Abstract
The Eisenberg plot or hydrophobic moment plot methodology is one of the most frequently used methods of bioinformatics. Bioinformatics is more and more recognized as a helpful tool in Life Sciences in general, and recent developments in approaches recognizing lipid binding regions in proteins are promising in this respect. In this study a bioinformatics approach specialized in identifying lipid binding helical regions in proteins was used to obtain an Eisenberg plot. The validity of the Heliquest generated hydrophobic moment plot was checked and exemplified. This study indicates that the Eisenberg plot methodology can be transferred to another hydrophobicity scale and renders a user-friendly approach which can be utilized in routine checks in protein–lipid interaction and in protein and peptide lipid binding characterization studies. A combined approach seems to be advantageous and results in a powerful tool in the search of helical lipid-binding regions in proteins and peptides. The strength and limitations of the Eisenberg plot approach itself are discussed as well. The presented approach not only leads to a better understanding of the nature of the protein–lipid interactions but also provides a user-friendly tool for the search of lipid-binding regions in proteins and peptides.
Keywords: amphitropic proteins, Eisenberg plot, hydrophobic moment plot, Heliquest, lipid binding regions, protein-lipid interactions, transmembrane proteins
1. Introduction
The Eisenberg plot or hydrophobic moment plot is one of the most beautiful examples of where bioinformatics really started off. In the search for methods to translate the primary sequence into more advanced structural information about the structure and folding of proteins, Eisenberg and co-workers developed their methodology [1,2]. Over the past two-three decades, it has become one of the most frequently used approaches in bioinformatics. In essence, the Eisenberg plot pictures the mean hydrophobicity (a measure for the overall hydrophobicity of the sequence) against the mean hydrophobic moment (a measure for the way polar and non-polar amino acids in the sequence are distributed). With the use of the so-called normalized consensus scale, both parameters of a sequence are calculated and windows of varying length between 7–20 amino acids are reported in the literature [2]. The way in which hydrophobicity is fluctuating along a sequence within a protein can be calculated and plotted also in a modified approach [3]. Whether a protein sequence region belongs to a globular, surface seeking or transmembrane protein is a frequently used application of the Eisenberg plot methodology (see [4] for a review). Particulary the search for surface seeking regions in proteins and peptides has received a lot of attention [5]. More recently approaches have been developed that have a special feature to recognize lipid binding regions in proteins [6–8].
Lipids and lipid–protein interactions play an increasingly appreciated and recognized role in many biological processes (see for reviews [9–11]). One interesting recent development is the bioinformatics approach, which enables the identification of lipid binding helical regions in proteins using the Heliquest web-server [6]. A recent example of this approach has been demonstrated for protein translocation motor proteins [12] with the identification of a possible general feature of these motor proteins: the possession of multiple lipid binding regions. The recent finding that multiple lipid binding regions can be identified in a protein translocation motor protein like E. coli SecA [12] corresponds with and possibly expands the earlier findings that specific SecA-lipid interactions could be demonstrated using different approaches [13–15].
This briefly exemplifies the potential power of the Heliquest-based bioinformatics method [6,12]. A closer look at the Heliquest software suggests additional possibilities of this program for the use in the Eisenberg plot methodology since the Heliquest software gives details about, the net charge (z), the mean hydrophobicity (<H>) and the mean hydrophobic moment (μH). In this study the Heliquest approach, though specialized in identifying lipid binding helical regions in proteins, was used to obtain the “original” Eisenberg plot. For this purpose the influence of using another hydrophobicity scale, the Fauchere and Pliska scale [16] instead of the normalized scale of Eisenberg [2], was examined. This study indicates that the Eisenberg plot methodology can be transferred to another hydrophobicity scale and can provide a user-friendly approach. The relevance of this particular methodology is checked on a number of individual cases. The strength and limitations of the Eisenberg plot approach, alone or in combination with the Heliquest lipid-binding feature, are discussed as well.
2. Results and Discussion
2.1. The Eisenberg Plot Approach Using the Original Databases
The Eisenberg plot methodology used an algorithm for detecting hydrophobic polypeptide sequence segments and discriminates between surface-seeking and transmembrane regions. This study checked whether the Heliquest data can give valid results according to the Eisenberg plot methodology [1,2], and whether various regions in a polypeptide could be divided by boundary lines, resulting in three possible alpha-helical properties: transmembrane, lipid surface-seeking and globular. In order to detect whether the data obtained by the Heliquest program allow detection of possible lipid membrane binding and hydrophobic motifs according to the Eisenberg plot methodology, the original databases were investigated [1,2]. For this purpose the corresponding sequences were run through the Heliquest program.
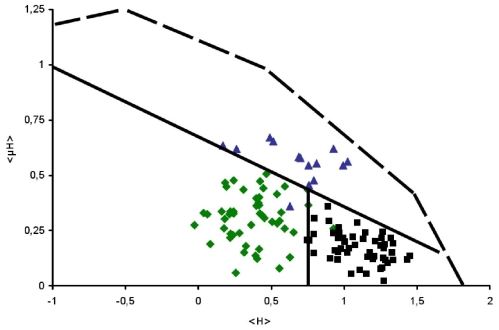
The results found with Heliquest generated data (Figure 1) correspond well with the overall picture of the original Eisenberg approach (see Table S1 and Table S2 for detailed description of all data used). This indicates that the data obtained by the Heliquest program are applicable and that the use of another hydrophobicity scale [16] with the Heliquest generated Eisenberg plot approach is valid. Obviously the scale and absolute numbers for the individual segments differ due to the use of this other hydrophobicity scale. It is interesting to note that the surface seeking regions can be distinguished even better by the Heliquest generated approach than in the original plots.
Figure 1.
Eisenberg plot as obtained by Heliquest generated data based on the original databases of Eisenberg and co-workers [1,2]. The originally identified Globular (
 ), Surface seeking (
), Surface seeking (
 ) and TransMembrane (■) segments are depicted.
) and TransMembrane (■) segments are depicted.
