Abstract
As newly synthesized proteins emerge from the ribosome, they interact with a variety of co-translational cellular machineries that facilitate their proper folding, maturation and localization. These interactions are essential for proper function of the cell and the ability to study these events is crucial to understanding cellular protein biogenesis. To this end, we have developed a highly efficient method to generate ribosome nascent chain complexes (RNC) site-specifically labeled with a fluorescent dye on the nascent polypeptide. The fluorescent RNC provided real-time, quantitative information on its co-translational interaction with the Signal Recognition Particle and will be a valuable tool in elucidating the role of the translating ribosome in numerous biochemical pathways.
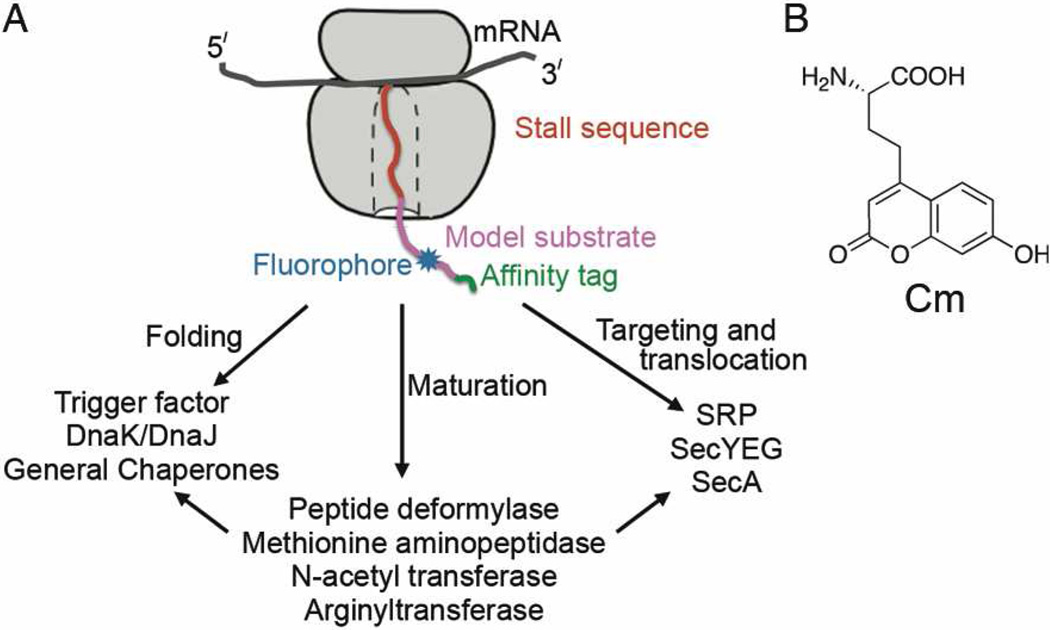
The ribosome is the central molecular machine that translates genetic information to proteins. As newly synthesized proteins emerge from the ribosomal exit tunnel, they interact with a host of cellular factors that facilitate the folding, localization, maturation, and quality control of the nascent proteins. These include chaperones1 such as trigger factor, Hsp70 (DnaK/J in bacteria) and the nascent chain-associated complex; modification and processing enzymes2 such as methionine aminopeptidase (or peptide deformylase in bacteria), N-acetyl transferase, and arginyl transferase; protein transport machineries3 such as the Signal Recognition Particle (SRP), the Sec61p (or secYEG) protein conducting channel, and even possibly SecA4 (Scheme 1A). These interactions, which often take place at the ribosome exit site, are essential for the proper function of the cell. A molecular understanding of the dynamic interactions of these machineries with the ribosomenascent chain complex (RNC) is crucial to understanding cellular protein biogenesis, yet such information is challenging to obtain due to the lack of suitable tools. To directly and quantitatively monitor these interactions, we have developed an efficient method to generate synchronized RNCs site-specifically labeled with a fluorophore on the nascent protein. Large quantities of fluorescent RNC can be generated using this method, enabling kinetic and thermodynamic studies of early events during the biogenesis of the nascent chain as it emerges from the ribosome.
