Abstract
In Saccharomyces cerevisiae, replication stress induced by hydroxyurea (HU) and methyl methanesulfonate (MMS) activates DNA integrity checkpoints; in checkpoint-defective yeast strains, HU treatment also induces morphological aberrations. We find that the sphingolipid pathway gene ISC1, the product of which catalyzes the generation of bioactive ceramides from complex sphingolipids, plays a novel role in determining cellular morphology following HU/MMS treatment. HU-treated isc1Δ cells display morphological aberrations, cell-wall defects, and defects in actin depolymerization. Swe1, a morphogenesis checkpoint regulator, and the cell cycle regulator Cdk1 play key roles in these morphological defects of isc1Δ cells. A genetic approach reveals that ISC1 interacts with other checkpoint proteins to control cell morphology. That is, yeast carrying deletions of both ISC1 and a replication checkpoint mediator gene including MRC1, TOF1, or CSM3 display basal morphological defects, which increase following HU treatment. Interestingly, strains with deletions of both ISC1 and the DNA damage checkpoint mediator gene RAD9 display reduced morphological aberrations irrespective of HU treatment, suggesting a role for RAD9 in determining the morphology of isc1Δ cells. Mechanistically, the checkpoint regulator Rad53 partially influences isc1Δ cell morphology in a dosage-dependent manner.
THE baker’s yeast Saccharomyces cerevisiae is dimorphic, existing in budding or pseudohyphal form, depending on its environment. In response to environmental cues such as nitrogen starvation or the presence of short-chain alcohols, diploid and certain haploid strains of yeast undergo morphological differentiation from budding to pseudohyphal forms (Gimeno et al. 1992; Lorenz et al. 2000; Lew 2003; Bharucha et al. 2008). MAPK and cAMP pathways are important in inducing such pseudohyphal growth in response to these environmental cues (Liu et al. 1993; Roberts and Fink 1994; Ward et al. 1995; Lengeler et al. 2000; Lorenz et al. 2000; Pan et al. 2000; Pan and Heitman 2002; Bharucha et al. 2008).
A morphogenesis checkpoint allows the cell to monitor defects in bud morphology, actin cytoskeleton perturbations, and cell-wall synthesis (Lew and Reed 1995) through its key regulator, Swe1 protein kinase (Lee et al. 2005; Keaton et al. 2007). Swe1 phosphorylates and inactivates Cdk1 at Tyr19 to cause cell cycle delay and to control morphogenetic irregularities. Swe1 accumulation is initiated in early S phase and its degradation must occur at the end of the G2 phase for the G2/M transition to occur (Sia et al. 1998; Lee et al. 2005). Persistence of Swe1 causes prolonged inhibition of Cdk1, which, in turn, can induce pseudohyphal growth (Pruyne and Bretscher 2000a,b).
Exposure to hydroxyurea (HU) or methyl methanesulfonate (MMS), both of which slow DNA synthesis, has been shown to induce minor morphological aberrations in yeast, specifically semifilamentous growth in certain wild-type strains (Jiang and Kang 2003), although most haploid wild-type strains tested undergo no morphological changes after HU exposure (Enserink et al. 2006). Both HU and MMS impede progression of DNA replication machinery, slow S-phase progression, and can induce DNA damage (Tercero and Diffley 2001; Katou et al. 2003; Zegerman and Diffley 2003). Cells respond to these genotoxic agents by activating checkpoints that cause cell cycle arrest while activating DNA repair machinery (Weinert and Hartwell 1988; Branzei and Foiani 2007). Furthermore, recent studies have shown that checkpoint proteins also play a role in morphogenesis in S. cerevisiae and Candida albicans (Jiang and Kang 2003; Enserink et al. 2006; Smolka et al. 2006; Shi et al. 2007) in addition to their role in cell cycle arrest and DNA repair (Wang 2009).
Many genes are involved in DNA damage checkpoint activity and morphogenesis, only some of which have been identified. In S. cerevisiae, DNA damage is identified by sensor proteins Mec1 and Tel1, which signal through the mediator Rad9 to downstream effectors Rad53 and Chk1 (Putnam et al. 2009). Three important components of the DNA replication machinery—Mrc1, Tof1, and Csm3—act as the replication checkpoint mediators in the place of Rad9 (Alcasabas et al. 2001; Katou et al. 2003; Bando et al. 2009). These mediators appear to function differently during normal DNA replication from when they are activated as part of a checkpoint (Katou et al. 2003; Calzada et al. 2005; Szyjka et al. 2005; Tourriere et al. 2005; Mohanty et al. 2006; Bando et al. 2009; Tanaka et al. 2009). Genome-wide studies reveal that, in addition to genes controlling cell cycle checkpoints, genes from other pathways such as amino acid, carbohydrate, and lipid metabolism also contribute to HU and MMS resistance (Chang et al. 2002; Hanway et al. 2002; Parsons et al. 2004). Genes in the sphingolipid pathway have been found to confer resistance to HU and MMS (Chang et al. 2002; Hanway et al. 2002).
Sphingolipids not only have major structural roles in the cell, but also are important bioactive molecules involved in signaling (Futerman and Riezman 2005; Riezman 2006; Milhas et al. 2009). Isc1 is the sole inositol phosphosphingolipid-phospholipase C protein identified in yeast that converts complex sphingolipids to ceramides; it is the ortholog of the mammalian neutral sphingomyelinases (Sawai et al. 2000; Matmati and Hannun 2008). Deletion of ISC1 in yeast causes sensitivity to HU and MMS and G2/M arrest (Matmati et al. 2009). HU-mediated G2/M block of isc1Δ cells can be rescued by deleting the SWE1 gene or by expressing a nonphosphorylatable Tyr-19 mutant of Cdk1 (Matmati et al. 2009).
We report that deletion of the ISC1 gene leads to morphological aberrations in S. cerevisiae cells upon exposure to various agents such as HU, MMS, galactose, or butanol. Morphological defects occurring after treatment with HU, galactose, or butanol are associated with stabilization of the morphogenesis checkpoint regulator Swe1; deletion of the SWE1 gene abolishes defects under all stress conditions tested. The aberrations induced upon replication stress by HU are associated with modification of the actin cytoskeleton and cell wall. Deletion of the replication checkpoint mediator genes MRC1, TOF1, or CSM3 does not reduce morphological defects significantly in isc1Δ cells after HU treatment; instead, these cells have morphological irregularities and cell-wall defects under unperturbed conditions. In contrast, deletion of RAD9 in isc1Δ cells reduces morphological defects significantly with HU treatment, although it does not reduce HU sensitivity. Finally, checkpoint effector Rad53 plays an important role in morphological defects of isc1Δ cells under HU stress. Such results indicate the importance of a sphingolipid gene in the control of cellular morphogenesis under various stress conditions.
Materials and Methods
Strains and plasmids
All strains and plasmids are listed in Table 1. Gene deletions were produced using G418 and phleomycin cassettes (Longtine et al. 1998; Gueldener et al. 2002).
Table 1 . Strains and plasmids.
| Strains | Genotype | Reference |
|---|---|---|
| Jk9-3d a | MATatrp1 leu2-3 his4 ura3 ade2 rme1 | Matmati et al. (2009) |
| JK9-3d a isc1Δ | JK9-3d a isc1::KanMX | Matmati et al. (2009) |
| JK9-3d a tof1Δ | JK9-3d a tof1:: KanMX | This study |
| JK9-3d a mrc1Δ | JK9-3d a mrc1::KanMX | This study |
| JK9-3d a csm3Δ | JK9-3d a csm3::KanMX | This study |
| JK9-3d a rad9Δ | JK9-3d a rad9::KanMX | This study |
| JK9-3d a swe1Δ | JK9-3d a swe1::KanMX | This study |
| JK9-3d a isc1Δtof1Δ | JK9-3d a isc1:: KanMX tof1Δ::Phl | This study |
| JK9-3d a isc1Δmrc1Δ | JK9-3d a isc1:: KanMX mrc1Δ::Phl | This study |
| JK9-3d a isc1Δcsm3Δ | JK9-3d a isc1:: KanMX csm3Δ::Phl | This study |
| JK9-3d a isc1Δswe1Δ | JK9-3d a isc1:: KanMX swe1Δ::Phl | This study |
| JK9-3d a isc1Δbem1Δ | JK9-3d a isc1:: KanMX bem1Δ::Phl | This study |
| JK9-3d a isc1Δbni1Δ | JK9-3d a isc1:: KanMX bni1Δ::Phl | This study |
| JK9-3d a isc1Δrad9Δ | JK9-3d a isc1:: KanMX rad9Δ::Phl | This study |
| JK9-3d α | MATα trp1 leu2-3 his4 ura3 ade2 rme1 | Sawai et al. (2000) |
| JK9-3d α isc1Δ | JK9-3d α isc1:: KanMX | Sawai et al. (2000) |
| BY4741 | MATahis3Δ1; leu2Δ0; met15Δ0; ura3Δ0 | Invitrogen |
| BY4741 isc1Δ | BY4741 isc1:: KanMX | Invitrogen |
| Plasmids | Gene | Reference |
|---|---|---|
| pRS316-ISC1 | ISC1 | Vaena de Avalos et al. (2004) |
| pBG999 | COF1-GFP | Gandhi et al. (2009) |
| pJM1042 [CDC28] | WT CDK1 | McMillan et al. (1999) |
| pAL88 [CDC28Y19F] | Cdk1Y19F | McMillan et al. (1999) |
| pRS315 | LEU2-CEN-ARS4 | Sikorski and Heiter (1989) |
| pCla6 | RAD53-CEN-LEU2 | Diani et al. (2009) |
Construction of double-deletion strains in isc1Δ background
Plasmid pRS416-ISC1 containing the ISC1 gene, including its endogenous promoter (Vaena De Avalos et al. 2004), was transformed into the isc1Δ derivative of Jk9-3d a. The CSM3, TOF1, MRC1, or RAD9 gene was then deleted from this strain using a phleomycin cassette. To subsequently select for loss of pRS416-ISC1, cells were grown in SD/Ura− media followed by growth in YPD media, and cells were plated on SC plates containing 5-FOA.
