5.
Characterization of mesenchymal stem cells (MSCs) generated from MSC-derived neural stem cells (NSCs). (A) Morphology of adherent cells generated from MSC-derived NSCs in MSC culture condition. (B) Flow cytometric analysis for the expression of characteristic MSC antigens on the adherent cells generated from MSC-derived NSCs in MSC culture condition. Characteristic MSC antigens include CD29, CD44, CD73, CD90, CD105, and CD166. Red line represents the isotype control and green line represents the staining against each specified antibodies. (C) Differentiation of adherent cells generated from MSC-derived NSCs into three mesodermal lineages: adipocytes (oil red O staining), chondrocytes (collagen II antibody staining), and osteoblasts (von Kossa staining for calcium deposits). Scale bars: (A) 75 μm; (C) adipocyte 2.5 μm, osteoblast 15 μm, and chondrocyte 50 μm.
We next investigated whether all or only a fraction of the MSC-derived NSCs had the potential to generate MSCs. MSC-derived neurospheres were harvested from the NSC culture medium, trypsinized, and resuspended in MSC culture medium. Limiting dilutions of cells from donor 1 (900, 300, and 100 cells per well) were grown under MSC culture conditions, and the number of adherent cell clusters (>50 cells) was scored at 7–10 days. Only 4–5% of cells within these MSC-derived neurospheres adhered to the culture flasks, proliferated, and differentiated into MSCs when grown in MSC culture conditions (Table 2).The data from the other two donors were almost identical to the data from donor 1 and showed no noticeable variation in the frequency of MSCs generated from MSC-derived NSCs (Table 2).These MSCs have been propagated up to the 10th passage, and the expanded MSCs have maintained all MSC characteristics, including the expression of characteristic MSC antigens such as CD29, CD44, CD73, CD90, CD105, and CD166, and the ability to differentiate into cells of multiple mesodermal lineages, including adipocytes, chondrocytes, and osteoblasts. Taken together, these results demonstrate that a small fraction (4–5%) of cells within the MSC-derived NSC population maintained the ability to differentiate into MSCs when grown in MSC culture conditions.
Table 2.
Frequency of Cells Capable of Adhering and Proliferating within the MSC-Derived NSCs
| Cell dose | 900 | 300 | 100 |
|---|---|---|---|
| Donor | |||
| 1 | 45.6 ± 4.2 | 14.8 ± 1.4 | 4.8 ± o.6 |
| 2 | 43.2 ± 3.8 | 14.2 ± 2.2 | 4.3 ± 0.4 |
| 3 | 45.3 ± 4.1 | 14.5 ± 1.5 | 4.6 ± 0.5 |
Abbreviations: MSC, mesenchymal stem cell; NSC, neural stem cell.
Dilutions of cells (900, 300, and 100 cells per well) were plated in well of 24-well plates with 1 mL MSC culture medium. These wells were scored 7–10 days later for the number of adherent cell clusters (>50 cells). The total number of wells analyzed for each cell dose is 60. Data for the number of adherent cell clusters per well are presented as the mean ± SD in each cell dose. These data are compiled from three independent experiments using MSCs derived from different donor samples.
Discussion
Many common neurological disorders, such as Parkinson's disease, stroke, and multiple sclerosis, are caused by the loss of neurons and glial cells. It is hoped that NSCs will provide a long-lasting source of neurons and glials for therapies aimed at cell replacement in disorders affecting the brain and spinal cord. Two recent reports demonstrated that clonal NSCs were effectively converted from BM-derived MSCs [29,30]. In one study, the data demonstrated that majority of MSCs (>60%) were converted to NSCs, suggesting that the conversion of MSCs into NSCs is a transdifferentiation phenomenon [30]. Since these protocols have considerable implications for using autologous NSCs generated from clinically accessible BM-derived MSCs for treating neurodegenerative diseases, we repeated the experimental protocol from Hermann et al. [30] as a prelude to further dissecting the underlying mechanisms of how human adult MSCs can be converted to NSCs.