Scheme 1.
(A) A schematic depiction of the fluorescent RNC and some cellular factors that interact with the ribosome-associated nascent protein. (B) Structure of the fluorescent amino acid, Cm, used in this study.
Several factors need to be considered for efficient labeling and purification of ribosome nascent chain complexes. The ribosome comprises numerous proteins, which precludes labeling strategies based on protein chemistry; hence selective labeling on the nascent chain necessitates the use of a bioorthogonal reactivity handle. Additionally, the fluorophore should be small to minimize perturbations due to probe incorporation. Finally, to ensure that intact RNC is obtained, we need to minimize harsh and lengthy procedures during labeling and purification. An approach that satisfies these requirements is amber suppression, which incorporates a fluorophore into the nascent chain co-translationally in response to the UAG (amber) stop codon.5,6 Current methods for generating fluorescent RNCs7,8 often use purified aminoacylated tRNA; however, the yield is low as the aminoacylated tRNA is chemically labile. Instead, we chose the M. jannaschii tyrosyl-tRNA synthetase (aaRS) developed by Schultz, which aminoacylates a cognate tRNACUA with the fluorescent amino acid L-(7-hydroxycoumarin-4-yl)ethylglycine (Cm, Scheme 1B).9,10 The use of a cognate aaRS allows continuous regeneration of the aminoacylated tRNACUA, resulting in higher yield.
This method, originally developed for in vivo production of proteins, is not optimal for the generation of fluorescent RNC for several reasons. Firstly, during the generation of a stalled RNC, the ribosome remains stably bound to the mRNA and the nascent chain and hence cannot be reused for multiple rounds of protein synthesis, resulting in low yields. Secondly, during amber suppression, release factor-1 (RF-1) competes with the suppressor tRNACUA for decoding the amber codon, which terminates protein synthesis and further reduces the yield. Finally, a high concentration of Cm (typically 1 mM) is required in in vivo experiments. This not only limits the scale of RNC production but also complicates subsequent purification of the RNC, as hydrophobic fluorophores can bind to the ribosome non-specifically at high concentrations. To address these problems, we generated labeled RNC in a highly efficient in vitro transcription-translation system.11–13 Cell-free translation permits the use of an RF-1 inhibitor to improve suppression yield, a technique that might be toxic in vivo. Further, the concentration of Cm required for efficient amber suppression is much lower (see below), which facilitates both labeling and purification. Finally, in vitro protein synthesis offers the flexibility to generate multiple RNCs with different nascent chain composition, length, and location of the fluorescent dye using the same cell lysate. This greatly reduces the time and effort required to explore different nascent chain constructs compared to that in vivo.
To test and optimize the efficiency of amber suppression in vitro, we started with a model protein chloramphenicol acetyl-transferase (CAT). We generated S30 cell lysates from E. coli KC6 strain14 that constitutively expressed tRNACUA.15,16 An amber suppression reaction (see SI for experimental details), with an amber codon engineered within the coding sequence of CAT, produced the full-length protein in 45–80% yield compared to wild-type CAT (SI Figure 1). In the absence of aaRS or Cm, < 2% of the full-length product was obtained, indicating highly specific incorporation of Cm into the protein. Addition of an RNA aptamer17 that inhibits RF-1 (RF-apt) increased the yield of the full-length product two-fold (SI Figure 2). Interestingly, in a less efficient suppression system using an earlier generation tRNACUA,9 addition of RF-apt provided greater enhancement in suppression yield (SI Figure 3).
We next used this method to generate a synchronized RNC complex, site-specifically labeled with Cm (Scheme 1A, blue) on the nascent chain. To ensure a homogeneous population of nascent chains bound to the ribosome, a SecM stall sequence that arrests translation elongation at a specific point19 was appended to the C-terminus of the protein (Scheme 1A, red). The nascent chain also contained a triple strep tag at the N-terminus for affinity purification (Scheme 1A, green).18 As a model protein substrate (Scheme 1A, magenta), we used the N-terminal region of a variant of alkaline phosphatase (phoA), called 1A9L.20 In vitro transcription-translation of a 1A9L template, with an amber codon at position 56, generated a polypeptide that matched the size expected for the full-length translation product. The suppression yield was close to 100% compared to wild-type 1A9L (Figure 1A, cf lanes 6 and 7 vs. 1). About 5–6% background suppression was observed in the absence of aaRS or Cm (Figure 1A, lanes 3–4); this background is likely lower in the presence of the orthogonal aminoacylation machinery.