Microscopy
Live cells grown in rich or minimal media were visualized under a Nikon Eclipse (TE2000-5) microscope with ×400 magnification. For all other purposes, cells were fixed with 3.7% formaldehyde, washed with phosphate buffer (50 mm, pH 7), and suspended in phosphate buffer. Formaldehyde-fixed cells were stained with calcofluor white (CFW; Sigma) at a final concentration of 50 μg/ml and visualized by ×1000 magnification at excitation and emission wavelength of 350 and 550 nm, respectively. For visualization of the actin cytoskeleton, fixed cells were stained with rhodamine–phalloidin (Invitrogen) according to the manufacturer’s instructions and visualized by ×1000 magnification at excitation and emission wavelengths of 525–545 and 565 nm, respectively. For visualization of nuclei, cells were stained with DAPI (Vectashield) and examined by ×1000 magnification at excitation and emission wavelengths of 355 and 455/525 nm, respectively.
Analysis of morphological aberrations
Overnight cultures were inoculated into fresh YPD at 1:20 dilution and grown at 30° to an A600 of 0.4, when agents were added to the following final concentrations: 12.5–200 mM HU (Sigma), 0.033% v/v MMS (Sigma), 1% v/v 1-butanol, or 2.0% D-galactose (Sigma). For galactose experiments, log-phase cells were pelleted, washed with medium containing yeast extract and peptone (without glucose), and grown in yeast extract–peptone–galactose medium. Cells containing pRS315 or pCla6 were grown overnight in SD/Leu− medium, inoculated into fresh SD/Leu− medium, and grown to an A600 of 0.2 when HU was added to 12.5 or 25 mm. Cells were collected at 5 and 22 hr after HU/MMS exposure or after 17 hr of butanol and galactose exposure, fixed with 3.7% formaldehyde, and washed with phosphate buffer before visualization. A bud was considered elongated if its length was more than two times its width (Enserink et al. 2006). The percentage of elongated buds or cells with abnormal morphology was calculated from large-budded populations only; populations containing only unbudded and small-budded cells were not considered.
Growth rates
Overnight cultures were inoculated in fresh YPD to an A600 of 0.2, and absorbance (A600) was measured at 3, 6, 9, and 12 hr growth at 30°. Cells containing pRS315 or pCla6 were grown in SD/Leu− medium as described above. Experiments were repeated at least five times.
Sensitivity to HU, MMS, and CFW
YPD plates containing 100 or 200 mm HU, 0.033% (v/v) MMS, or 8 mm CFW were prepared and used within 48 hr of preparation. Overnight cultures were inoculated in fresh YPD medium at an A600 of 0.2 and grown at 30°. Log-phase cultures were adjusted to an A600 of 0.4 before making 10-fold serial dilutions and spotting 2.5 μl each on the plates. Cells containing plasmids such as pCla6 (Diani et al. 2009) were grown in SD/Leu− medium; SD/Leu− plates contained 25 or 50 mm HU.
Western blot analysis
Cell extracts were prepared from log-phase cultures as described previously (Matmati et al. 2009). An equal amount of each protein extract was fractionated by SDS-PAGE, blotted, and probed for Swe1 protein as previously described (Matmati et al. 2009). The Pstaire antibody (Santa Cruz) that recognizes amino acid residues 45–51 of Cdc2-p34 was used as a control in all Western blot analyses. Samples were also run through SDS-PAGE in parallel to compare protein concentrations using Coomassie blue staining.
Results
Replication stress induces aberrant morphology in isc1Δ cells
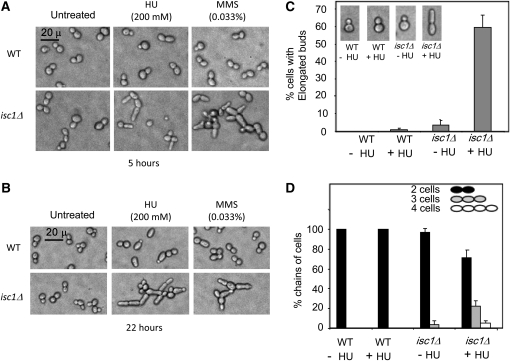
Strains containing ISC1 gene deletions are sensitive to genotoxic agents HU and MMS in long-term exposure tests (Matmati et al. 2009). We observed that isc1Δ cells developed many morphological aberrations after exposure to either HU or MMS. Wild-type (WT) cells exposed to HU or MMS display modest elongation of mother cell or buds only after 22 hr of HU exposure. In contrast, isc1Δ cells had many morphological abnormalities after exposure to either HU or MMS for 5 or 22 hr (Figure 1, A and B). The morphological changes could even be seen as early as 3 hr after genotoxic treatment (data not shown). Abnormalities included elongated buds, seen in 60% of cells 22 hr post-HU treatment, and daughter cells that did not separate from mother cells, resulting in chain-like structures of three or more cells (Figure 1C). After 22 hr of HU treatment, ∼22% of the cells were found in three-cell chains and ∼5% in four-cell chains (Figure 1D). Often the bud attached to the mother cell was highly elongated. In the absence of HU treatment, a small population of isc1Δ cells (∼3%) had elongated buds and three-cell chains (Figure 1, C and D; 200–500 cells were counted in each sample for morphological defects). Perhaps these cells have experienced replication stress, DNA damage, or other types of stress and are more sensitive to that stress in the absence of isc1.
Figure 1 .
HU and MMS induce morphological aberrations in isc1Δ cells. (A) Cellular morphology after a 5-hr exposure of WT and isc1Δ cells to HU or MMS (phase contrast ×400). (B) Cellular morphology after a 22-hr exposure to HU and MMS (phase contrast ×400). (C) Bars depict the frequency of elongated bud formation in isc1Δ cells exposed to HU for 22 hr. (D) Bars depict the frequency of chains containing three or more cells in isc1Δ cells after HU exposure for 22 hr.
The pattern of HU-induced morphological abnormalities was also observed in isc1Δ cells of the Jk9-3d “α” mating type as well as in isc1Δ cells of BY4741; wild-type cells of each strain did not undergo significant morphological change, whereas isc1Δ cells formed elongated buds and chains of incompletely separated cells. These experiments suggest that Isc1 suppresses morphological irregularities under replication stress induced by HU and MMS.
Abnormal chitin deposition in stressed isc1Δ cells
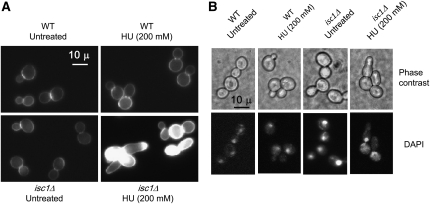
The cell wall is responsible for maintaining cell shape; therefore, morphological abnormalities may indicate alterations in cell-wall dynamics, including chitin distribution, during morphogenesis (De Groot et al. 2001; Roncero 2002). Because HU- and MMS-treated isc1Δ cells were often misshapen and had elongated buds or attached daughter cells, we investigated whether chitin deposition was altered in these cells. We stained cells with CFW that binds specifically to chitin in the cell wall. Regardless of HU treatment, wild-type cells displayed a thin line of chitin deposition on the cell wall and significant fluorescence at the bud neck (Figure 2A). Untreated isc1Δ cells had a similar pattern of chitin deposition (Figure 2A). However, after HU exposure, the elongated buds and chains of cells of the isc1Δ strain showed a high level of fluorescence at different locations on the cell surface, including the tips of the elongated buds (Figure 2A), suggesting increased chitin deposition and abnormal cell-wall architecture. Our results suggest that Isc1 is needed for proper chitin deposition or cell-wall architecture in stressed cells.
Figure 2 .
Cell-wall defects and DAPI staining of isc1Δ cells after HU exposure. (A) WT and isc1Δ cells treated with HU were stained with CFW (×1000-fold magnification). Only HU-treated isc1Δ cells display abnormal CFW staining. (B) Nucleus is stuck at the bud neck in HU-treated WT and isc1Δ cells while bud continues polarized growth only in the isc1Δ cells (phase-contrast and DAPI-stained cells, ×1000).
Nuclear division and bud morphogenesis in HU-treated isc1Δ cells
Wild-type cells are known to slow DNA synthesis and delay nuclear and cell division following replication stress such as that induced by HU treatment (Slater 1973). Generally DNA replication and nuclear division coordinate transition from polar bud growth to isotropic bud growth. We wanted to analyze the status of nuclear division following HU treatment of isc1Δ cells. After 5 hr of HU exposure, the shape of wild-type cells remained normal but nuclei were found at the bud neck (Figure 2B). Untreated isc1Δ cells each had a nucleus but, after HU treatment, nuclei did not divide and remained at the bud neck (Figure 2B). Although HU treatment delayed nuclear division in both WT and isc1Δ cells, in the majority of isc1Δ cells, buds continued to elongate (polar growth) in isc1Δ cells. The results suggest that transition from polar bud growth to isotropic growth does not occur in isc1Δ cells after HU treatment.