In the original Eisenberg plot methodology two features were extracted. First of all, a surface seeking propensity for surface helices are thought to exist for points close to the line <μH> = 0.600 – 0.342 <H>. Secondly, potential transmembrane helices are assumed if the mean hydrophobicity <H> is greater than 0.51 and the mean hydrophobic moment is below the line as defined above [2]. The corresponding features in the plot obtained by Heliquest generated data are <μH> = 0.654 – 0.324<H> and <H> above 0.75 respectively.
2.2. The Validity Check of the “New” Eisenberg Plot
In order to check the validity of the newly obtained Eisenberg plot one step further, a number of more recent examples were checked which were not included in the Eisenberg databases [1,2]. In Table 1, a number of examples are depicted with more recent data that used the original Eisenberg approach and which were compared with the Heliquest generated Eisenberg plot.
Table 1.
Representative examples of clearly identified transmembrane (M) and surface seeking (S) regions of proteins and peptides as reported in the literature in the period 1990–2010.
| Name | Sequence | z | <H> | <μH> | D | Conf. |
|---|---|---|---|---|---|---|
| RW16 | RRWRRWWRRWWRRWRR | 10 | 0.213 | 0.975 | YES | S [17] |
| RL16 | RRLRRLLRRLLRRLRR | 10 | 0.006 | 0.824 | YES | S [17] |
| Pbuy | FRKLFRVYSNFLRGKLKL | 6 | 0.280 | 0.650 | YES | S [18] |
| Pill | KQLIRFLKRLDRNLWGLA | 4 | 0.447 | 0.633 | YES | S [18] |
| Pc9k | NRLARHFRDIAGRVNQRL | 4 | 0.096 | 0.591 | YES | S [18] |
| Pqc7 | LKDVEEAQQKIINIIRRL | 1 | 0.280 | 0.650 | YES | S [18] |
| Pc3c | WYSEMKRNVQRLERAIEE | 0 | 0.113 | 0.615 | NO | S [18] |
| Pihf | RDAKELVELFFEEIRRAL | −1 | 0.276 | 0.566 | NO | S [18] |
| KL | KLLKLLLKLLKLLLKLLL | 5 | 0.953 | 0.659 | YES | S [19] |
| CRAMP18 | GEKLKKIGQKIKNFFQKL | 5 | 0.164 | 0.674 | YES | S [19] |
| SPLN14–27 | SLSRYAKLANRLA | 3 | 0.254 | 0.530 | YES | S [20] |
| SPLN28–41 | PKLLETFLSKWIG | 1 | 0.712 | 0.596 | YES | S [20] |
| Histatin 5 | SHAKRHHGYKRKFHEKHH | 5 | −0.157 | 0.263 | YES | G [21] |
| PGLaa | SKAGAIAGKIAKVALKAL | 3 | 0.398 | 0.501 | YES | S [21] |
| SP-B(7–24) | YCWLCRALIKRIQAMIPK | 4 | 0.747 | 0.434 | YES | S [22] |
| PC-TP196- | VPNFLKDMARACQNYLKK | 3 | 0.295 | 0.677 | YES | S [23] |
| Equinatoxin II | ASLSFDILKTVLEALGNV | −1 | 0.591 | 0.458 | NO | S [24] |
| KL4 | KLLLLKLLLLKLLLLKLL | 4 | 1.102 | 0.157 | YES | M [25] |
| KALP23 | KKLALALALALALALALA | 2 | 0.783 | 0.154 | YES | M [26] |
| WALP23 | WWLALALALALALALALA | 0 | 1.143 | 0.107 | NO | M [26] |
| Glycophorin A (92–114) | ITLIIFGVMAGVIGTILLI | 0 | 1.133 | 0.213 | NO | M [27] |
| TMX31 | WISFAISCFLLCVVLLGF | 0 | 1.321 | 0.216 | NO | M [28] |
| MHCClassII | VLVALLLAGQATTAYFLY | 0 | 0.899 | 0.115 | NO | M [29] |
This region is analyzed with a window of 11 AA in accordance to the original reference [21].
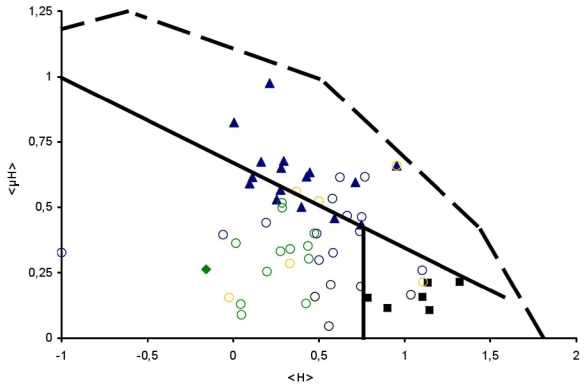
All data confirmed the findings obtained with the original Eisenberg approach (see Figure 2), which strongly substantiates the applicability of the Heliquest generated hydrophobic moment plot methodology. For example all surface seeking (S) regions of proteins and peptides were identified as such in the Heliquest generated approach and are found situated in or close to the surface seeking area of the Eisenberg plot.
Figure 2.
Eisenberg plot of a number of successfully identified proteins and peptides in which Surface seeking (
 ), Globular (
), Globular (
 ) and TransMembrane (■) segments are depicted, see Table 1 for details. Examples of signal peptides (SP) (circles, black), lipid-binding peptides (LBP) (circles, blue), amphitropics (circles, green) and others (circles, orange) are depicted, see Table 2 for details.
) and TransMembrane (■) segments are depicted, see Table 1 for details. Examples of signal peptides (SP) (circles, black), lipid-binding peptides (LBP) (circles, blue), amphitropics (circles, green) and others (circles, orange) are depicted, see Table 2 for details.
It has previously been discussed that the Heliquest lipid binding discrimination factor, when used in the analysis mode, cannot be used to identify transmembrane regions [12]. According to the results depicted in Table 1, it is clear that the Eisenberg methodology identified the transmembrane regions, since the Heliquest generated <H> is in all these cases above 0.75. Additionally the lipid discrimination factor D identified a substantial amount of all depicted (Table 1) experimentally demonstrated lipid binding regions [17–29]. The combination of the Heliquest discrimination factor and the Heliquest generated Eisenberg plot data was able to predict and identify all potential lipid binding regions. For example the lipid binding capability of WALP23 [26] is missed by the Heliquest discrimination factor but is recognized as transmembrane region by the Heliquest generated Eisenberg plot approach. The lipid binding capacity of Histatin 5 [21] is not identified by the Eisenberg plot approach but is well recognized by the Heliquest discrimination factor. This strongly suggests that in general the confirmative value of the combination of these two approaches would be even higher than the already impressive positive prediction value of 86% of the Heliquest discrimination factor alone [6].