Figure 1.
(A) Translation and amber suppression of a model substrate, 1A9L, without (lane 1) or with (lanes 3 – 7) an amber codon at residue 56 from the N-terminus. Translation products were visualized by labeling with 35S-methionine and SDS-PAGE. The full-length band was quantified relative to that of wild-type 1A9L (lane 1) by autoradiography. The lower molecular weight band (marked with an asterisk) is from the polysome fraction.18 (B) The suppression yield of full-length RNC1A9L at different concentrations of Cm, quantified as in part A. (C) Translation of a 125 amino acid-long nascent chain of firefly luciferase (Luc), without (lane 1) and with (lanes 3–8) the amber codon placed at various locations in the nascent chain. When the amber codon is placed at position 85 (lane 4), the fluorophore is estimated to be near the peptide exit site.
The practical large-scale generation of fluorescent RNC is limited by the high concentration of Cm typically required for in vivo amber suppression. So, we examined the dependence of the suppression yield on the concentration of Cm in the in vitro reaction. A suppression yield close to saturation was obtained at ~50 µM (Figure 1A, lanes 6 and 7 and Figure 1B), ~20-fold lower than that in vivo. This, combined with the smaller volume of in vitro translation reactions, reduced the consumption of Cm over two orders of magnitude. The use of lower concentration of Cm also minimized nonspecific binding of the fluorescent dye to the ribosome and simplified subsequent purification of the fluorescently labeled RNC.
To evaluate the generality of the method, we tested amber suppression in RNCs carrying another nascent protein, the N-terminal region of firefly luciferase (RNCLuc).20 Furthermore, to test the dependence of suppression yield on the sequence context, we varied the position of the amber codon in the open reading frame. Highly efficient incorporation of Cm to generate the full-length nascent chain was observed for RNCLuc at virtually all the positions tested (Figure 1C), even when the amber codon was placed near the C-terminus of the nascent polypeptide (Figure 1C, lane 4). Thus, this method is highly robust and versatile, and allows incorporation of the fluorescent probe at any desired location. In comparison, an established method that uses chemically labeled Met-tRNAfMet to fluorescently label the RNCs is restricted to labeling the N-terminus of proteins.8
To obtain large quantities of RNC for biophysical measurements, we scaled up the translation reaction and purified fluorescently labeled RNC in a single step using a Strep-Tactin column.18,21 The yields, measured by OD260 (Table 1), varied from one substrate protein to another and mirrored the translation efficiency of the corresponding wild-type construct. Notably, 200–500 µg of purified, fluorescently labeled RNC were obtained per ml of translation (equivalent to 2–4 nmol from one liter of cell culture), comparable to the yields of unlabeled RNC.18 The purified RNC generated the fluorescence emission profile expected for 7-hydroxycoumarin (SI Figure 4 and Figure 2B). To test non-specific binding of Cm to the ribosome, RNC was generated from a wild-type 1A9L template, translated in the presence of Cm and purified in parallel. This RNC showed ≤15% fluorescence background (SI Figure 4), indicating specific incorporation of Cm into the nascent chain in response to the amber codon.
Table 1.
Yields of fluorescent RNC complexes after affinity purification. 3A7L is an SRP substrate similar to 1A9L.20 The number after the protein name indicates the position of the amber codon from the N-terminus. Luciferase is abbreviated as Luc. NA: not applicable.
| RNC | RNC yields (mg/5ml translation) | |
|---|---|---|
| − tRNACUA | + tRNACUA | |
| 1A9L (wt) | 2 | 1.4 |
| 1A9L 56 amb | NA | 1.4 |
| 3A7L 56 amb | NA | 0.86 |
| Luc 56 amb | NA | 2.1 |
| Luc 65 amb | NA | 2.6 |
| Luc 75 amb | NA | 2.8 |
| Luc 85 amb | NA | 2.1 |
Figure 2.