Role of Isc1 in actin dynamics during replication stress
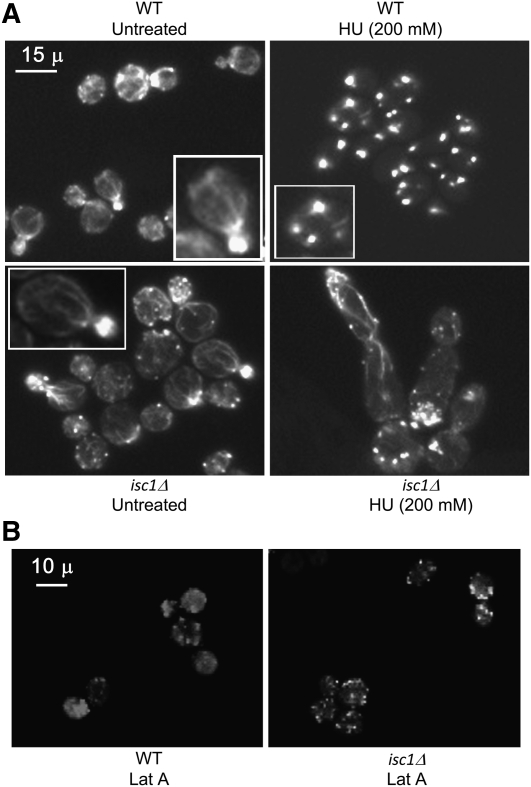
The actin cytoskeleton plays a major role in bud growth and many other cellular events and is a key component of the morphogenesis checkpoint (Lew 2003). Interestingly, actin shows a deleterious complex haploinsufficiency with Isc1 (Haarer et al. 2007). Therefore, we investigated whether Isc1 plays any role in actin dynamics during replication stress by HU. Rhodamine–phalloidin staining revealed that, in WT cells, the actin cytoskeleton was present as polarized cables spreading from the mother cell to the bud (Figure 3). Actin depolarization was notable after 3 hr of HU treatment (data not shown) and after 5 hr almost all cells had depolarized actin, seen as punctate staining throughout the cell (Figure 3). Rhodamine–phalloidin staining in untreated isc1Δ cells was identical to that of WT cells. However, HU treatment did not cause any actin depolymerization in the isc1Δ cells (Figure 3A). In these cells, actin cables were clearly visible and extended from mother cell to the bud tip. The results suggest that Isc1 plays an important role in actin cytoskeletal reorganization during replication stress, and it may also control cellular morphogenesis. To be sure that the lack of actin depolarization in isc1Δ cells was not simply due to an actin depolarization defect, but to mediation of replication stress by isc1Δ, we treated WT and isc1Δ cells with latrunculin A (LatA), a compound known to induce actin depolarization (McMillan et al. 1998), for 5 hr and then analyzed actin distribution. We observed punctate actin staining in both WT and isc1Δ cells after LatA treatment (Figure 3B), indicating depolarization. It is clear from these experiments that Isc1 controls actin depolarization in cells under replication stress.
Figure 3 .
Defects in actin dynamics in isc1Δ cells with HU treatment. HU-treated cells were stained with rhodamine–phalloidin and observed at ×1000. (A) WT untreated cells have actin cables extending from mother cells to buds. After HU treatment (WT HU, 200 mm) for 5 hr, actin depolymerized (white dots). Actin cables are present in untreated isc1Δ cells (isc1Δ untreated) and in treated cells (isc1Δ HU, 200 mm). Insets show a magnified cell. (B) In both WT (WT LatA) and isc1Δ cells (isc1Δ LatA), actin depolymerized after 5 hr of latrunculin A treatment.
Isc1 acts in parallel with replication checkpoint mediators to maintain cell growth and morphology
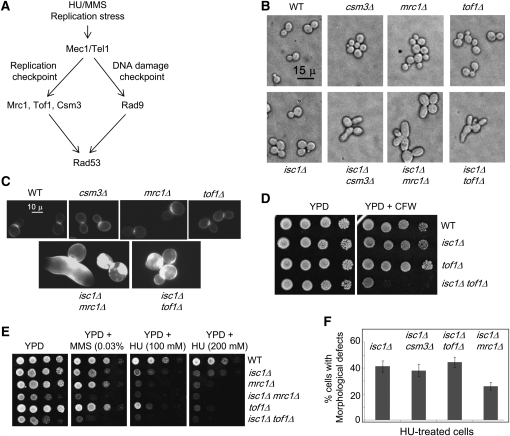
HU treatment activates the replication checkpoint (Figure 4A) and causes DNA replication arrest in an Mrc1-Tof1-Csm3-dependent manner (Katou et al. 2003; Bando et al. 2009; Tanaka et al. 2009) in which Csm3 forms heterotrimers with Tof1 and Mrc1 (Mayer et al. 2004; Xu et al. 2007; Bando et al. 2009). MMS blocks progression of the replication fork, causes DNA damage, and activates the DNA damage checkpoint through Rad9 (Putnam et al. 2009) (Figure 4A) as well as the S-phase checkpoint. Interestingly, Rad9 can function at the replication fork in the absence of Mrc1 or Tof1 (Foss 2001; Katou et al. 2003). We investigated whether the defects of HU-treated isc1Δ cells depend on these proteins by deleting MRC1, TOF1, CSM3, and RAD9 in isc1Δ cells. Genome-wide studies had already shown that simultaneous deletion of ISC1 and CSM3 induces synthetic growth defects (Tong et al. 2004; Pan et al. 2006). We constructed isc1Δcsm3Δ, isc1Δmrc1Δ, and isc1Δtof1Δ strains and found them to be viable although slow growing (Supporting Information, Figure S1).
Figure 4 .
Genetic interactions of ISC1 with replication checkpoint mediators MRC1, TOF1, and CSM3 and control cell morphology. (A) Model shows the replication checkpoint and DNA damage checkpoint pathways. Mec1 and Tel1 are sensors and Rad53 is the major effector in both DNA replication checkpoint and DNA damage checkpoint (Chk1 effector is not shown). Whereas Mrc1, Tof1, and Csm3 act as the replication checkpoint mediators, Rad9 is the DNA damage checkpoint mediator. (B) Morphology of indicated strains by phase-contrast microscopy (×400). (C) CFW staining reveals cell-wall defects in isc1Δmrc1Δ and isc1Δtof1Δ cells. (D) Spot test with WT, isc1Δ, tof1Δ, and isc1Δtof1Δ cells on YPD and YPD + CFW plates reveals a high sensitivity of isc1Δtof1Δ cells to CFW. (E) Spot tests of the WT, isc1Δ, mrc1Δ, isc1Δmrc1Δ, tof1Δ, and isc1Δtof1Δ cells on YPD, YPD + 0.033% MMS, 100 mm HU, and 200 mm HU plates show that isc1Δmrc1Δ and isc1Δtof1Δ cells grow slowly compared with other strains and are more sensitive to HU and MMS. (F). Bars indicate the percentage of cells with HU-induced morphological aberrations (elongated buds and chains of cells) in isc1Δcsm3Δ, isc1Δmrc1Δ, and isc1Δtof1Δ strains compared to the isc1Δ strain.
Cell morphology of double-mutant strains of isc1Δmrc1Δ, isc1Δtof1Δ, and isc1Δcsm3Δ was assessed with and without HU treatment (Figure 4, B–F). Untreated single-mutant isc1Δ, csm3Δ, mrc1Δ, or tof1Δ strains did not display major morphological aberrations compared to untreated WT cells. However, double-mutant isc1Δmrc1Δ, isc1Δtof1Δ, and isc1Δcsm3Δ strains frequently formed large and sometimes misshapen mother cells and buds in comparison to WT cells and to the single-deletion derivatives even in the absence of genotoxic treatment (Figure 4B). However, some major differences were observed in cell shape and size between HU-treated isc1Δ cells and the untreated double-deletion strains isc1Δmrc1Δ, isc1Δtof1Δ, and isc1Δcsm3Δ (compare Figure 1, A and B with Figure 4B). In addition, the double-mutant strains had significant chitin accumulation in the cell wall as evidenced by enhanced CFW staining compared to staining of WT and single-mutant cells (Figure 4C). Cells with morphological aberrations are known to be sensitive to CFW (De Groot et al. 2001; Enserink et al. 2006); thus we assessed CFW sensitivity in our strains. We found that CFW sensitivity was not significantly different in WT, isc1Δ, or tof1Δ cells. However, isc1Δtof1Δ cells were very sensitive to 25 μg/ml of CFW. We also tested mrc1Δ and isc1Δmrc1Δ strains and observed that the isc1Δmrc1Δ strain was very sensitive to low concentrations of CFW (8 μg/ml) and that the MRC1 deletion alone caused sensitivity to higher concentrations of CFW (25 μg/ml; data not shown).