2.3. The Meaning of the Eisenberg Plot for Novel Classes of Proteins and Peptides
The results obtained using the Heliquest generated Eisenberg plot methodology demonstrated it to be a valid and equally powerful approach as compared to the original Eisenberg plot methodology. However, over the last two decades numerous examples of experimentally demonstrated lipid-binding of proteins and peptides have been reported where the Eisenberg plot approach did not always identify them as either surface seeking or transmembrane [5,30].
In other words, there is evidence for novel classes or subclasses of proteins and peptides which cannot be classified as Globular, Surface seeking or Membrane protein. The data as depicted in Figure 2 and Table 2 used solely examples of experimentally demonstrated lipid binding of proteins and peptides [14,31–59]. For example some of the depicted signal sequences, all well described in literature for their ability to bind to (anionic) phospholipids [31–35], were found to be located in the globular protein region. The results of the depicted signal sequences obtained by the Heliquest generated data were found to correspond with the results as described and discussed in a thorough signal sequence analysis performed with the original Eisenberg plot methodology [60]. Thanks to the pioneer work of Von Heijne and co-workers, who performed statistical analysis of signal sequence and presequences [61–63], it is well-known that for example mitochondrial targeting sequences form amphiphilic helices and are identified by the Eisenberg plot methodology as surface seeking [61]. Eukaryotic signal sequences frequently can be found in the transmembrane region in an Eisenberg plot, probably due to their longer hydrophobic region compared to the signal sequences present in prokaryotic organisms [60,62,63]. Since the introduction of the hydrophobic moment plot methodology, numerous other novel peptides summarized as lipid binding peptides (LBP peptides) have been analyzed systematically by the Eisenberg approach. A few typical examples are depicted in Table 2, for example Aurein [36,37] a typical α-AMP peptide and penetratin [43] a typical cell penetratin peptide. A large number of these peptides were found to be located in the globular protein area of an Eisenberg plot. In the case of the α-AMP peptides, a specific area has been identified in the globular protein area of an Eisenberg plot where such peptides are often found and a possible use of this dedicated area for identification purposes has been postulated [5,39,64]. All sequences, being part of the amphitropic protein family, were not recognized by the Eisenberg plot methodology as either surface seeking or membrane protein, while the Heliquest lipid binding discrimination factor interestingly enough identified all these regions as lipid-binding. For protein translocation motor proteins multiple lipid-binding regions were predicted which are apparently required for a reversible membrane binding and proper functioning [12]. Multiple lipid binding were found in other amphitropic proteins like FtsY [12,49,50], and apocytochrome c [12,51] as well, indicating a specific feature of these members of the amphitropic protein family. It can be concluded that more recently recognized types of proteins and peptides that are classified as for example amphitropic, signal sequences or (α-) AMP peptide, cannot always be detected by the Eisenberg approach due to its novel and more complex features. Intriguingly, the Heliquest discrimination factor often identified the lipid binding regions in such proteins and peptides.
Table 2.
Examples of demonstrated lipid-binding proteins and peptides, which are not always identified by the Eisenberg plot methodology. The results of using the lipid-binding discrimination factor of the Heliquest program are included.
| Name | Sequence | z | <H> | <μH> | D | Conf. |
|---|---|---|---|---|---|---|
| SP & LBP: | ||||||
| 1. prePhoE | KKSTLALVVMGIVASASV | 2 | 0.558 | 0.045 | Y | [31] |
| 2. preLamB | RKLPLAVAVAAGVMSAQA | 2 | 0.478 | 0.157 | Y | [32] |
| 3. proOmpA | KKTAIAIAVALAGFATVA | 2 | 0.569 | 0.204 | Y | [33] |
| 4. prePhoA | TIALALLPLLPTPVTKAR | 2 | 0.744 | 0.197 | Y | [34] |
| 5. Ovalbumin | IFYCPIAIMSALAMVTLG | 0 | 1.036 | 0.165 | N | [35] |
| 6. Aurein 1.2 | GLFDIKKVASVIGGL | 1 | 0.583 | 0.326 | N | [36,37] |
| 7. Citropin | GLFDVIKKVASVIGGL | 1 | 0.623 | 0.614 | Y | [36,37] |
| 8. Maculatin 1.1 | GLFGVLAKVAAHVVPAIA | 1 | 0.738 | 0.408 | Y | [36,37] |
| 9. VP1 | GTAMRILGGVI | 1 | 0.665 | 0.468 | Y | [38] |
| 10. HA2 FP | FGAIAGFIENGWEGMIDG | −3 | 0.579 | 0.533 | N | [38] |
| 11. AP1 | GEQGALAQFGEWL | −2 | 0.488 | 0.399 | N | [39] |
| 12. SIV peptide | GVFVLGFLGFLA | 0 | 1.102 | 0.259 | N | [40] |
| 13. Gaegurin 5 | LGALFKVASKVLPSVCAI | 2 | 0.749 | 0.463 | Y | [41] |
| 14. PBP5 | GNFFGKIIDYIKLMFHHW | 1 | 0.768 | 0.616 | Y | [42] |
| 16. Penetratin | RQIKIWFQNRRMKWKK | 7 | 0.193 | 0.327 | Y | [43] |
| 17. Polyarginine-R9 | RRRRRRRRR | 9 | −1.010 | 0.146 | Y | [44] |