A FRET assay for measuring the binding parameters of the RNC-SRP interaction. (A) The crystal structure of SRP (PDB ID:2ffh) with its signal sequence binding M-domain in blue and the helix containing the acceptor fluorophore in red. The arrow marks the hydrophobic groove that binds the signal sequence on the nascent chain. (B) Fluorescence emission spectra of 20 nM RNC1A9L labeled with Cm (black), 30 nM BODIPY-Fl labeled SRP (blue), and their complex (red).
To test whether the fluorescent RNC was functional, we developed a FRET assay to monitor the interaction of RNC1A9L with SRP. SRP is a well-characterized, co-translational protein targeting machinery that recognizes a hydrophobic signal sequence on its substrate proteins. The protein component of E. coli SRP, Ffh, binds the signal sequence via a hydrophobic groove on its methionine-rich M-domain (Figure 2A, blue).22,23 1A9L, in which the natural signal sequence of phoA was replaced with a more hydrophobic core, serves as a model substrate for SRP.20 RNC1A9L with Cm placed two residues downstream of the signal sequence served as the donor in the FRET assay. As the acceptor, we introduced BODIPY-Fl at a non-conserved residue (Figure 2A, gold) on the helix lining the signal sequence-binding groove of Ffh using cysteine-maleimide chemistry.
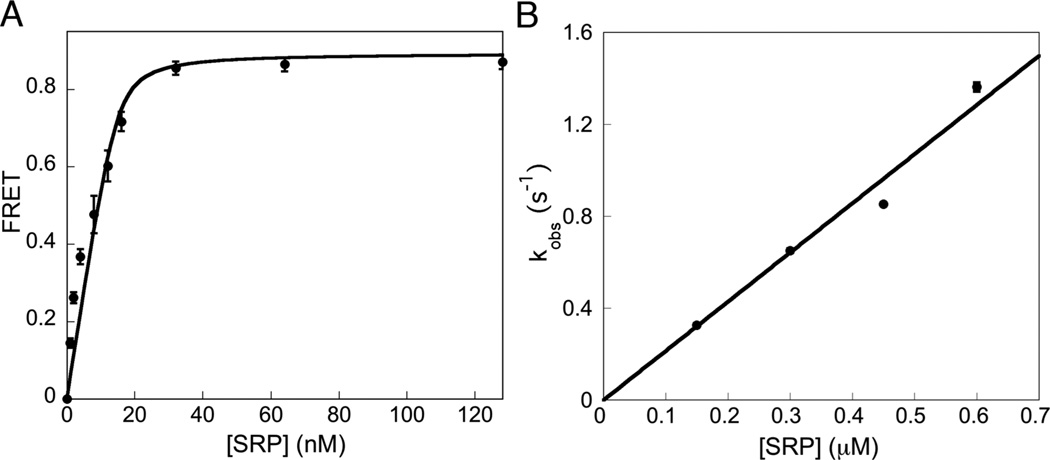
Efficient energy transfer was observed when BODIPY-Fl labeled SRP was added to Cm-labeled RNC1A9L (Figure 2B); this FRET signal could be competed off by excess unlabeled SRP (SI Figure 5). At saturating concentrations of labeled SRP, the FRET efficiency with RNC1A9L was ~ 0.8 (Figure 2B & 3A). Equilibrium titrations based on the FRET signal gave a Kd value of 0.73 ± 0.43 nM (Figure 3A), in good agreement with reported values.7,20,24 The FRET assay further allowed us to measure the kinetics of RNC•SRP complex assembly on the millisecond time scale. The assembly of the complex fit well to single exponential kinetics (SI Figure 6). The observed rate constant increased linearly with increasing SRP concentration, giving a kon value, as measured by the slope of the concentration dependence, of 2.2 × 106 M−1s−1 (Figure 3B).
Figure 3.