The HU sensitivity of double-mutant isc1Δmrc1Δ, isc1Δtof1Δ, and isc1Δcsm3Δ cells was assessed. Single-mutant isc1Δ, mrc1Δ, and tof1Δ cells were sensitive to both HU and MMS compared to WT cells. However, isc1Δmrc1Δ and isc1Δtof1Δ strains were much more sensitive to these genotoxins than was the WT strain or strains containing single deletions (Figure 4E). Notably, double-deletion strains grew slowly—even when cultures of the same absorbance were cultured on YPD plates (Figure S1). Because isc1Δmrc1Δ, isc1Δtof1Δ, and isc1Δcsm3Δ cells were slow growing, they were treated with a low concentration of HU (25 mm) to preserve viability while we assessed their morphology. As expected from analyses of untreated double-mutant strains, HU treatment induced severe morphological defects, including increased cell size, elongated buds, and chains of connected cells in isc1Δmrc1Δ, isc1Δtof1Δ, and isc1Δcsm3Δ cells (Figure 4F). However, whereas the extent of cell elongation and chain formation in the HU-induced isc1Δtof1Δ (n = 440 cells) and isc1Δcsm3Δ (n = 278 cells) cells was not less than that of HU-treated isc1Δ cells, the HU-treated isc1Δmrc1Δ cells (n = 314 cells) showed ∼30% less bud elongation and chain formation than the HU-treated isc1Δ cells (n = 415 cells); all the HU-treated double-mutant cells were bigger in size than the HU-treated isc1Δ cells. The results suggest that the absence of MRC1 partially compromised HU-generated signal transduction. These results suggest that Tof1 and Csm3 do not play a role in the morphological defects of HU-treated isc1Δ cells, whereas Mrc1 may play a minor role in promoting bud elongation and chain formation following HU treatment. Although the morphological aberrations in double-mutant strains are somewhat different from the HU-induced defects in isc1Δ cells, we conclude that Isc1 acts in parallel with the replication checkpoint mediators to maintain cell growth and morphology in the absence of genotoxic treatment.
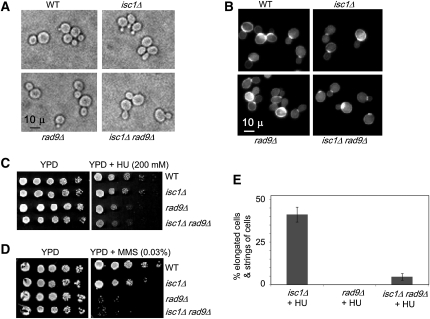
Rad9 mediates signals of replication stress in isc1Δ cells
Although Mrc1 is the main replication checkpoint mediator functioning in HU treatment, Rad9 is known to function in its absence (Katou et al. 2003). To explore a possible role for Rad9 in cellular signaling during replication stress, an isc1Δrad9Δ strain was constructed and characterized. The growth pattern of the double-mutant strain was reduced compared to wild type or either single-mutant strain (Figure S2), but its cellular morphology (Figure 5A) and CFW staining pattern (Figure 5B) were normal. Both the isc1Δrad9Δ strain and rad9Δ strain were sensitive to HU and MMS (Figure 5, C and D). Because these cells were highly sensitive to HU, we tested various concentrations of HU to identify a low concentration that induced morphological aberrations in isc1Δ cells without killing the rad9Δ and isc1Δrad9Δ cells. It was observed that 25 mm HU was sufficient to induce morphological aberrations in isc1Δ cells. When cells were treated with 25 mm HU, WT and rad9Δ cells displayed no morphological irregularities (n = 214 cells; data not shown) whereas isc1Δ cells had severe morphological defects in 41% of the cells (n = 415 cells; Figure 5E). Interestingly, significantly fewer isc1Δrad9Δ cells—only 4.6%—displayed morphological defects, and cells remained viable (n = 277 cells). Following treatment with 100 mm HU, we found that, whereas ∼64% of isc1Δ cells had morphological defects, only ∼12% of isc1Δrad9Δ cells had similar defects (data not shown). These results suggest that Rad9 plays an important role in the transmission of replication stress signals generated in HU-treated isc1Δ cells.
Figure 5 .
Signals generated in isc1Δ cells after HU treatment pass through Rad9 to control cellular morphology. (A) Phase-contrast microscopy (×1000) revealed no morphological aberrations of isc1Δrad9Δ cells when compared to WT, isc1Δ, or rad9Δ cells in the absence of genotoxic treatment. (B) CFW staining of the cell wall does not differ among WT, isc1Δ, rad9Δ, and isc1Δrad9Δ cells without genotoxic treatment. (C and D) Spot tests of serial dilutions of WT, isc1Δ, rad9Δ, and isc1Δrad9Δ cells on YPD, YPD + HU, and YPD + MMS plates showing that isc1Δrad9Δ cells are sensitive to both HU and MMS. (E) Bars show reduction in HU-induced morphological aberrations in isc1Δrad9Δ cells in comparison to isc1Δ cells.
Role of Rad53 in HU sensitivity and morphological aberrations of isc1Δ cells
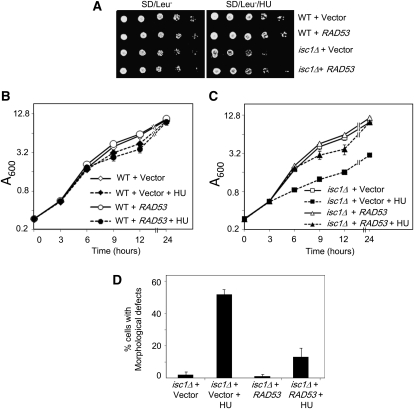
We explored the possible involvement of the Rad53 effector in this pathway for the following reasons: (1) HU and MMS activate DNA integrity checkpoints in which signals from Mrc1-Tof1-Csm3-Rad9 converge at the effector protein Rad53 (Alcasabas et al. 2001; Foss 2001); (2) a reduction of cellular Rad53 concentration causes increased HU sensitivity of WT cells (Cordon-Preciado et al. 2006); (3) Rad53 has been shown to control cellular morphology through Swe1 activity (Enserink et al. 2006; Smolka et al. 2006; Diani et al. 2009); and finally, (4) RAD9, implicated (above) in this pathway, is an activator of RAD53. To investigate the role of Rad53, we transformed either an empty vector or a vector carrying RAD53 into WT and isc1Δ strains. As expected, WT cells containing the vector alone were resistant to HU; however, WT cells carrying an extra copy of RAD53 had modestly increased resistance to HU. Similarly, whereas isc1Δ cells with an empty vector were sensitive to HU, isc1Δ cells containing an extra copy of RAD53 showed a modest increase in resistance to HU (Figure 6A). These data suggest that RAD53 dosage partially controls HU sensitivity of isc1Δ cells.
Figure 6 .
Increase in RAD53 dosage rescues HU sensitivity and HU-induced morphological aberrations of isc1Δ cells. (A) WT and isc1Δ cells were transformed with empty vector pRS315 or a plasmid containing a WT RAD53 gene expressed under the endogenous promoter. Fivefold dilutions of log-phase cells were spotted on SD/Leu− and SD/Leu−/HU and incubated at 30°. Both WT and isc1Δ cells containing RAD53 had increased resistance to HU compared to cells containing the empty vector. (B) WT cells containing either an empty vector or a RAD53 plasmid were grown in SD/Leu− and SD/Leu−/HU liquid media, and absorbance of all cultures was monitored at 0, 3, 6, 9, 12, and 24 hr. Vector, Control plasmid; RAD53, a plasmid containing an extra copy of RAD53 gene. (C) The isc1Δ cells were grown and analyzed as in B. (D) isc1Δ cells containing either an empty vector or a RAD53 plasmid were grown in SD/Leu− and SD/Leu−/HU liquid media (isc1Δ cells + vector, n = 261; isc1Δ cells + vector + HU, n = 368; isc1Δ cells + RAD53 + HU, n = 381; isc1Δ cells + RAD53 + HU, n = 403). Cells were collected 22 hr after HU exposure, fixed with formaldehyde, and analyzed for morphological aberrations by phase-contrast microscopy (×400).
We conducted a quantitative analysis of the effects of an extra copy of RAD53 on the HU sensitivity of isc1Δ cells. Because HU slows DNA replication and growth, WT cells carrying an empty vector or an extra copy of RAD53 had a slower growth rate after HU exposure compared to untreated cells (Figure 6B). However, by 24 hr, cells exposed to HU reached the concentration of the untreated cells as the untreated cells slowly reached the stationary phase. In contrast, isc1Δ cells containing only the chromosomal copy of RAD53 had severely reduced growth (75%) when exposed to HU compared to untreated cells. An extra copy of RAD53 restored the growth of cells treated with HU to the degree of untreated cells by 24 hr (Figure 6C). We calculated the generation times of the cultures during a 3- to 6-hr growth period and observed that isc1Δ cells containing an empty vector had generation times of ∼1.8 and 4.8 hr without HU and with HU treatment, respectively. In contrast, isc1Δ cells carrying a RAD53 plasmid showed a generation time of ∼1.8 hr in HU during the same time period. These experiments also indicate a role for Rad53 in the growth of isc1Δ cells in HU.
We also assessed the effect of RAD53 gene dosage on cellular morphology after HU treatment. Whereas isc1Δ cells containing the chromosomal copy of RAD53 displayed increased morphological aberrations (in 52% of cells), an additional copy of RAD53 significantly reduced their HU-induced morphological irregularities to only 13% of cells (Figure 6D). We found such differences not only after 22 hr of HU exposure but also after 6 or 12 hr of HU exposure. To confirm that the extra copy of RAD53, and not a mutation in the isc1Δ cells, was responsible for these results, we grew isc1Δ cells containing the RAD53 plasmid from the above experiment in rich (YPD) medium for several generations to evict the plasmid and then treated the resulting cells with HU as above. These cells displayed morphological aberrations, as did isc1Δ cells that had always lacked RAD53 plasmid. Results of this experiment eliminate the possibility of a second mutation in the RAD53-transformed isc1Δ cells, causing the observed phenotypes.