| 18. Substance-P | RPKPQQFFGLM | 2 | 0.501 | 0.298 | Y | [45] |
| 19. Dermaseptin B2 | IKEVGKEAAKAAAKAAGK | 3 | −0.058 | 0.395 | Y | [46] |
| Amphitropics: | ||||||
| 20. SecA(1–21) | MLIKLLTKVFGSRNDRTL | 3 | 0.442 | 0.303 | Y | [14] |
| 21. SecA(108–125) | KTLTATLPAYLNALTGKG | 2 | 0.437 | 0.352 | Y | [47] |
| 22. SecA(593–614) | ALMRIFASDRVSGMMRKL | 3 | 0.425 | 0.131 | Y | [48] |
| 23. SecA(865–882) | AAAAALAAQTGERKVGRN | 2 | 0.049 | 0.088 | Y | [14] |
| 24. FtsY(1–18) | MAKEKKRGFFSWLGFGQK | 4 | 0.277 | 0.332 | Y | [49] |
| 25. FtsY(188–208) | KPTKEGFFARLKRSLLKT | 5 | 0.198 | 0.254 | Y | [50] |
| 26. Apocyt c2–21 | VEKGKKIFVQKCAQCHTV | 3 | 0.333 | 0.341 | Y | [51] |
| 27. Apocyt c80–101 | AGIKKKTEREDLIAYLKK | 3 | 0.046 | 0.129 | Y | [51] |
| 28. EcMinD251–269 | RPFRFIEEEKKGFLKRLF | 3 | 0.287 | 0.498 | Y | [52] |
| 29. α-synuclein1–15 | MDVFMKGLSKAKEGV | 1 | 0.285 | 0.517 | Y | [53] |
| 30. ARF1 | MGNIFANLFKGLFGKKEM | 2 | 0.474 | 0.400 | Y | [54] |
| 31. K-segment dehydrins | EKKGIMDKIKEKLPG | 2 | 0.017 | 0.363 | Y | [55] |
| Miscellaneous: | ||||||
| 32. Kes 1p (7–29) | SSSWTSFLKSIASFNGDL | 0 | 0.500 | 0.523 | N | [56] |
| 33. PBP4 | RRIPLVRFESRLYKDIYQNN | 3 | 0.331 | 0.285 | Y | [42] |
| 34. KCNQ1354–372 | KVQQKQRQKHFNRQIPAA | 5 | −0.023 | 0.154 | Y | [57] |
| 35. ABP280(49–71) | FTRWCNEHLKCVSKRIAN | 3 | 0.370 | 0.560 | Y | [58] |
| 36. L15K7 | KLLKLLLKLLKLLLKLLLKLLK | 5 | 0.953 | 0.659 | Y | [59] |
2.4. Examples Illustrating the Power of the Total Approach
This study indicated the power of the combined use of the Heliquest lipid binding discrimination factor and the Heliquest generated Eisenberg plot methodology. This aspect of the development of the most complete approach in the search for potential lipid binding regions was investigated for some additional examples.
The first example is the well-known and thoroughly studied M13 coat protein [65,66]. The Heliquest lipid binding discrimination factor identified clearly two predicted lipid-binding regions (Table 3). Additionally the Heliquest generated Eisenberg plot approach identified one of these regions as transmembrane. Both these predicted findings correspond well with what was demonstrated experimentally [65,66].
Table 3.
Examples of the use of a combined Heliquest discrimination factor and a Heliquest generated Eisenberg plot methodology in the identification of potential lipid-binding regions.
| Name | Sequence | z | <H> | <μH> | D | Confirmed |
|---|---|---|---|---|---|---|
| M13 coat protein: | ||||||
| 2KKSLVLKASVAVATLVPM19 | 3 | 0.559 | 0.072 | YES | [65] | |
| 47YAWAMVVVIVGATIGIKL64 | 1 | 0.923 | 0.062 | NO | [65] | |
| 54VIVGATIGIKLFKKFTSK71 | 4 | 0.553 | 0.288 | YES | - | |
| Ffh: | ||||||
| (P0AGD7) | 1MFDNLTDRLSRTLRNISG18 | 1 | 0.255 | 0.663 | YES | - |
| 44ALPVVREFINRVKEKAVG61 | 2 | 0.313 | 0.365 | YES | - | |
| 166QKPVDIVNAALKEAKLKF183 | 2 | 0.272 | 0.331 | YES | - | |
| 309SKVDRAQAEKLASKLKKG326 | 4 | −0.118 | 0.297 | YES | - | |
| 336EQLRQMKNMGGMASLMGK353 | 2 | 0.218 | 0.261 | YES | - | |
| 395KGSRKRRIAAGCGMQVQD412 | 4 | 0.008 | 0.140 | YES | - | |
| 415RLLKQFDDMQRMMKKMKK432 | 5 | 0.064 | 0.606 | YES | - | |
| 428KKMKKGGMAKMMRSMKGM445 | 7 | 0.039 | 0.327 | YES | - | |
| Fis1: | ||||||
| (P40515) | 35PTATIQSRFNYAWGLIKS52 | 2 | 0.514 | 0.349 | YES | [67] |
| 60LGVKILTDIYKEAESRRR77 | 2 | 0.147 | 0.326 | YES | - | |
| 108RNNKQVGALKSMVEDKIQ125 | 2 | 0.023 | 0.305 | YES | - | |
| 133VVAGGVLAGAVAVASFFL150 | 0 | 0.811 | 0.145 | YES | [67] |
Since it has been demonstrated experimentally that FtsY contains lipid-binding regions [49,50], and recently novel lipid binding regions have been predicted [12], the closely related protein Ffh was investigated. There are no reports indicating the possible lipid-binding regions in Ffh, however there is some experimental evidence for an existing protein-lipid interaction when it comes to Ffh membrane binding (see [68,69]). The Heliquest discrimination factor identified multiple novel lipid binding regions in Ffh (Table 3), seeming divided over four lipid binding domains (LBD), regions ranging from AA 1–61, AA 166–183, AA 309–353 and AA 395–445. The Heliquest generated Eisenberg plot identified two possible binding regions as surface seeking helices, the lipid binding regions AA1–18 and AA415–432.
A recent report indicated that the cytosolic domain of Fis1 binds reversibly to lipids and might be another member of the rapid growing family of amphitropic proteins [67]. The Heliquest lipid binding discrimination factor identified four lipid binding regions (Table 3). The Heliquest generated Eisenberg plot analysis identified one possible lipid-binding region as surface seeking, region AA 35–52, and one lipid binding region as transmembrane, AA 133–150. Indeed the region AA 133–150 has been identified before as transmembrane [70] and upon binding to lipids a recent report about the cytosolic domain of Fis1 indicated a more non-polar environment for two Trp-residues, close to the AA 35–52 region.