In this study, using MSCs derived from three human adult BM samples, we have confirmed that MSCs can generate clonal NSCs when cultured in NSC culture conditions (Fig. 2), as described in a previous report [30]. However, our data showed that only a small fraction (on average, 8%) of MSCs possess the ability to generate NSCs (Fig. 2). These MSC-derived NSCs showed typical NSC characteristics, including the expression of the characteristic NSC antigens, self-renewal, and multilineage differentiation into neurons, astrocytes, and oligodendrocytes (Fig. 3 and Table 1).To further examine the functional aspects on these MSC-derived NSCs, immunocytochemistry, EM, and electrophysiology were employed to demonstrate that neurons derived from these MSC-derived NSCs are electrically active and exhibit functional synaptic transmission (Fig. 4). In addition, these MSC-derived NSCs also stopped expressing characteristic antigens of MSCs and lost the ability to differentiate into cells of mesodermal lineages, including adipocytes, chondrocytes, and osteoblasts (data not shown). Furthermore, we have demonstrated that a small fraction (4–5%) of MSC-derived NSCs maintains the potential to generate MSCs when they are switched back to the MSC culture conditions (Fig. 5 and Table 2).Those MSCs generated from MSC-derived NSCs, express the characteristic MSC antigens, including CD29, CD44, CD73, CD90, CD105, and CD166, proliferate, and differentiate into multiple mesodermal lineages, including adipocytes, chondrocytes, and osteoblasts [Fig. 5].
Organ-specific stem cells have been identified in a variety of mammalian tissues [43,44]. These cells hold great promise for cellular therapy, if they can reliably produce functional progeny of specific lineages. A central dogma in development has been that organ-specific stem cells are restricted to making the differentiated cell types of the tissue from which they are isolated. One of the major achievements in stem cell research over the last 10 years is the discovery that BM and blood stem cells are able to generate not only blood cells but also cells belonging to many different tissues such as brain, liver, lung, kidney, heart, and pancreas [44,45]. These studies suggest that BM and blood stem cells possess a broader developmental potential than originally thought. Since BM and blood stem cell transplantation have been well established over the last 40 years as an effective treatment option for patients with various blood disorders, this new discovery suggests that BM and blood stem cells may some day be used to treat patients with neurodegenerative disorders, liver diseases, heart failure, and diabetes. However, there is a large body of conflicting results regarding the broader developmental potential of BM and blood stem cells and it remains a controversial issue in the stem cell research field [44–47].
To clarify this controversial issue in stem cell plasticity, two specific areas need to be appreciated. The first area is the complexity of the stem cell populations in the BM and blood. BM and blood contain several different stem cell populations, including hematopoietic stem cells [48], MSCs [18], mesodermal adult progenitor cells [49,50], and others [51–53]; however, each stem cell population represents only a very minor population (e.g., 0.01–0.001%). It is also well accepted in the stem cell field that all stem cell populations are heterogeneous. The second area is the lack of common standards. Several stem cells could be derived from different tissues, by diverse isolation protocols, cultured and expanded in different media and conditions. All of these variables could (1) influence the selection of cell types and the composition of heterogeneous subpopulations; (2) selectively favor expansion of various cell populations with substantially different potentials; or (3) alter the long-term fate of adult stem cells upon in vitro culture. It is likely that the complexity of stem cell populations and the lack of common standards could have significantly contributed to the generation of conflicting results from different laboratories. We are taking a simplified approach to investigate if BM stem cells can differentiate into neural cells by using a subpopulation of stem cells instead of whole BM or blood cells. It has been reported that all identified stem cell populations in the BM, including hematopoietic stem cells [44–47], MSCs [21–28], mesodermal adult progenitor cells [50], and others [51–53], possess the potential to differentiate into neural cells. However, MSCs are the most well-characterized stem cell population for the generation of neural cells, both in vitro and in vivo, and are the choice of stem cell population in this study.
A small fraction (usually <5%) of MSCs possesses the potential to differentiate into cells expressing neuronal and glial markers, both in vitro [21–23] and in vivo [24–27]. However, the identity of these cells remains unknown [21–28]. Several lines of evidence have recently reported and further demonstrated that MSCs are heterogeneous. Colter et al. reported that single cell-derived colonies of MSCs contained two morphologically distinct cell types: spindle-shaped cells and large flat cells [54]. Tremain et al. reported that MicroSAGE analysis of 2,353 expressed genes in a single cell-derived colony of undifferentiated human MSC revealed the expression of mRNAs from multiple cell lineages, including chondrocytes, myoblasts, osteoblasts, and endothelial, epithelial, and neural cell lineages [55]. In addition, Woodbury et al. reported that RT-PCR analysis shows that adult BM stromal cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis [56]. The work of Pochampally et al. showed that serum deprivation of human marrow stromal cells selects for a subpopulation of early progenitor cells with enhanced expression of Oct-4 and other embryonic genes [57]. Taking all of these published data together, it is generally appreciated that populations of MSCs are heterogeneous [54–56], contain a small fraction (<5%) of cells that are capable of differentiating into neural cells [21–28], and may also contain a subpopulation of more primitive Oct-4+ stem cells [57]. However, the identities of these cells within the MSCs that are capable of differentiating into neural cells remain not well defined.