(A) Equilibrium titration for binding of 20 nM fluorescently labeled RNC1A9L to BODIPY-Fl labeled SRP. The data were fit to Eq 2 (SI) and gave a dissociation constant of 0.73 ± 0.43 nM. (B) The observed rate constants of association of RNC1A9L with SRP in the presence of a non-hydrolyzable GTP analogue, GppNHp (SI Figure 6), were plotted against the SRP concentration. A linear fit of the data (SI, Eq 3) gave a kon value of 2.2 × 106 M−1s−1. Measurements were performed in triplicate and are representative of several experiments. Error bars are depicted but are not visible in B.
The specificity of the SRP-nascent chain interaction was tested by comparison with RNCLuc, whose nascent chain lacks a hydrophobic signal sequence. Addition of BODIPY-Fl labeled SRP to RNCLuc with Cm incorporated at several different positions resulted in no significant change in the fluorescence signal (SI Figure 7), indicating the lack of interaction between the SRP M-domain and the nascent chain of an SRP-independent substrate.
The generation of large quantities of fluorescently labeled RNC has allowed, for the first time, the direct measurement of the dynamic interactions of the nascent polypeptide with the SRP. This method can be applied to study a variety of co-translational biochemical pathways by engineering the fluorophore at a designated site in the nascent protein of interest. Since the fluorescent dye is on the protein, this method can also be used to study nascent-chain dynamics on the ribosome25,26 or to follow the fate of newly synthesized proteins in post-translational pathways. Compared to in vivo amber suppression, the in vitro setting offers higher flexibility in generating RNCs with different nascent chain composition and fluorophore position, and eliminates problems with cellular uptake and toxicity of the unnatural amino acid. In principle, other unnatural amino acids, can also be inserted into RNC using this method, e.g. the photocrosslinker p-benzoylphenylalanine 27 for cross-linking experiments. Finally, this method can also be used to generate site-specifically labeled fluorescent proteins that are difficult to produce in vivo due to their toxicity, protease susceptibility, or low solubility.
Supplementary Material
ACKNOWLEDGMENT
We thank Prof. Swartz (Stanford University) for help with generation of S30 extracts, Prof. Schultz (TSRI) for providing the pEVOL plasmid containing the tRNACUA/aaRS pair, Prof. Chapman (TSRI) for a sample of Cm, Profs Tirrell, Barton and Dougherty (Caltech) for use of their facilities, Prof. Sando (Kyushu University) for the RF-1 inhibitor aptamer sequence, and D. Dougherty and members of the Shan group for helpful comments on the manuscript. This work was supported by NIH grant GM078024 to S.S. S.S. was supported by the Henry Dreyfus teacher-scholar award, the Beckman Young Investigator award, and the Packard and Lucile award in science and engineering.
Footnotes
ASSOCIATED CONTENT
Supporting Information available. Experimental protocols and supplementary figures. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
I.S. and S.-o.S. designed research; I.S. performed research with assistance from S.C. and D.Z.; I.S. and S.-o.S. wrote the paper.