3. Method Section
3.1. Primary and Secondary Structures Identification
The primary structure of the proteins was obtained from either the Swiss-Prot sequence database or the indicated references. The primary structures of the corresponding regions identified as lipid binding helix were collected. The included regions were checked for the extent of helicity either using the available crystallographic data and/or via secondary structure prediction using the program SOPMA [71], available at http://npsa-pbil.ibcp.fr/. In the 18-residue window at least 50% helicity of the sequence must be predicted.
3.2. Determination Lipid-Binding Potential
The lipid binding potential is performed as described before [12]. In essence, the mean hydrophobicity (<H>), the hydrophobic moment (μH) and the net charge (z) were calculated. In the analysis, 18-residue windows were used, and for each sequence under investigation the window with the highest discrimination factor was selected. The ultimate classification rule renders the discrimination factor (D):
When this discrimination factor is above 0.68, the corresponding can be considered to be a (potential) lipid-binding region. See [12] for detailed information about the way the discrimination factor is defined.
3.3. Eisenberg Plot Approach
The Eisenberg plot approach was essentially performed as described in the original study [1]. Both the mean hydrophobicity (<H>) and the hydrophobic moment (μH) were extracted from the Heliquest program [6] and subsequently plotted. In the analysis, 18-residue windows were used. The basic difference with the original approach is the hydrophobicity scale used, which was the Fauchere and Pliska scale [16] instead of the original normalized ‘consensus’ scale by Eisenberg [2]. This study used the data set compiled by Eisenberg and co-workers [1,2]. The used segments are summarized in Table S1 and Table S2. The criteria used to select more recent examples were the presence of experimental evidence for the existence of protein–lipid or peptide–lipid interactions and the described use of the original Eisenberg plot methodology. The used segments are summarized in Table 1 and Table 2.
4. Conclusions
The data presented here indicates that Heliquest generated data can be utilized for a hydrophobic moment plot analysis. A comparison of both the original databases [1,2] used by Eisenberg and co-workers and the newly generated database (this study) of recent examples of well described lipid-binding proteins and peptides clearly demonstrates the validation of the Heliquest generated Eisenberg plot. One important advantage of the use Heliquest generated data plot is the fact that it utilizes a freely available and user-friendly software package [6].
During the introduction of the Eisenberg plot [1,2] there was consensus about the alpha-helical classification, either surface active, globular or transmembrane. The finding that numerous lipid-binding regions of experimentally demonstrated lipid-binding peptides and proteins were found to be located in the globular protein area of the Eisenberg plot is intriguing. The extension of the classical threefold classification has been postulated for the so-called oblique orientated α-helices [5,30,39]. For peptides, additional novel classes have been proposed such as the signal peptides [72], the helical antimicrobial peptides α-AMP [39,73] and cell-penetrating peptides [74]. For proteins, the new class is the amphitropic protein family [75–77]. Protein translocation motor proteins like SecA [12,78], BiP, and mtHsp70 [12] have been postulated to be members of this family. It seems that membrane dynamic processes involving proteins such as FtsY [12,50], Ffh [68] and Fis1 [67], are members of the amphitropic family. Taking all results together, it seems that protein classification has been significantly broadened since the introduction of the Eisenberg plot methodology.
There is a growing perception that membrane proteins can also possess the so-called non-annular lipid-binding sites, where specific anionic phospholipids bind tightly to the protein and have been demonstrated to be involved in the formation of homo-oligomeric structures [79] and hetero-oligomeric structures [80] of proteins. How these particular lipid-binding sites fit into the possible search for lipid-binding regions in proteins will be investigated in future investigations.
Based on all the sequences investigated in this study, a positive discrimination value above 80% was found for the Heliquest lipid binding discrimination factor, while the combined approach was able to identify all sequences as potential lipid binding. All sequences investigated were well reported examples of experimentally confirmed lipid-binding proteins or peptides. What the positive prediction value will be for not yet experimentally confirmed protein-lipid interacting proteins remains to be seen. This study clearly indicates however that the combined use of the Heliquest lipid binding discrimination factor and the Heliquest generated Eisenberg plot methodology provides a powerful tool for the search of possible lipid-binding regions in proteins. The presented bioinformatics approach might serve as a starting point for studying proteins which have not yet been characterized in detail when it comes to protein–lipid interactions.