Several possible mechanisms have been proposed for the apparent stem cell plasticity, including cell fusion [58,59], transdifferentiation of tissue specific stem cells, the coexistence of multiple stem cells with different potentials, or resident totipotent stem cells in these tissues [43,44,60]. In this study, we convincingly demonstrated that multipotent NSCs can be generated from human BM-derived MSCs when they are cultured in NSC culture conditions and that those MSC-derived NSCs can further differentiate into functional neurons that form synapses and can fire action potentials. We further demonstrated that only 8% of MSCs are capable of differentiating into NSCs, suggesting that 8% of MSCs are committed multipotent NSCs. These 8% committed multipotent NSCs within MSCs may account for the well-established phenomenon that <5% of MSCs that are capable of differentiating into cells that express neuronal and glial markers. It remains possible that MSCs may also contain further committed neural progenitor cells that cannot proliferate and be seen in the NSC culture conditions; however, the frequency for these cells should be minimal. In addition, we have demonstrated that 4–5% of MSC-derived NSCs retain the potential to differentiate into MSCs when they are cultured in MSC culture conditions, suggesting that there is another subpopulation of stem cells within those committed multipotent MSC-derived NSCs that are more primitive and possess the potentials to differentiate into multipotent NSCs and multipotent MSCs depending on the culture conditions. Data presented in this study suggest that the possible mechanisms for the differentiation of MSCs into neural cells are the presence of committed multipotent NSCs and the more primitive bipotential stem cell population in the heterogeneous MSCs. Experiments are in progress in our laboratory to further define the identities of those committed multipotent NSCs and the more primitive bipotential stem cell population within the heterogeneous MSCs and to further assess the function and safety of these committed multipotent NSCs and the more primitive bipotential stem cell population in vivo. The ability to identify, purify, and expand those committed multipotent NSCs and the more primitive bipotential stem cells will facilitate the realization of autologous NSC transplant strategies for neurodegenerative disorders.
Acknowledgments
We thank personnel in the Flow Cytometry Core Laboratory for their assistance in FACS analysis; personnel in the Light Microscopy Digital Imaging Core Laboratory for their assistance in microscopy imaging; Keely Walker in the Office of Faculty and Institutional Support, City of Hope, for her assistance in preparation of the manuscript. This investigation was supported by grants from the NIH (NCI PPG CA 30206 and NCI P30 CA 33572), The Ella Fitzgerald Charitable Foundation, The California Community Foundation, and The Rosalinde and Arthur Gilbert Foundation.
References
- 1.Forman MS. Trojanowski JQ. Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O. Kokaia Z. Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders––how to make it work. Nat Med. 2004;10(Suppl):S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 3.Lindvall O. Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 4.Kim BJ. Auerbach JM. Rodriguez-Gomez JA. Velasco I. Gavin D. Lumelsky N. Lee SH. Nguyen J. Sanchez-Pernaute R. Bankiewicz K. McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 5.Pluchino S. Quattrini A. Brambilla E. Gritti A. Salani G. Dina G. Galli R. Del Carro U. Amadio S. Bergami A. Furlan R. Comi G. Vescovi AL. Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 6.Lee JP. Jeyakumar M. Gonzales RI. Takahashi H. Lee PJ. Baek RC. Clark D. Rose H. Fu G. Clarke J. McKercher S. Meerloo J. Muller FJ. Park KI. Butters TD. Dwek RA. Schwartz P. Tong G. Wenger D. Lipton SA. Seyfried TN. Platt FM. Snyder EY. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 7.Kordower JH. Freeman TB. Snow BJ. Vingerhoets FJG. Mufson EJ. Sanberg PR. Hauser RA. Smith DA. Nauert GM. Perl DP. Olanow CW. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 8.Piccini P. Brooks DJ. Bjorklund A. Gunn RN. Grasby PM. Rimoldi O. Brundin P. Hagell P. Rehncrona S. Widner H. Lindvall O. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 9.Lindvall O. Hagell P. Clinical observations after neural transplantation in Parkinson's disease. Prog Brain Res. 2000;127:299–320. doi: 10.1016/s0079-6123(00)27014-3. [DOI] [PubMed] [Google Scholar]