Role of Swe1 and Cdk1 proteins in determining cellular morphology during stress
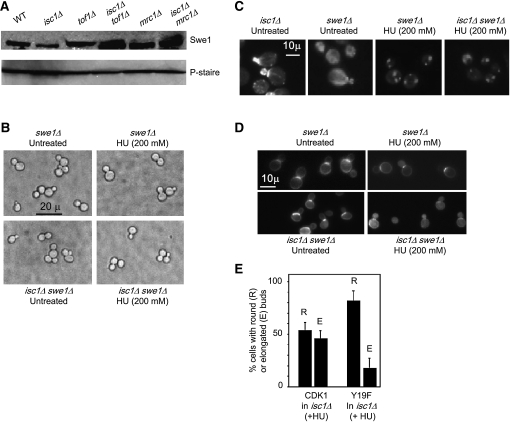
Because stabilization of the morphogenesis checkpoint regulator Swe1 occurs in isc1Δ cells upon exposure to HU/MMS (Matmati et al. 2009), we investigated whether Swe1 is associated with morphological aberrations in isc1Δ cells. As expected, stabilization of Swe1 was observed in both isc1Δtof1Δ and isc1Δmrc1Δ cells in comparison to WT, isc1Δ, and tof1Δ strains in the absence of genotoxic treatment (Figure 7A). We also observed that morphological aberrations seen in isc1Δ cells upon exposure to HU were dependent on the presence of Swe1 (Figure 7, B–D). Unlike the isc1Δ strain, the isc1Δswe1Δ strain did not differ in cellular morphology, CFW staining, or actin depolymerization from swe1Δ and WT strains (compare Figure 7, B–D isc1Δswe1Δ with Figure 1, A and B, Figure 2, and Figure 3). We stained HU-treated swe1Δ and isc1Δswe1Δ cells with DAPI and observed that most of the cells had nuclei at the bud neck and no morphological irregularities (data not shown). These results suggest that the morphological aberrations in isc1Δ cells under replication stress require Swe1.
Figure 7 .
Role of Swe1 in determining morphology of isc1Δ cells under replication stress. (A) Expression profile of Swe1 in the WT, isc1Δ, tof1Δ, isc1Δtof1Δ, mrc1Δ, and isc1Δmrc1Δ strains. P-STAIRE antibody probing of the same samples shows equal loading of protein in various samples. (B) Phase-contrast microscopy reveals similar cell morphology of indicated strains regardless of HU treatment. (C) Rhodamine–phalloidin staining shows that SWE1 deletion in isc1Δ cells restores actin depolymerization upon HU treatment as in WT cells. (D) CFW staining shows that swe1Δ and isc1Δswe1Δ cells have no cell-wall defect. (E) Expression of the Cdk1Y19F mutant, but not WT CDK1, rescues isc1Δ cells to a large extent from budding defects after HU exposure.
Because Swe1 controls G2/M arrest by inactivating Cdk1 in isc1Δ cells (Matmati et al. 2009), we tested whether the morphological aberrations of isc1Δ cells occurred due to inactivation of Cdk1. As expected, expression of a nonphosphorylatable mutant of CDK1, Cdk1Y19F (Y19F, n = 407; in comparison to WT Cdk1, n = 404), in isc1Δ cells prevented the induction of morphological aberrations by HU to a large extent (Figure 7E). These results strongly suggest that Isc1 protein controls cellular morphology during HU exposure by destabilizing Swe1 such that Cdk1 remains active.
Isc1 functions through actin regulators during budding
Actin assembly and disassembly is regulated by proteins in more than one pathway (Pruyne and Bretscher 2000a,b; Rodal et al. 2005; Moseley and Goode 2006). For example, formin homologs Bni1 and Bnr1 are downstream targets of Rho proteins and function in actin cable nucleation (Imamura et al. 1997). Whereas Bni1 controls actin cable nucleation in the bud, Bnr1 functions at the bud neck (Pruyne et al. 2004). Bni1 is a member of the polarisome complex and has been implicated in bud elongation during nitrogen starvation of diploid cells (Bidlingmaier and Snyder 2004; Liu et al. 2010). We wanted to test whether Bni1 functions downstream of Isc1 during HU stress. BNI1 was deleted in isc1Δ cells, and the resulting cells were characterized upon HU treatment. Whereas isc1Δ cells showed elongated buds and polarized actin, isc1Δbni1Δ cells had shorter buds, and some cells showed a few depolarized actin spots (Figure S3A). However, the reversal was not complete, suggesting that additional proteins play a role in actin cable nucleation in isc1Δ cells.
To identify other proteins involved in this process, we looked to the Cdk1 pathway. Cdk1 controls actin cable organization through Cdc42 that, in turn, controls actin organization by forming a complex with Cdc42 and Bem1 http://www.yeastgenome.org/cgi-bin/locus.fpl?dbid=S000000404(Wang 2009). BEM1 and ISC1 have been shown to share positive genetic interactions (Fiedler et al. 2009), and they may function in a common pathway to control bud morphogenesis. To test this possibility, we constructed an isc1Δbem1Δ strain, treated it with HU, and found that bud elongation occurred much faster than in the isc1Δ strain (Figure S3B), suggesting that the two genes may function in parallel pathways to control bud elongation during replication stress.
To determine whether Isc1 functions through the proteins known to control actin disassembly such as cofilin, coronin, and Aip1 (Lin et al. 2010) during replication stress, a plasmid containing GFP-tagged COF1 (Gandhi et al. 2009) was transformed into WT and isc1Δ cells. Following HU treatment, Cof1-GFP dynamics were compared to actin dynamics via rhodamine–phalloidin staining (Figure S3C). In untreated WT cells, actin cables spread from mother to daughter cells with the highest phalloidin staining seen in the daughter cells; Cof1-GFP was fairly evenly distributed in mother and daughter cells. After HU treatment, actin cables in WT cells were disassembled to form punctate structures and Cof1-GFP also appeared in a punctate pattern in most cells (Figure S3C). In the isc1Δ cells, actin and Cof1-GFP were similarly distributed regardless of whether the cells were treated with HU (Figure S3C). All these data suggest that Isc1 functions through actin regulators to control actin depolymerization during replication stress.
Response to galactose- or butanol-induced stress is also Swe1 dependent
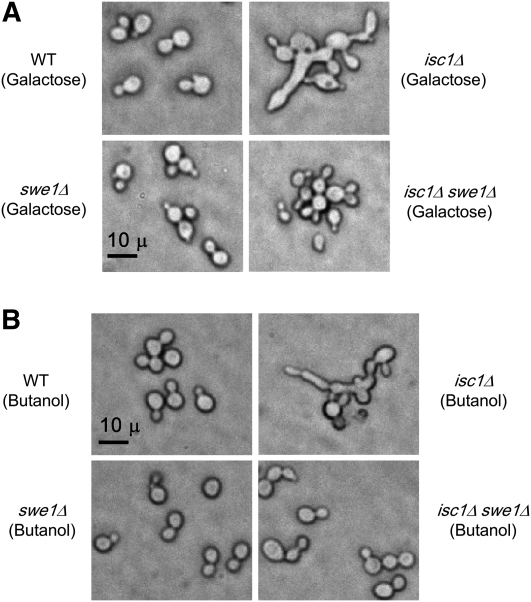
Because the isc1Δ cells displayed morphological aberrations under HU and MMS stress, we investigated whether they would show similar phenotypes under other stress conditions. Cells were grown in the presence of either galactose or butanol, both of which induced extensive morphological aberrations in isc1Δ cells compared to WT cells (Figure 8, A and B). It is known that a small proportion (2–3%) of WT cells display morphological aberrations after treatment with galactose (Palecek et al. 2002). In contrast, butanol has been shown to induce morphological aberrations in haploid WT yeast in a strain-specific manner. Although morphological aberrations were induced in Σ1278b and W303 strains, butanol did not induce aberrations in the S288c strain (Lorenz et al. 2000). In WT cells of the Jk9-3d (“a” type) strain used in this study, we observed morphological aberrations in <1% of cells after treatment with either galactose or butanol. However, >70% of isc1Δ cells displayed morphological aberrations after treatment (Figure 8, A and B).
Figure 8 .
Galactose and butanol induce morphological aberrations in isc1Δ cells in a Swe1-dependent manner. (A) WT, isc1Δ, swe1Δ, and isc1Δswe1Δ cells were grown in 2.0% D-galactose for 17 hr before fixing with formaldehyde and visualization with phase-contrast microscopy (×400). (B) WT, isc1Δ, swe1Δ, and isc1Δswe1Δ cells were grown in 1.0% 1-butanol for 17 hr before fixing with formaldehyde and microscopic visualization (×400).
Because we found that Swe1 controls the morphology of HU-treated isc1Δ cells (Figure 7), we investigated whether Swe1 plays a similar role after galactose or butanol treatment. If so, deletion of SWE1 in isc1Δ cells should abolish the morphological defects. Unlike isc1Δ cells, neither swe1Δ nor isc1Δswe1Δ cells displayed morphological aberrations after galactose or butanol treatment. These experiments show that both galactose and butanol induced morphological aberrations in isc1Δ cells in a Swe1-dependent manner.
These experiments, taken together, strongly suggest that, under several stress conditions, the absence of the ISC1 gene leads to morphological defects and that these events are Swe1 dependent. Isc1 also cooperates with various DNA integrity and morphogenesis checkpoint proteins and with several actin regulators to control cellular morphology under replication stress. Finally, Rad9 and Rad53 control the HU-dependent morphological aberrations of isc1Δ cells.
