Abstract
The 5′-UTR serves as the loading dock for ribosomes during translation initiation and is the key site for translation regulation. Many genes in the yeast Saccharomyces cerevisiae contain poly(A) tracts in their 5′-UTRs. We studied these pre-AUG poly(A) tracts in a set of 3274 recently identified 5′-UTRs in the yeast to characterize their effect on in vivo protein abundance, ribosomal density, and protein synthesis rate in the yeast. The protein abundance and the protein synthesis rate increase with the length of the poly(A), but exhibit a dramatic decrease when the poly(A) length is ≥12. The ribosomal density also reaches the lowest level when the poly(A) length is ≥12. This supports the hypothesis that a pre-AUG poly(A) tract can bind to translation initiation factors to enhance translation initiation, but a long (≥12) pre-AUG poly(A) tract will bind to Pab1p, whose binding size is 12 consecutive A residues in yeast, resulting in repression of translation. The hypothesis explains why a long pre-AUG poly(A) leads to more efficient translation initiation than a short one when PABP is absent, and why pre-AUG poly(A) is short in the early genes but long in the late genes of vaccinia virus.
POLY(A) tracts in 5′-UTR have been recognized recently as important sites for translation regulation. These poly(A) tracts, referred to hereafter as pre-AUG AN, where N stands for the number of consecutive A nucleotides, can interact with translation initiation factors or poly(A) binding proteins (PABP) to either increase or decrease translation efficiency. Pre-AUG AN can enhance internal ribosomal entry both in the presence of PABP and eIF-4G in the yeast, Saccharomyces cerevisiae (Gilbert et al. 2007), and in the complete absence of PABP and eIF-4G (Shirokikh and Spirin 2008). Translation initiation factors eIF-4B and eIF-4F can bind to poly(A) tracts (Gallie and Tanguay 1994), and exogenous poly(A) added to an in vitro translation system can inhibit translation initiation (Lodish and Nathan 1972; Jacobson and Favreau 1983; Grossi De Sa et al. 1988), most probably by sequestering the translation initiation factors (Gallie and Tanguay 1994) and PABP (Gilbert et al. 2007). The inhibiting effect of exogenous poly(A) on translation can be removed by addition of either translation initiation factors eIF-4B and eIF-4F (with eIF-4A) in combination (Gallie and Tanguay 1994) or PABP (Grossi De Sa et al. 1988; Gilbert et al. 2007) which presumably would bind to the exogenous poly(A) and free translation initiation factors sequestered by the exogenous poly(A).
While pre-AUG AN may improve translation efficiency, a few studies (Wu and Bag 1998; Bag 2001; Melo et al. 2003a,b; Patel et al. 2005; Ma et al. 2006; Patel and Bag 2006; Bag and Bhattacharjee 2010) suggest an inhibitory effect of PABP when it binds to a long pre-AUG AN and presumably interferes with the scanning mechanism of translation initiation. The binding site of Pab1p in the yeast is about 12 consecutive A nucleotides, with the binding affinity decreasing rapidly with shorter poly(A) until 8, below which there is little affinity (Sachs et al. 1987). This suggests that mRNAs with pre-AUG AN of different lengths may interact differently with yeast Pab1p and have different translation efficiencies.
Several recent technological breakthroughs have eliminated two fundamental difficulties in characterizing the relationship between pre-AUG AN and translation efficiency in S. cerevisiae. First, the transcription start site (TSS) necessary for the accurate delineation of 5′-UTR and pre-AUG AN has been characterized for thousands of yeast genes by direct mapping of the capped yeast mRNA sequences (Miura et al. 2006). This approach to delineate TSSs is conceptually more direct and technologically more accurate than the serial analysis of gene expression (SAGE) approach (Zhang and Dietrich 2006). A merged set of TSSs from these two studies has recently been compiled (Lawless et al. 2009).
The second difficulty in quantifying the relationship between pre-AUG AN and translation efficiency is the lack of large-scale characterization of protein production and ribosomal density on mRNA. This difficulty is alleviated by a recent proteomic study in yeast (Ghaemmaghami et al. 2003) and several studies characterizing the ribosomal density and the protein synthesis rate for thousands of yeast genes (Arava et al. 2003; MacKay et al. 2004; Ingolia et al. 2009).