Supplementary Information
References
- 1.Eisenberg D, Weiss RM, Terwilliger TC. The helical hydrophobic moment: A measure of the amphiphilicity of a helix. Nature. 1982;299:371–374. doi: 10.1038/299371a0. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg D, Schwarz E, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984;15:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg D, Weiss RM, Terwilliger TC. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci USA. 1984;81:140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 5.Phoenix DA, Harris F. The hydrophobic moment and its use in the classification of amphiphilic structures (review) Mol Membr Biol. 2002;19:1–10. doi: 10.1080/09687680110103631. [DOI] [PubMed] [Google Scholar]
- 6.Gautier R, Douguet D, Anthonny B, Drin G. Heliquest: A web-server to screen sequences with specificα–helical properties. Bioinformatics. 2008;24:2101–2102. doi: 10.1093/bioinformatics/btn392. [DOI] [PubMed] [Google Scholar]
- 7.Scott DL, Diez G, Goldmann WH. Protein-lipid interactions: Correlation of a predictive algorithm for lipid-binding sites with three-dimensional structural data. Theor Biol Med Model. 2006;3 doi: 10.1186/1742-4682-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brzeska H, Guag J, Remmert K, Chacko S, Korn ED. An experimentally based computer search identifies unstructured membrane-binding sites in proteins: Application to class I myosins, PAKS, and CARMIL. J Biol Chem. 2010;285:5738–5747. doi: 10.1074/jbc.M109.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cserhati T. Interaction of phospholipids with proteins and peptides. New advances III. Int J Biochem. 1993;25:123–131. doi: 10.1016/0020-711x(93)90001-u. [DOI] [PubMed] [Google Scholar]
- 10.Dowhan W. Molecular basis for membrane phospholipid diversity: Why are there so many lipids? Annu Rev Biochem. 1997;66:199–232. doi: 10.1146/annurev.biochem.66.1.199. [DOI] [PubMed] [Google Scholar]
- 11.Sudhahar CG, Haney RM, Xue Y, Stahelin RV. Cellular membranes and lipid-binding domains as attractive targets for drug development. Curr Drug Targets. 2008;9:603–613. doi: 10.2174/138945008785132420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller RCA. The prediction of novel multiple lipid-binding regions in protein translocation motor proteins: A possible general feature. Cell Mol Biol Lett. 2011;16:40–54. doi: 10.2478/s11658-010-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulbrandt ND, London EL, Oliver DB. Deep penetration of a portion of Escherichia coli SecA protein into model membranes is promoted by anionic phospholipids and by partial unfolding. J Biol Chem. 1992;267:15184–15192. [PubMed] [Google Scholar]
- 14.Breukink E, Keller RCA, de Kruijff B. Nucleotide and negatively charged lipid-dependent vesicle aggregation caused by SecA. FEBS Lett. 1993;331:19–24. doi: 10.1016/0014-5793(93)80289-7. [DOI] [PubMed] [Google Scholar]
- 15.Ahn T, Kim H. SecA of Escherichia coli traverses lipid bilayer of phospholipid vesicles. Biochem Biophys Res Commun. 1994;203:326–330. doi: 10.1006/bbrc.1994.2185. [DOI] [PubMed] [Google Scholar]
- 16.Fauchere J, Pliska V. Hydrophobic parameters p of amino-acid side chains from the partitioning of N-acetyl-amino-acid amides. Eur J Med Chem. 1983;8:369–375. [Google Scholar]
- 17.Derossi D, Chassaing G, Prochiantz A. Trojan peptides: The penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- 18.Sivakamasundari C, Nagaraj R. Interaction of 18-residue peptides derived from amphipathic helical segments of globular proteins with model membranes. J Biosci. 2009;34:239–250. doi: 10.1007/s12038-009-0028-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim YS, Cha HJ. Disperse distribution of cationic amino acids on hydrophilic surface of helical wheel enhances antimicrobial peptide activity. Biotechn Bioeng. 2010;107:216–223. doi: 10.1002/bit.22810. [DOI] [PubMed] [Google Scholar]
- 20.Sitaram N, Subbalakshmi C, Nagaraj R. Identification of a second membrane-active 13-residue peptide segment in the antimicrobial protein, bovine seminalplasmin. FEBS Lett. 1993;328:239–242. doi: 10.1016/0014-5793(93)80935-n. [DOI] [PubMed] [Google Scholar]
- 21.Helmerhors EJ, van’t Hof W, Breeuweri P, Veerman ECI, Abee T, Troxler RF, Nieuw Amerongen AV, Oppenheim FG. Characterization of Histatin 5 with respect to amphipathicity, hydrophobicity, and effects on cell and mitochondrial membrane integrity excludes a candidacidal mechanism of pore formation. J Biol Chem. 2001;276:5643–5649. doi: 10.1074/jbc.M008229200. [DOI] [PubMed] [Google Scholar]
- 22.Bruni R, Taeusch HW, Waring AJ. Surfactant protein B: Lipid interactions of synthetic peptides representing the amino-terminal amphipathic domain. Proc Natl Acad Sci USA. 1991;88:7451–7455. doi: 10.1073/pnas.88.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng L, Chan WW, Roderick SL, Cohen DE. High-level expression and mutagenesis of recombinant human phosphatidylcholine transfer protein using a synthetic gene: Evidence for a C-terminal membrane binding domain. Biochemistry. 2000;39:15399–15409. doi: 10.1021/bi001076a. [DOI] [PubMed] [Google Scholar]
- 24.Belmonte G, Menestrina G, Perderzolli C, Krizaj I, Gubensek F, Macek P. Primary and secondary structure of a pore-forming toxin from the sea anemone, Actinia equina L., and its association with lipid vesicles. Biochim Biophys Acta. 1994;1192:197–204. doi: 10.1016/0005-2736(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson M, Vandenbussche G, Cursted T, Ruysschaert J-M, Johansson J. The 21-residue surfactant peptide (LysLeu4)4Lys(KL4) is a transmembrane α-helix with a mixed nonpolar/polar surface. FEBS Lett. 1996;384:185–188. doi: 10.1016/0014-5793(96)00290-6. [DOI] [PubMed] [Google Scholar]
- 26.de Planque MRR, Kruijtzer JAW, Liskamp RMJ, Marsh D, Greathouse DV, Koeppe RE, de Kruijff B, Killian JA. Different membrane anchoring positions of Tryptophan and lysine in synthetic transmembrane alpha-helical peptides. J Biol Chem. 1999;274:20839–20846. doi: 10.1074/jbc.274.30.20839. [DOI] [PubMed] [Google Scholar]
- 27.Treutlein HR, Lemmon MA, Engelman DM, Brunger AT. The glycophorin A transmembrane domain dimer: Sequence-specific propensity for a right-handed supercoil of helices. Biochemistry. 1992;31:12726–12732. doi: 10.1021/bi00166a003. [DOI] [PubMed] [Google Scholar]