Discussion
The goal of the present study was to dissect the role of ISC1 in determining cellular morphology during replication stress in yeast. Our results suggest that ISC1 is a key regulator of cellular morphogenesis under a broad range of environmental stressors. The results show that the absence of ISC1 leads to morphological aberrations, cell-wall defects, and defects in actin depolymerization during HU treatment. The replication checkpoint mediators Tof1 or Csm3 (and to a large extent Mrc1) do not play a major role in transmission of the signals generated in isc1Δ cells during HU treatment. However, Isc1 functions in parallel with these mediators to control cell growth and morphology in unperturbed cells. The DNA damage checkpoint mediator Rad9 was found to control signals generated by HU treatment in isc1Δ cells, leading to morphological defects. The checkpoint effector Rad53, activated by Rad9 upon DNA damage, also controls HU-dependent morphological aberrations of isc1Δ cells, suggesting that DNA damage checkpoint proteins are active under these conditions. However, there is no evidence that the DNA damage checkpoint itself controls morphology. Interestingly, Swe1 and Cdk1 were found to control morphological defects of isc1Δ cells. Finally, the role of ISC1 in cellular morphology was not limited to replication stress; this sphingolipid gene was also found to control cell morphology under other stress conditions such as during galactose or butanol treatment.
We find that Rad9 and Swe1 function differently to control morphology in response to HU stress in isc1Δ cells. Deletion of RAD9 reduced morphological aberrations to a large extent in isc1Δ cells during HU stress. In C. albicans, genotoxin-induced morphological aberrations are reduced by RAD9 deletion (Shi et al. 2007). Rad9 is known to cause G2/M arrest upon DNA damage, and rad9Δ cells are MMS/HU sensitive. Although isc1Δrad9Δ cells had fewer morphological defects at low HU concentrations, they are not resistant to HU because rad9Δ cells are also partially sensitive to HU. In contrast, isc1Δswe1Δ cells showed no morphological defects as well as increased HU resistance. Although both Swe1 and Rad9 control HU-induced morphological aberrations in isc1Δ cells, we do not know if they are acting in a single pathway or in two different pathways (see Figure 9). Because our experiments demonstrate that the effect of Swe1 in isc1Δ cells is greater than the individual effects of Rad9, Rad53, or Cdk1, Swe1 may operate through various effector molecules to cause cell elongation in isc1Δ cells during HU stress.
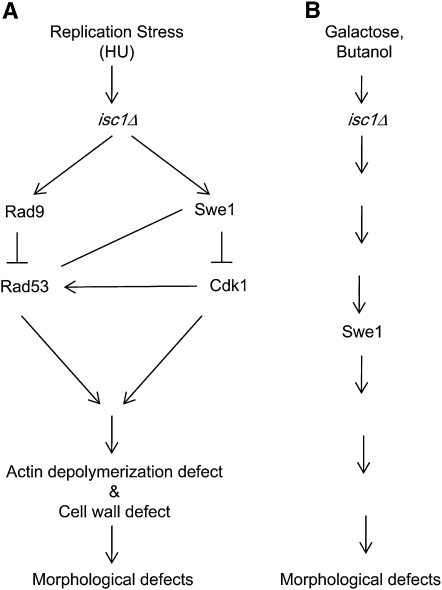
Figure 9 .
Model depicts mechanisms by which morphological defects are induced in isc1Δ cells under stress conditions. (A) In one pathway, HU treatment of isc1Δ cells generates signals that are recognized by Rad9 and passed to Rad53, inhibiting the latter and leading to overproduction and/or stabilization of Swe1. This leads to Cdk1 phosphorylation and inactivation, resulting in a G2/M arrest and defects in actin dynamics. Furthermore, ISC1 gene deletion causes cell-wall defects under HU stress. The combination of the actin defect and the cell-wall defect leads to morphological aberrations. Alternatively, HU treatment of isc1Δ cells leads to stabilization of Swe1, which inactivates Cdk1; also HU treatment of isc1Δ cells causes signaling through Rad9 and Rad53. Both Cdk1 and Rad53 (see parallel pathways in Figure 9A) pathways finally lead to actin and cell-wall defects, causing morphological aberrations. Finally, Cdk1 is known to phosphorylate Rad53 to control cellular morphology. In isc1Δ cells, this pathway may also be active (arrow from Cdk1 to Rad53). The role of Mrc1, Tof1, and Csm3 is not shown. (B) Galactose or butanol treatment of isc1Δ cells causes Swe1 stabilization that, in turn, causes morphological aberrations.
Rad53 has been shown to control cellular morphology (Enserink et al. 2006, 2009; Diani et al. 2009). Our study shows that increasing Rad53 gene dosage decreases the morphological aberrations of isc1Δ cells to a large extent. How does Isc1 control Rad53? Isc1 may partially regulate Rad53 function by altering its concentration, activity, and phosphorylation status. Although it is possible that Isc1 controls the morphological functions of Rad53 through Rad9, the DNA damage checkpoint functions of Rad9 and Rad53 may or may not be involved in this process.
At present, the proteins that transmit HU-induced signals in isc1Δ cells to Rad9/Rad53 are not known, but two different models can explain our results. On one hand, it is possible that Isc1 controls morphological functions of Rad53 function, which in turn, may control Swe1, and ultimately control Cdk1. On the other hand, Rad53 has phosphorylation targets of Cdk1, and a specific amino acid residue on Rad53 has been implicated in its role in certain aspects of morphogenesis (Diani et al. 2009). It has been shown that both Swe1 and Cdk1 control HU-mediated G2/M arrest of isc1Δ cell sensitivity (Matmati et al. 2009), and we show here that they control morphological aberrations of isc1Δ cells under HU stress. It is possible that Rad53 activity is affected in HU-treated isc1Δ cells through Cdk1 and Rad9 via two independent pathways. This may control Swe1 stabilization and activity in the isc1Δ cells. It is known that Swe1 accumulation leading to aberrant morphology occurs in both untreated and HU-treated rad53Δ cells (Enserink et al. 2006). Regulation of one or more proteins from among Rad53, Rad9, Cdk1, and Swe1 by phosphorylation is an attractive possibility since Isc1 generates ceramide that may, in turn, activate protein phosphatases. Detailed mutational analysis of ISC1 and related sphingolipid genes is necessary to understand the possible role of the sphingolipid pathway in controlling Rad53 activity. Similarly, mutational analyses as well as biochemical analyses of Rad53 will show how Isc1 controls Rad53 activity. Experiments are underway to understand the mechanism of action of Isc1 on Rad53 function.
Our findings clearly indicate that both replication checkpoint and DNA damage checkpoint proteins play significant roles in cellular morphogenesis. Although Mrc1, Tof1, and Csm3 do not play a significant role in the morphological defects of isc1Δ cells upon HU exposure, simultaneous deletions of ISC1 and MRC1/TOF1/CSM3 caused slow growth and frequent basal morphogenetic aberrations in the absence of genotoxic treatment (albeit in a somewhat different manner than that of isc1Δ cells during HU stress). These findings suggest the following: (1) that ISC1 functions redundantly with replication checkpoint mediator genes MRC1, TOF1, and CSM3 to control cellular morphology and cell growth; (2) that Swe1 stability plays an important downstream role in this process; and (3) that Tof1 and Csm3 play key roles in cellular morphology, a role revealed in the absence of ISC1. Previous studies had implicated other checkpoint proteins such as Mec1 and Tel1, Mrc1 and Rad9, and Rad53 in cellular morphology (Jiang and Kang 2003; Enserink et al. 2006) in S. cerevisiae and Mec1, Rad9, and Rad53 in C. albicans (Shi et al. 2007).
Results from this study also specifically connect Isc1 to the regulation of actin cytoskeleton dynamics. Whereas wild-type cells undergo actin depolymerization upon exposure to HU, isc1Δ cells do not, suggesting that Isc1 controls actin depolymerization specifically during replication stress. Several studies have shown that ISC1 interacts genetically with actin. ISC1 and ACT1 genes share complex haploinsufficiency interactions, suggesting complementary roles for each of the two genes in the maintenance of cell growth and viability (Haarer et al. 2007). Diploid cells containing a single copy each of ISC1 and ACT1 show a severe growth defect, and similar defects in both cell growth and morphology also occur when the wild-type ACT1 gene is replaced by act1-105 or act1-111 (Wertman et al. 1992; Cali et al. 1998; Haarer et al. 2007). Furthermore, defects in cell growth and actin depolarization in a slm1Δslm2ts mutant were abolished by deletion of the ISC1 gene along with the calcineurin gene (Tabuchi et al. 2006). These results and the current findings suggest that Isc1 plays a key role in maintenance of the actin cytoskeleton and controls cellular morphology under DNA replication stress.
As shown by a previous work (Enserink et al. 2006), we observed that HU treatment caused actin depolarization in WT cells, indicated by punctuate staining (that was not seen in isc1Δ cells). It is possible that the punctate staining of actin is a reflection of actin patches. When actin patches switch from a polar to an isotropic pattern, no polar actin patterns or actin cables are seen. Many HU-treated isc1Δ cells are growing in a polar manner, and thus actin cables and high concentrations of patches in the early buds or tips of elongated cells are expected. Thus, the influence of HU on actin in isc1Δ cells could be indirect.
Several other sphingolipid pathway genes such as LCB1, RVS161, and RVS167 have been implicated in actin dynamics (Munn et al. 1995; Zanolari et al. 2000). However, the finding that ISC1 controls actin dynamics upon HU exposure is novel. Deletion of BNI1 in isc1Δ cells partially abolishes the actin defect, suggesting that Isc1 plays a key role in actin depolarization in HU by affecting actin regulators. However, since deletion of BNI1 confers only a partial effect, there are other genes playing redundant roles to control actin disruption mediated by ISC1. These data suggest that studies of other actin regulators are needed to determine how Isc1 might control actin dynamics under HU stress.