- 10.Freed CR. Greene PE. Breeze RE. Tsai WY. DuMouchel W. Kao R. Dillon S. Winfield H. Culver S. Trojanowski JQ. Eidenberg D. Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 11.Hagell P. Piccini P. Bjorklund A. Brundin P. rehncrona S. Widner H. Crabb L. Pavese N. Oertel WH. Quinn N. Brooks DJ. Lindvall O. Dyskinesia after neural transplantation in Parkinson's disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 12.Bjorklund A. Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 13.Carvey PM. Ling ZD. Sortwell CE. Pitzer MR. McGuire SO. Collier TJ. A clonal line of mesencephalic progenitor cells converted to dopamine neurons by hematopoietic cytokines: a source of cells for transplantation in Parkinson's disease. Exp Neurol. 2001;171:98–108. doi: 10.1006/exnr.2001.7735. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds BA. Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds BA. Weiss S. Clonal and population analysis demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S. Werning M. Duncan ID. Brustle O. Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 17.Reubinoff BE. Itsykson P. Turetsky T. Pera MF. reinhartz E. Itzik A. Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Caplan AI. Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends in Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 20.Bianco P. Riminucci M. Gronthos S. Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Ramos JR. Song S. Cardozo-Pelaez F. Hazzi C. Stedeford T. Willing A. Freeman TB. Saporta S. Janssen W. Patel N. Cooper DR. Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 22.Woodbury D. Schwarz EJ. Prockop DJ. Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Buzanska L. Machaj EK. Zablocka B. Pojda Z. Domanska-Janik K. Human cord blood-derived cells attain neuronal and glial feasures in vitro. J Cell Sci. 2002;115:2131–2138. doi: 10.1242/jcs.115.10.2131. [DOI] [PubMed] [Google Scholar]
- 24.Zhao LR. Duan WM. Reyes M. Keene CD. Verfallie CM. Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 25.Kopen GC. Prockop DJ. Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes, after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azizi SA. Stokes DA. Augelli BJ. DiGirolamo C. Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats-similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofstetter CP. Schwarz EJ. Hess D. Widenfalk J. El Manira A. Prockop DJ. Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blondheim NR. Levy YS. Ben-Zur T. Burshtein A. Cherlow T. Kan I. Barzilai R. Bahat-Stromza M. Barhum Y. Bulvik S. Melamed E. Offen D. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006;15:141–164. doi: 10.1089/scd.2006.15.141. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H. Taguchi T. Tanaka H. Kataoka H. Li Z. Muramatsu K. Gondo T. Kawai S. Neurospheres induced from bone marrow stromal cells are multipotent for differentiation into neuron, astrocyte, and oligodendrocyte phenotypes. Biochem Biophys Res Comm. 2004;322:918–922. doi: 10.1016/j.bbrc.2004.07.201. [DOI] [PubMed] [Google Scholar]
- 30.Hermann A. Gastl R. Liebau S. Popa MO. Fiedler J. Boehm BO. Maisel M. Lerche H. Schwarz J. Brenner R. Storch A. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 31.Hockfield S. McKay RDG. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneko Y. Sakakibara S. Imai T. Suzuki A. Nakamura Y. Sawamoto K. Ogawa Y. Toyama Y. Miyata T. Okano H. Musashi 1: an envolutionally conversed marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22:139–153. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 33.Shih CC. Hu MCT. Hu J. Medeiros J. Forman SJ. Long-term ex vivo maintenance and expansion of transplantable human hematopoietic stem cells. Blood. 1999;94:1623–1636. [PubMed] [Google Scholar]
- 34.Shih CC. MCT Hu. Hu J. Yazaki PJ. Weng Y. Meideros J. Forman SJ. A secreted and LIF-mediated stromal cell-derived activity that promotes ex vivo expansion of human hematopoietic stem cells. Blood. 2000;95:1957–1966. [PubMed] [Google Scholar]
- 35.Shih CC. Weng Y. Mamelak A. LeBon T. Hu MCT. Forman SJ. Identification of a candidate human neurohematopoietic stem-cell population. Blood. 2001;98:2412–2422. doi: 10.1182/blood.v98.8.2412. [DOI] [PubMed] [Google Scholar]