- 28.Tatulian SA, Tamm LK. Secondary structure, orientation, oligomerization and lipid interactions of the transmembrane domain of Influenza Hemagglutinin. Biochemistry. 2000;39:496–507. doi: 10.1021/bi991594p. [DOI] [PubMed] [Google Scholar]
- 29.Kukol A, Torres J, Arkin IT. A structure for the trimeric MHC class II-associated invariant chain transmembrane domain. J Mol Biol. 2002;320:1109–1117. doi: 10.1016/s0022-2836(02)00563-6. [DOI] [PubMed] [Google Scholar]
- 30.Phoenix DA, Harris F, Daman OA, Wallace J. The prediction of amphiphilic α-helices. Curr Prot Pept Sci. 2002;3:201–221. doi: 10.2174/1389203024605368. [DOI] [PubMed] [Google Scholar]
- 31.Keller RCA, Killian JA, de Kruijff B. Anionic phospholipids are essential for α-helix formation of the signal peptide of prePhoE upon interaction with phospholipid vesicles. Biochemistry. 1992;31:1672–1677. doi: 10.1021/bi00121a014. [DOI] [PubMed] [Google Scholar]
- 32.Jones JD, Gierasch LM. Effect of charged residue substitutions on the thermodynamics of signal peptide-lipid interactions for the Escherichia coli LamB signal sequence. Biophys J. 1994;67:1546–1561. doi: 10.1016/S0006-3495(94)80628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizo J, Blanco FJ, Kobe B, Bruch MD, Gierasch LM. Conformational behavior of Escherichia coli OmpA signal peptides in membrane mimetic environments. Biochemistry. 1993;32:4881–4894. doi: 10.1021/bi00069a025. [DOI] [PubMed] [Google Scholar]
- 34.Golovastov VV, Nesmeyanova MA. Effect of membrane phospholipid composition and charge of the signal peptide of Escherichia coli alkaline phosphatase on efficiency of its secretion. Biochemistry (Mosc) 2003;68:1089–1096. doi: 10.1023/a:1026302510912. [DOI] [PubMed] [Google Scholar]
- 35.Fidelio GD, Austen BM, Chapman D, Lucy JA. Interactions of ovalbumin and of its putative signal sequence with phospholipid monolayers. Possible importance of differing lateral stabilities in protein translocation. Biochem J. 1987;244:295–301. doi: 10.1042/bj2440295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcotte I, Wegener KL, Lam Y-H, Chia BCS, de Planque MRR, Bowie JH, Auger M, Separovic F. Interaction of antimicrobial peptides from Australian amphibians with lipid membranes. Chem Phys Lipids. 2003;122:107–120. doi: 10.1016/s0009-3084(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 37.Ambroggio EE, Separovic F, Bowie JH, Fidelio GD, Bagatolli LA. Direct visualization of membrane leakage induced by the antibiotic peptides: Maculatin, citropin, and aurein. Biophys J. 2005;89:1874–1881. doi: 10.1529/biophysj.105.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brandenburg K, Harris F, Dennison S, Seydel1 U, Phoenix D. Domain V of m-calpain shows the potential to form an oblique-orientated α-helix, which may modulate the enzyme’s activity via interactions with anionic lipid. Eur J Biochem. 2002;269:5414–5422. doi: 10.1046/j.1432-1033.2002.03225.x. [DOI] [PubMed] [Google Scholar]
- 39.Dennison SR, Morto LH, Brandenburg K, Harris F, Phoenix DA. Investigations into the ability of an oblique alpha-helical template to provide the basis for design of an antimicrobial anionic amphiphilic peptide. FEBS J. 2006;273:3792–3803. doi: 10.1111/j.1742-4658.2006.05387.x. [DOI] [PubMed] [Google Scholar]
- 40.Bradshaw JP, Darkes MJM, Harroun TA, Katsaras J, Epand RM. Oblique membrane insertion of viral fusion peptide probed by neutron diffraction. Biochemistry. 2000;39:6581–6585. doi: 10.1021/bi000224u. [DOI] [PubMed] [Google Scholar]
- 41.Park S-H, Kim H-E, Kim C-M, Yun H-J, Choi E-C, Lee B-J. Role of praline, cysteine and a disulphide bridge in the structure and activity of the anti-microbial peptide gaegurin 5. Biochem J. 2002;368:171–182. doi: 10.1042/BJ20020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris F, Brandenburg K, Seydel U, Phoenix D. Investigations into the mechanisms used by the C-terminal anchors of Escherichia coli penicillin-binding proteins 4, 5, 6 and 6b for membrane interaction. Eur J Biochem. 2002;269:5821–5829. doi: 10.1046/j.1432-1033.2002.03295.x. [DOI] [PubMed] [Google Scholar]
- 43.Duplaa H, Convert O, Sautereau AM, Tocanne JF, Chassaing G. Binding of substance P to monolayers and vesicles made of phosphatidylcholine and/or phosphatidylserine. Biochim Biophys Acta. 1992;1107:12–22. doi: 10.1016/0005-2736(92)90323-e. [DOI] [PubMed] [Google Scholar]
- 44.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 45.Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. J Pept Res. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 46.Galanth C, Abbassi F, Lequin O, Ayala-Sanmartin J, Ladram A, Nicolas P, Amiche M. Mechanism of antibacterial action of Dermaseptin B2: Interplay between helix-hinge-helix structure and membrane curvature strain. Biochemistry. 2009;48:313–327. doi: 10.1021/bi802025a. [DOI] [PubMed] [Google Scholar]
- 47.Hu H-J, Holley J, He J, Harrison RW, Yang H, Tai PC, Pan Y. To be or not to be: Predicting soluble SecAs as membrane proteins. IEEE Trans NanoBiosci. 2007;6:168–179. doi: 10.1109/tnb.2007.897486. [DOI] [PubMed] [Google Scholar]
- 48.Cooper DB, Smith VF, Crane JM, Roth HC, Lilly AA, Randall LL. SecA, the motor of the secretion machine, binds diverse partners on one interactive surface. J Mol Biol. 2008;382:74–87. doi: 10.1016/j.jmb.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiche B, Bürk J, Angelini S, Schiltz E, Thumfart J-O, Koch H-G. A cleavable N-terminal membrane anchor is involved in membrane binding of the Escherichia coli SRP receptor. J Mol Biol. 2008;28:761–773. doi: 10.1016/j.jmb.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 50.Parlitz R, Eitan A, Stjepanovic G, Bahari L, Bange G, Bibi E, Sinning I. Escherichia coli signal recognition particle receptor FtsY contains an essential and autonomous membrane-binding amphipathic helix. J Biol Chem. 2007;44:32176–32321. doi: 10.1074/jbc.M705430200. [DOI] [PubMed] [Google Scholar]
- 51.Jordi W, de Kruijff B, Marsh D. Specificity of the interaction of amino- and carboxy-terminal fragments of the mitochondrial precursor protein apocytochrome c with negatively charged phospholipids. A spin-label electron spin resonance study. Biochemistry. 1989;28:8998–9005. doi: 10.1021/bi00449a007. [DOI] [PubMed] [Google Scholar]