Isc1 also seems to play an important role in cell-wall synthesis. Chitin accumulates to high levels in the cell wall and bud tips during exposure to HU in isc1Δ cells as well as in isc1Δmrc1Δ, isc1Δtof1Δ, and isc1Δcsm3Δ cells. Isc1, along with the replication checkpoint mediators, may be involved in a cell-wall checkpoint (Harvey and Kellogg 2003). Recently, it was observed that the cell-wall synthesis gene CWP1 was upregulated during the diauxic shift in isc1Δ cells (Kitagaki et al. 2009). In addition, deletion of the ISC1 homolog CSS1 in Schizosaccharomyces pombe caused severe cell-wall defects, including unusual accumulation of glucans in the periplasmic space, suggesting that Css1 plays a key role in cell-wall synthesis (Feoktistova et al. 2001). These observations strongly suggest that there is a link between sphingolipid metabolism and cell-wall synthesis in both yeast species. However, at present we do not have direct evidence of Isc1 controlling cell-wall synthesis. Moreover, changes in actin dynamics can affect cell-wall dynamics, and isc1Δ cells show defects in actin dynamics in HU. Thus the defect in cell walls of isc1Δ cells may be indirect.
Another major finding is that Isc1 regulates cellular morphology not only under HU/MMS stress, but also during galactose- and butanol-induced stress, and that Swe1 controls this response. Isc1 may act not only along with checkpoint proteins but also with or through various proteins of the cAMP and MAPK pathways to control cell morphology. Future experiments will elucidate the detailed mechanism of action of Isc1 in determining cell morphology under various stress conditions.
Our current understanding of the control of cellular morphology by Isc1 can be summarized in several models (see Figure 9). For example, in the absence of ISC1 (Figure 9A), HU stress may act on Rad9 to control Rad53. Inhibition of Rad53 stabilizes Swe1 such that Cdk1 is inactivated. Loss of Cdk1 activity in turn causes defects in actin dynamics and cell-wall synthesis, resulting in morphological aberrations. Alternatively, HU exposure of isc1Δ cells can cause Swe1 accumulation (by a yet-unknown mechanism) leading to Cdk1 inactivation. In parallel, HU exposure of isc1Δ cells activates the Rad9-Rad53 pathway. Both pathways contribute to actin defects and cell-wall defects leading to morphological aberrations. These parallel pathways may influence each other or act independently. A third possibility is that treatment of isc1Δ cells with HU may also lead to Swe1 accumulation inducing Cdk1 phosphorylation, which can affect Rad53 phosphorylation. Cdk1-dependent Rad53 phosphorylation already has been implicated in morphogenesis in yeast (Diani et al. 2009). All three pathways may be used simultaneously to affect cell morphology also. Finally, a pathway supported by our findings suggests that deletion of MRC1, TOF1, or CSM3 in an isc1Δ strain also leads to Swe1 stabilization and morphological aberrations.
In conclusion, we have shown for the first time that a sphingolipid pathway gene (ISC1) controls cellular morphogenesis under various stress conditions. We have further demonstrated that the DNA replication checkpoint mediators Mrc1, Tof1, and Csm3 function in parallel with Isc1 to monitor cell-wall and cellular morphology. Also, we find that the Isc1 protein coordinates the checkpoint mediator Rad9, the checkpoint effector Rad53, the stability of the morphogenesis checkpoint regulator Swe1, the activity of the cell cycle regulator Cdk1, and actin dynamics to control cellular morphology under HU stress. Finally, the Isc1 protein controls cell morphology under various stress conditions through the Swe1 protein.
Acknowledgments
We thank Bruce Goode, Dan Lew, Achille Pellicioli, David Pellman, and their laboratory members for supplying plasmids and strains; Maurizio Del Poeta and his lab members for providing microscopy facility; Elizabeth De Stasio for her valuable time and help in preparation of the manuscript; and the anonymous reviewers for their valuable comments and advice for revising and improving the manuscript. This work was supported in part by the South Carolina Center of Biomedical Research Excellence (COBRE) in Lipidomics and Pathobiology [P20 RR17677 from National Center for Research Resources (NCRR)] for B.K.M. and W.J.Z., an American Cancer Society–Institutional Research Grant (ACS-IRG 97-219-08) from the Hollings Cancer Center at the Medical University of South Carolina for B.K.M., a PhRMA foundation starter grant to W.J.Z., an American Society for Biochemistry and Molecular Biology travel grant to K.T., National Institutes of Health (NIH) grant GM063265 to N.M., and NIH grants GM43825 and GM63265 to Y.A.H.
Literature Cited
- Alcasabas A. A., Osborn A. J., Bachant J., Hu F., Werler P. J., et al. , 2001. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat. Cell Biol. 3: 958–965 [DOI] [PubMed] [Google Scholar]
- Bando M., Katou Y., Komata M., Tanaka H., Itoh T., et al. , 2009. Csm3, Tof1, and Mrc1 form a heterotrimeric mediator complex that associates with DNA replication forks. J. Biol. Chem. 284: 34355–34365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha N., Ma J., Dobry C. J., Lawson S. K., Yang Z., et al. , 2008. Analysis of the yeast kinome reveals a network of regulated protein localization during filamentous growth. Mol. Biol. Cell 19: 2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmaier S., Snyder M., 2004. Regulation of polarized growth initiation and termination cycles by the polarisome and Cdc42 regulators. J. Cell Biol. 164: 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzei D., Foiani M., 2007. Interplay of replication checkpoints and repair proteins at stalled replication forks. DNA Repair (Amst.) 6: 994–1003 [DOI] [PubMed] [Google Scholar]
- Cali B. M., Doyle T. C., Botstein D., Fink G. R., 1998. Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae. Mol. Biol. Cell 9: 1873–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada A., Hodgson B., Kanemaki M., Bueno A., Labib K., 2005. Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev. 19: 1905–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M., Bellaoui M., Boone C., Brown G. W., 2002. A genome-wide screen for methyl methanesulfonate-sensitive mutants reveals genes required for S phase progression in the presence of DNA damage. Proc. Natl. Acad. Sci. USA 99: 16934–16939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Preciado V., Ufano S., Bueno A., 2006. Limiting amounts of budding yeast Rad53 S-phase checkpoint activity results in increased resistance to DNA alkylation damage. Nucleic Acids Res. 34: 5852–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot P. W., Ruiz C., Vazquez de Aldana C. R., Duenas E., Cid V. J., et al. , 2001. A genomic approach for the identification and classification of genes involved in cell wall formation and its regulation in Saccharomyces cerevisiae. Comp. Funct. Genomics 2: 124–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diani L., Colombelli C., Nachimuthu B. T., Donnianni R., Plevani P., et al. , 2009. Saccharomyces CDK1 phosphorylates Rad53 kinase in metaphase, influencing cellular morphogenesis. J. Biol. Chem. 284: 32627–32634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink J. M., Smolka M. B., Zhou H., Kolodner R. D., 2006. Checkpoint proteins control morphogenetic events during DNA replication stress in Saccharomyces cerevisiae. J. Cell Biol. 175: 729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink J. M., Hombauer H., Huang M. E., Kolodner R. D., 2009. Cdc28/Cdk1 positively and negatively affects genome stability in S. cerevisiae. J. Cell Biol. 185: 423–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistova A., Magnelli P., Abeijon C., Perez P., Lester R. L., et al. , 2001. Coordination between fission yeast glucan formation and growth requires a sphingolipase activity. Genetics 158: 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler D., Braberg H., Mehta M., Chechik G., Cagney G., et al. , 2009. Functional organization of the S. cerevisiae phosphorylation network. Cell 136: 952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss E. J., 2001. Tof1p regulates DNA damage responses during S phase in Saccharomyces cerevisiae. Genetics 157: 567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Riezman H., 2005. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 15: 312–318 [DOI] [PubMed] [Google Scholar]
- Gandhi M., Achard V., Blanchoin L., Goode B. L., 2009. Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Mol. Cell 34: 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno C. J., Ljungdahl P. O., Styles C. A., Fink G. R., 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68: 1077–1090 [DOI] [PubMed] [Google Scholar]
- Gueldener U., Heinisch J., Koehler G. J., Voss D., Hegemann J. H., 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 30: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer B., Viggiano S., Hibbs M. A., Troyanskaya O. G., Amberg D. C., 2007. Modeling complex genetic interactions in a simple eukaryotic genome: actin displays a rich spectrum of complex haploinsufficiencies. Genes Dev. 21: 148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanway D., Chin J. K., Xia G., Oshiro G., Winzeler E. A., et al. , 2002. Previously uncharacterized genes in the UV- and MMS-induced DNA damage response in yeast. Proc. Natl. Acad. Sci. USA 99: 10605–10610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S. L., Kellogg D. R., 2003. Conservation of mechanisms controlling entry into mitosis: budding yeast wee1 delays entry into mitosis and is required for cell size control. Curr. Biol. 13: 264–275 [DOI] [PubMed] [Google Scholar]
- Imamura H., Tanaka K., Hihara T., Umikawa M., Kamei T., et al. , 1997. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16: 2745–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. W., Kang C. M., 2003. Induction of S. cerevisiae filamentous differentiation by slowed DNA synthesis involves Mec1, Rad53 and Swe1 checkpoint proteins. Mol. Biol. Cell 14: 5116–5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katou Y., Kanoh Y., Bando M., Noguchi H., Tanaka H., et al. , 2003. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 424: 1078–1083 [DOI] [PubMed] [Google Scholar]