- 36.Ai C. Todorov I. Slovak ML. Digiusto D. Forman SJ. Shih CC. Human marrow-derived mesodermal progenitor cells generate insulin-secreting islet-like clusters in vivo. Stem Cells Dev. 2007;16:757–770. doi: 10.1089/scd.2007.0038. [DOI] [PubMed] [Google Scholar]
- 37.Song H. Stevens CF. Gage FH. Nueral stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 38.Schikorski T. Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- 39.Bekkers JM. Stevens JM. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA. 1991;88:7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzsimonds RM. Song HJ. Poo MM. Propagation of activity-dependent synaptic depression in simple neural networks. Nature. 1997;388:439–448. doi: 10.1038/41267. [DOI] [PubMed] [Google Scholar]
- 41.Kandel ER. Schwartz JH. Jessell TM. Principles of Neural Science. McGraw-Hill; New York: 2000. [Google Scholar]
- 42.Zaheer A. Zhong W. Uc EY. Moser DR. Lim R. Expression of mRNAs of multiple growth factors and receptors by astrocytes and glioma cells: detection with reverse transcription-polymerase chain reaction. Cell Mol Neurobiol. 1995;15:221–237. doi: 10.1007/BF02073330. [DOI] [PubMed] [Google Scholar]
- 43.Shih CC. Digiusto D. Mamelak A. LeBon T. Forman SJ. Hematopoietic potential of neural stem cells: plasticity versus heterogeneity. Leukemia Lymphoma. 2002;43:2263–2268. doi: 10.1080/1042819021000040215. [DOI] [PubMed] [Google Scholar]
- 44.Raff M. Adult stem cell plasticity: fact or artifact? Annu Rev Cell Dev Biol. 2003;19:1–22. doi: 10.1146/annurev.cellbio.19.111301.143037. [DOI] [PubMed] [Google Scholar]
- 45.Sell S. Adult stem cell plasticity: introduction to the first issue of Stem Cell Reviews. Stem Cell Rev. 2005;1:1–8. doi: 10.1385/SCR:1:1:001. [DOI] [PubMed] [Google Scholar]
- 46.Quesenberry PJ. Dooner G. Colvin G. The stem cell continuum: considerations on the heterogeneity and plasticity of marrow stem cells. Stem Cell Rev. 2005;1:29–36. doi: 10.1385/SCR:1:1:029. [DOI] [PubMed] [Google Scholar]
- 47.Vieyra DS. Jackson KA. Goodell MA. Plasticity and tissue regenerative potential of bone marrow-derived cells. Stem Cell Rev. 2005;1:65–70. doi: 10.1385/SCR:1:1:065. [DOI] [PubMed] [Google Scholar]
- 48.Spangrude GJ. Heimfeld S. Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:59–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 49.Reyes M. Lund T. Lenvik T. Aguiar D. Koodie L. Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 50.Jiang Y. Jahagirdar BN. Reinhardt RL. Schwartz RE. Keene CD. Ortiz-Gonzalerz XR. Reyes M. Lenvik T. lund T. Blackstad M. Du J. Aldrich S. Lisberg A. Low WC. Largaespada DA. Verfallie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 51.Kogler G. Sensken S. Airey JA. Trapp T. Muschen M. Feldhahn N. Liedtke S. Sorg RV. Fischer J. Rosenbaum C. Greschat S. Knipper A. Bender J. Degistirci O. Gao J. Caplan AI. Colletti EJ. Almeida-Porada G. Muller HW. Zanjani E. Wernet P. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'lppolito G. Diabira S. Howard GA. Menei P. Roos BA. Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 53.Kucia M. Ratajczak J. Ratajczak MZ. Bone marrow as a source of circulating CXCR4+ tissue-committed stem cells. Biol Cell. 2005;97:133–146. doi: 10.1042/BC20040069. [DOI] [PubMed] [Google Scholar]
- 54.Colter DC. Sekiya I. Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tremain N. Korkko J. Ibberson D. Kopen GC. DiGirolamo C. Phinney DG. MicroSAGE analysis of 2,353 expressed genes in a single cell-derived colony of undifferentiated human mesenchymal stem cells reveals mRNAs of multiple cell lineages. Stem Cells. 2001;19:408–418. doi: 10.1634/stemcells.19-5-408. [DOI] [PubMed] [Google Scholar]
- 56.Woodbury D. Reynolds K. Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002;69:908–917. doi: 10.1002/jnr.10365. [DOI] [PubMed] [Google Scholar]
- 57.Pochampally RR. Smith JR. Ylostalo J. Prockop DJ. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood. 2004;103:1647–1652. doi: 10.1182/blood-2003-06-1967. [DOI] [PubMed] [Google Scholar]
- 58.Ying QL. Nichols J. Evans EP. Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 59.Terada N. Hamazakim T. Oka M. Hoki M. Mastalerz DM. Nakano y. Meyer EM. Morel L. Peterson BE. Scott EW. Bone marrow cells adopt the phenotypes of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 60.Jang YY. Sharkis SJ. Stem cell plasticity: a rare cell, not a rare event. Stem Cell Reviews. 2005;1:45–52. doi: 10.1385/SCR:1:1:045. [DOI] [PubMed] [Google Scholar]