- 52.Szeto TH, Rowland SL, Rothfield LI, King GF. Membrane localization of MinD is mediated by a C-terminal motif that is conserved across eubacteria, archaea, and chloroplasts. Proc Natl Acad Sci USA. 2002;99:15693–15698. doi: 10.1073/pnas.232590599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of α-Synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 54.Rorat T. Plant dehydrins - Tissue location, structure and function. Cell Mol Biol Lett. 2006;11:536–556. doi: 10.2478/s11658-006-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies SMA, Harroun TA, Hauβ T, Kelly SM, Bradshaw JP. The membrane bound N-terminal domain of human adenosine diphosphate ribosylation factor-1 (ARF1) FEBS Lett. 2003;548:119–124. doi: 10.1016/s0014-5793(03)00638-0. [DOI] [PubMed] [Google Scholar]
- 56.Drin G, Casella J-F, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic α–helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–146. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- 57.Thomas AM, Harmer SC, Khambra T, Tinker A. Characterization of a Binding Site for Anionic Phospholipids on KCNQ1. J Biol Chem. 2011;286:2088–2100. doi: 10.1074/jbc.M110.153551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldmann WH, Teodoridis JM, Sharma CP, Hu B, Isenberg G. Fragments from Actin Binding Protein (ABP-280; Filamin) insert into reconstituted lipid layers. Biochem Biophys Res Commun. 1999;259:108–112. doi: 10.1006/bbrc.1999.0735. [DOI] [PubMed] [Google Scholar]
- 59.Cornut I, Büttner K, Dasseux J-L, Dufourcq J. Application to the de novo design of ideally amphipathic Leu, Lys peptides with haemolytic activity higher than that of melittin. FEBS Lett. 1994;349:29–33. doi: 10.1016/0014-5793(94)00621-0. [DOI] [PubMed] [Google Scholar]
- 60.Prabhakaran M. The distribution of physical, chemical and conformational properties in signal and nascent peptides. Biochem J. 1990;269:691–696. doi: 10.1042/bj2690691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986;5:1335–13342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Heijne G. Analysis of the distribution of charged residues in the N-terminal region of signal sequences: Implications for protein export in prokaryotic and eukaryotic cells. EMBO J. 1984;3:2315–2318. doi: 10.1002/j.1460-2075.1984.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Heijne G, Abrahmsen L. Species-specific variation in signal peptide design. Implications for secretion in foreign hosts. FEBS Lett. 1989;244:439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]
- 64.Dennison SR, Harris F, Phoenix DA. Are oblique orientated α-Helices used by antimicrobial peptides for membrane invasion? Prot Pept Lett. 2005;12:27–29. doi: 10.2174/0929866053406039. [DOI] [PubMed] [Google Scholar]
- 65.Gallusser A, Kuhn A. Initial steps in protein membrane insertion. Bacteriophage M13 procoat protein binds to the membrane surface by electrostatic interaction. EMBO J. 1990;9:2723–2729. doi: 10.1002/j.1460-2075.1990.tb07459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagler C, Nagler G, Kuhn A. Cysteine residues in the transmembrane regions of M13 Procoat protein suggest that oligomeric coat Proteins assemble onto phage progeny. J Bacteriol. 2007;189:2897–2905. doi: 10.1128/JB.01551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells RC, Hill RB. The cytosolic domain of Fis1 binds and reversibly clusters lipid vesicles. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moll RG. Protein–protein, protein–RNA and protein–lipid interactions of signal-recognition particle components in the hyperthermoacidophilic archaeon Acidianus ambivalens. Biochem J. 2003;374:247–254. doi: 10.1042/BJ20030475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reinau ME, Otzen DE. Stability and structure of the membrane protein transporter Ffh is modulated by substrates and lipids. Arch Biochem Biophys. 2009;492:48–53. doi: 10.1016/j.abb.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 70.Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, Hill RB, Basañez G, Hardwick JM. Mitochondrial Fission proteins regulate programmed cell death in Yeast. Genes Dev. 2004;18:2785–2797. doi: 10.1101/gad.1247904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geourjon C, Deleage G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- 72.Izard JW, Kendall DA. Signal peptides: Exquisitely designed transport promoters. Mol Microbiol. 1994;13:765–773. doi: 10.1111/j.1365-2958.1994.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 73.Giuliani A, Pirri G, Nicolette SF. Antimicrobial peptides: An overview of a promising class of therapeutics. Cent Eur J Biol. 2007;2:1–33. [Google Scholar]
- 74.Madani F, Lindberg S, Langel Ü, Futaki S, Gräslund A. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys. 2011;2011:414729. doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burn P. Amphitropic proteins: A new class of membrane proteins. Trends Biochem Sci. 1988;13:79–83. doi: 10.1016/0968-0004(88)90043-6. [DOI] [PubMed] [Google Scholar]
- 76.Johnson JE, Cornell RB. Amphitropic proteins: Regulation by reversible membrane interactions (review) Mol Membr Biol. 1999;16:217–235. doi: 10.1080/096876899294544. [DOI] [PubMed] [Google Scholar]
- 77.Halskau Ø, Muga A, Martínez A. Linking new paradigms in protein chemistry to reversible membrane-protein interactions. Curr Prot Pept Sci. 2009;10:339–359. doi: 10.2174/138920309788922199. [DOI] [PubMed] [Google Scholar]
- 78.Keller RCA, Snel MME, de Kruijff B, Marsh D. SecA restricts in a nucleotide-dependent manner acyl chain mobility up to the center of a phospholipid bilayer. FEBS Lett. 1995;358:251–254. doi: 10.1016/0014-5793(94)01439-8. [DOI] [PubMed] [Google Scholar]
- 79.Triano I, Barrera FN, Renart ML, Molina ML, Fernández-Ballester G, Poveda JA, Fernández AM, Encina JA, Ferrer-Montiel AV, Otzen D, et al. Occupancy of nonannular lipid binding sites on KcsA greatly increases the stability of the tetrameric protein. Biochemistry. 2010;49:5397–5404. doi: 10.1021/bi1003712. [DOI] [PubMed] [Google Scholar]
- 80.Gold VAM, Robson A, Baob H, Romantsovc T, Duong F, Collinson I. The action of cardiolipin on the bacterial translocon. Proc Natl Acad Sci USA. 2010;107:10044–10049. doi: 10.1073/pnas.0914680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.