- Keaton M. A., Bardes E. S., Marquitz A. R., Freel C. D., Zyla T. R., et al. , 2007. Differential susceptibility of yeast S and M phase CDK complexes to inhibitory tyrosine phosphorylation. Curr. Biol. 17: 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagaki H., Cowart L. A., Matmati N., Montefusco D., Gandy J., et al. , 2009. ISC1-dependent metabolic adaptation reveals an indispensable role for mitochondria in induction of nuclear genes during the diauxic shift in Saccharomyces cerevisiae. J. Biol. Chem. 284: 10818–10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Asano S., Park J. E., Sakchaisri K., Erikson R. L., 2005. Monitoring the cell cycle by multi-kinase-dependent regulation of Swe1/Wee1 in budding yeast. Cell Cycle 4: 1346–1349 [DOI] [PubMed] [Google Scholar]
- Lengeler K. B., Davidson R. C., D’Souza C., Harashima T., Shen W. C., et al. , 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64: 746–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., 2003. The morphogenesis checkpoint: how yeast cells watch their figures. Curr. Opin. Cell Biol. 15: 648–653 [DOI] [PubMed] [Google Scholar]
- Lew D. J., Reed S. I., 1995. Cell cycle control of morphogenesis in budding yeast. Curr. Opin. Genet. Dev. 5: 17–23 [DOI] [PubMed] [Google Scholar]
- Lin M. C., Galletta B. J., Sept D., Cooper J. A., 2010. Overlapping and distinct functions for cofilin, coronin and Aip1 in actin dynamics in vivo. J. Cell Sci. 123: 1329–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Larsson L., Caballero A., Hao X., Oling D., et al. , 2010. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell 140: 257–267 [DOI] [PubMed] [Google Scholar]
- Liu H., Styles C. A., Fink G. R., 1993. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science 262: 1741–1744 [DOI] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., III, Demarini D. J., Shah N. G., Wach A., et al. , 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961 [DOI] [PubMed] [Google Scholar]
- Lorenz M. C., Cutler N. S., Heitman J., 2000. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell 11: 183–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matmati N., Hannun Y. A., 2008. Thematic review series: sphingolipids. ISC1 (inositol phosphosphingolipid-phospholipase C), the yeast homologue of neutral sphingomyelinases. J. Lipid Res. 49: 922–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matmati N., Kitagaki H., Montefusco D., Mohanty B. K., Hannun Y. A., 2009. Hydroxyurea sensitivity reveals a role for ISC1 in the regulation of G2/M. J. Biol. Chem. 284: 8241–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Pot I., Chang M., Xu H., Aneliunas V., et al. , 2004. Identification of protein complexes required for efficient sister chromatid cohesion. Mol. Biol. Cell 15: 1736–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J. N., Sia R. A., Lew D. J., 1998. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J. Cell Biol. 142: 1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J. N., Sia R. A., Bardes E. S., Lew D. J., 1999. Phosphorylation-independent inhibition of Cdc28p by the tyrosine kinase Swe1p in the morphogenesis checkpoint. Mol. Cell. Biol. 19: 5981–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhas D., Clarke C. J., Hannun Y. A., 2010. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett. 584: 1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty B. K., Bairwa N. K., Bastia D., 2006. The Tof1p-Csm3p protein complex counteracts the Rrm3p helicase to control replication termination of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 103: 897–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J. B., Goode B. L., 2006. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol. Mol. Biol. Rev. 70: 605–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A. L., Stevenson B. J., Geli M. I., Riezman H., 1995. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 6: 1721–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek S. P., Parikh A. S., Huh J. H., Kron S. J., 2002. Depression of Saccharomyces cerevisiae invasive growth on non-glucose carbon sources requires the Snf1 kinase. Mol. Microbiol. 45: 453–469 [DOI] [PubMed] [Google Scholar]
- Pan X., Heitman J., 2002. Protein kinase A operates a molecular switch that governs yeast pseudohyphal differentiation. Mol. Cell. Biol. 22: 3981–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Harashima T., Heitman J., 2000. Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr. Opin. Microbiol. 3: 567–572 [DOI] [PubMed] [Google Scholar]
- Pan X., Ye P., Yuan D. S., Wang X., Bader J. S., et al. , 2006. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell 124: 1069–1081 [DOI] [PubMed] [Google Scholar]
- Parsons A. B., Brost R. L., Ding H., Li Z., Zhang C., et al. , 2004. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat. Biotechnol. 22: 62–69 [DOI] [PubMed] [Google Scholar]
- Pruyne D., Bretscher A., 2000a Polarization of cell growth in yeast. J. Cell Sci. 113(Pt. 4): 571–585 [DOI] [PubMed] [Google Scholar]
- Pruyne D., Bretscher A., 2000b Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113(Pt. 3): 365–375 [DOI] [PubMed] [Google Scholar]
- Pruyne D., Gao L., Bi E., Bretscher A., 2004. Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol. Biol. Cell 15: 4971–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam C. D., Jaehnig E. J., Kolodner R. D., 2009. Perspectives on the DNA damage and replication checkpoint responses in Saccharomyces cerevisiae. DNA Repair (Amst.) 8: 974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., 2006. Organization and functions of sphingolipid biosynthesis in yeast. Biochem. Soc. Trans. 34: 367–369 [DOI] [PubMed] [Google Scholar]
- Roberts R. L., Fink G. R., 1994. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8: 2974–2985 [DOI] [PubMed] [Google Scholar]
- Rodal A. A., Sokolova O., Robins D. B., Daugherty K. M., Hippenmeyer S., et al. , 2005. Conformational changes in the Arp2/3 complex leading to actin nucleation. Nat. Struct. Mol. Biol. 12: 26–31 [DOI] [PubMed] [Google Scholar]
- Roncero C., 2002. The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41: 367–378 [DOI] [PubMed] [Google Scholar]
- Sawai H., Okamoto Y., Luberto C., Mao C., Bielawska A., et al. , 2000. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae. J. Biol. Chem. 275: 39793–39798 [DOI] [PubMed] [Google Scholar]
- Shi Q. M., Wang Y. M., Zheng X. D., Lee R. T., Wang Y., 2007. Critical role of DNA checkpoints in mediating genotoxic-stress-induced filamentous growth in Candida albicans. Mol. Biol. Cell 18: 815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia R. A., Bardes E. S., Lew D. J., 1998. Control of Swe1p degradation by the morphogenesis checkpoint. EMBO J. 17: 6678–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. L., 1973. Effect of reversible inhibition of deoxyribonucleic acid synthesis on the yeast cell cycle. J. Bacteriol. 113: 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka M. B., Chen S. H., Maddox P. S., Enserink J. M., Albuquerque C. P., et al. , 2006. An FHA domain-mediated protein interaction network of Rad53 reveals its role in polarized cell growth. J. Cell Biol. 175: 743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyjka S. J., Viggiani C. J., Aparicio O. M., 2005. Mrc1 is required for normal progression of replication forks throughout chromatin in S. cerevisiae. Mol. Cell 19: 691–697 [DOI] [PubMed] [Google Scholar]
- Tabuchi M., Audhya A., Parsons A. B., Boone C., Emr S. D., 2006. The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol. Cell. Biol. 26: 5861–5875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Katou Y., Yagura M., Saitoh K., Itoh T., et al. , 2009. Ctf4 coordinates the progression of helicase and DNA polymerase alpha. Genes Cells 14: 807–820 [DOI] [PubMed] [Google Scholar]
- Tercero J. A., Diffley J. F., 2001. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature 412: 553–557 [DOI] [PubMed] [Google Scholar]
- Tong A. H., Lesage G., Bader G. D., Ding H., Xu H., et al. , 2004. Global mapping of the yeast genetic interaction network. Science 303: 808–813 [DOI] [PubMed] [Google Scholar]
- Tourriere H., Versini G., Cordon-Preciado V., Alabert C., Pasero P., 2005. Mrc1 and Tof1 promote replication fork progression and recovery independently of Rad53. Mol. Cell 19: 699–706 [DOI] [PubMed] [Google Scholar]
- Vaena de Avalos S., Okamoto Y., Hannun Y. A., 2004. Activation and localization of inositol phosphosphingolipid phospholipase C, Isc1p, to the mitochondria during growth of Saccharomyces cerevisiae. J. Biol. Chem. 279: 11537–11545 [DOI] [PubMed] [Google Scholar]
- Wang Y., 2009. CDKs and the yeast-hyphal decision. Curr. Opin. Microbiol. 12: 644–649 [DOI] [PubMed] [Google Scholar]
- Ward M. P., Gimeno C. J., Fink G. R., Garrett S., 1995. SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol. Cell. Biol. 15: 6854–6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A., Hartwell L. H., 1988. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 241: 317–322 [DOI] [PubMed] [Google Scholar]
- Wertman K. F., Drubin D. G., Botstein D., 1992. Systematic mutational analysis of the yeast ACT1 gene. Genetics 132: 337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Boone C., Brown G. W., 2007. Genetic dissection of parallel sister-chromatid cohesion pathways. Genetics 176: 1417–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanolari B., Friant S., Funato K., Sutterlin C., Stevenson B. J., et al. , 2000. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 19: 2824–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegerman P., Diffley J. F., 2003. Lessons in how to hold a fork. Nat. Struct. Biol. 10: 778–779 [DOI] [PubMed] [Google Scholar]