REFERENCES
- 1.Fedyukina DV, Cavagnero S. Annu. Rev. Biophys. 2011;40:337–359. doi: 10.1146/annurev-biophys-042910-155338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer G, Boehringer D, Ban N, Bukau B. Nat. Struct. Mol. Biol. 2009;16:589–597. doi: 10.1038/nsmb.1614. [DOI] [PubMed] [Google Scholar]
- 3.Cross BCS, Sinning I, Luirink J, High S. Nat. Rev. Mol. Cell. Biol. 2009;10:255–264. doi: 10.1038/nrm2657. [DOI] [PubMed] [Google Scholar]
- 4.Huber D, Rajagopalan N, Preissler S, Rocco MA, Merz F, Kramer G, Bukau B. Mol. Cell. 2011;41:343–353. doi: 10.1016/j.molcel.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Xie J, Schultz PG. Annu. Rev. Biophys. Biomol. Struct. 2006;35:225–249. doi: 10.1146/annurev.biophys.35.101105.121507. [DOI] [PubMed] [Google Scholar]
- 6.Crowley KS, Reinhart GD, Johnson AE. Cell. 1993;73:1101–1115. doi: 10.1016/0092-8674(93)90640-c. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan JJ, Chen JC, Miao YW, Shao YL, Lin JL, Bock PE, Johnson AE. J. Biol. Chem. 2003;278:18628–18637. doi: 10.1074/jbc.M300173200. [DOI] [PubMed] [Google Scholar]
- 8.Ellis JP, Bakke CK, Kirchdoerfer RN, Jungbauer LM, Cavagnero S. ACS Chem. Biol. 2008;3:555–566. doi: 10.1021/cb800059u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JY, Xie JM, Schultz PG. J. Amer. Chem. Soc. 2006;128:8738–8739. doi: 10.1021/ja062666k. [DOI] [PubMed] [Google Scholar]
- 10.Charbon G, Brustad E, Scott KA, Wang J, Lobner-Olesen A, Schultz PG, Jacobs-Wagner C, Chapman E. ChemBioChem. 2011;12:1818–1821. doi: 10.1002/cbic.201100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goerke AR, Swartz JR. Biotechnol. Bioeng. 2009;102:400–416. doi: 10.1002/bit.22070. [DOI] [PubMed] [Google Scholar]
- 12.Jewett MC, Swartz JR. Biotechnol. Bioeng. 2004;86:19–26. doi: 10.1002/bit.20026. [DOI] [PubMed] [Google Scholar]
- 13.Goerke AR, Swartz JR. Biotechnol. Bioeng. 2008;99:351–367. doi: 10.1002/bit.21567. [DOI] [PubMed] [Google Scholar]
- 14.Calhoun KA, Swartz JR. J. Biotechnol. 2006;123:193–203. doi: 10.1016/j.jbiotec.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Young TS, Ahmad I, Yin JA, Schultz PG. J. Mol. Biol. 2010;395:361–374. doi: 10.1016/j.jmb.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Guo JT, Melancon CE, Lee HS, Groff D, Schultz PG. Angew. Chem. Int. Ed. 2009;48:9148–9151. doi: 10.1002/anie.200904035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sando S, Ogawa A, Nishi T, Hayami M, Aoyama Y. Bioorg. Med. Chem. Lett. 2007;17:1216–1220. doi: 10.1016/j.bmcl.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Schaffitzel C, Ban N. J. Struct. Biol. 2007;158:463–471. doi: 10.1016/j.jsb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Evans MS, Ugrinov KG, Frese MA, Clark PL. Nat. Methods. 2005;2:757–762. doi: 10.1038/nmeth790. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Rashid R, Wang K, Shan SO. Science. 2010;328:757–760. doi: 10.1126/science.1186743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Schaffitzel C, Ban N, Shan SO. Proc. Nat. Acad. Sci. USA. 2009;106:1754–1759. doi: 10.1073/pnas.0808573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janda CY, Li J, Oubridge C, Hernandez H, Robinson CV, Nagai K. Nature. 2010;465:507–510. doi: 10.1038/nature08870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hainzl T, Huang SH, Merilainen G, Brannstrom K, Sauer-Eriksson AE. Nat. Struct. Mol. Biol. 2011;18:389–391. doi: 10.1038/nsmb.1994. [DOI] [PubMed] [Google Scholar]
- 24.Bornemann T, Jockel J, Rodnina MV, Wintermeyer W. Nat. Struct. Mol. Biol. 2008;15:494–499. doi: 10.1038/nsmb.1402. [DOI] [PubMed] [Google Scholar]
- 25.Hsu STD, Cabrita LD, Fucini P, Christodoulou J, Dobson CM. J. Amer. Chem. Soc. 2009;131:8366–8367. doi: 10.1021/ja902778n. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien EP, Christodoulou J, Vendruscolo M, Dobson CM. J. Amer. Chem. Soc. 2011;133:513–526. doi: 10.1021/ja107863z. [DOI] [PubMed] [Google Scholar]
- 27.Chin JW, Martin AB, King DS, Wang L, Schultz PG. Proc. Nat. Acad. Sci. USA. 2002;99:11020–11024. doi: 10.1073/pnas.172226299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.