Through a detailed analysis of sequence features in characterized 5′-UTRs and their relationship to in vivo protein production and ribosomal loading, two interesting patterns were revealed. First, the frequency of nucleotide A increased dramatically toward the initiation codon, with a concurrent decrease in nucleotide U. This trend is particularly pronounced in highly expressed genes relative to lowly expressed genes when gene expression is measured by either the protein abundance, by the predicted rate of protein synthesis based on mRNA abundance, by the ribosomal density, or by the codon adaptation index, which is highly correlated with transcript and protein abundance in the yeast (Duret and Mouchiroud 1999; Coghlan and Wolfe 2000). Second, the protein abundance and the protein synthesis rate both increase with the length of pre-AUG AN up to N = 11 and decrease dramatically for genes having a pre-AUG AN with N ≥ 12. The ribosomal density also reaches the lowest level for genes having a pre-AUG AN with N ≥ 12.
Materials and Methods
The 5′-UTR sequences were extracted by using the specification in table 4 of Miura et al. (2006) and the yeast genomic sequences retrieved from University of Tokyo Genome Browser (http://yeast.utgenome.org/). A total of 3274 genes have their TSS characterized, with many having multiple TSSs and consequently multiple 5′-UTRs. To avoid overrepresentation of the sequence patterns of genes with multiple 5′-UTRs, only the longest 5′-UTR for each gene is used. The 5′-UTR sequences are available as a FASTA file (MiuraTSS.FAS in Supporting Information, File S5).
Three measures of translation efficiency were used for quantifying the relationship between pre-AUG AN and translation efficiency. The first is codon adaptation index (CAI) (Sharp and Li 1987) with its improved version (Xia 2007) implemented in DAMBE (Xia 2001; Xia and Xie 2001). We retrieved the genomic sequences of the 16 chromosomes of S. cerevisiae from the genome database at National Center for Biotechnology Information, extracted coding sequences (CDSs) and calculated their corresponding CAI values by using DAMBE with the Eysc_h.cut codon usage table. The benefit of using CAI is that it can be computed for any gene with a codon frequency distribution, whereas experimentally measured protein expression or ribosomal loading data are often limited to relatively highly expressed proteins or transcripts.
The second measure of translation efficiency is the protein abundance, in units of molecules/cell, measured experimentally in a large-scale quantification of yeast protein abundance (Ghaemmaghami et al. 2003). The dataset contains protein abundance data for 3850 yeast genes after excluding 18 genes that do not have a matched name in the current yeast database. For characterizing the relationship between the protein abundance and the length of pre-AUG AN, the protein abundance was log transformed to stabilize the variance and to linearize the relationship. The protein abundance data used in this article is available as GhaemmaghamiProtein.xls in File S4.
The third measure of translation efficiency is based on large-scale characterizations of ribosomal loading on gene-specific mRNA sequences (Arava et al. 2003; MacKay et al. 2004; Ingolia et al. 2009). The experimental data for the ribosomal density and the predicted protein synthesis rate in two experimental conditions (mating pheromone treatment and control) were reliably measured for 3916 genes (supplemental table II in MacKay et al. 2004). Another data set characterizing ribosomal density, with a similar method but in a different laboratory (Arava et al. 2003), as well as a data set characterizing ribosomal loading with the quite different deep sequencing method (Ingolia et al. 2009), were also analyzed and compared against results from MacKay et al. (2004). These three data sets, referred to hereafter as MacKayData, AravaData, and IngoliaData are noteworthy in that they are highly concordant (Concordance.pdf in File S1 and File S2). All other data used this paper are in AllData.xls (File S3).
Results
Overrepresentation of A in 5′-UTRs
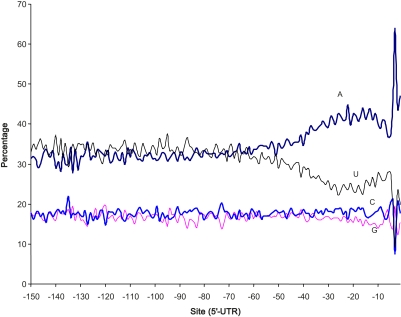
The nucleotide frequency distribution over 150 5′-UTR sites immediately upstream of the translation initiation codon shows a dramatic increase of nucleotide A toward the translation initiation codon starting from site −40, with a concurrent decrease in nucleotide U (Figure 1). The nucleotide frequency distribution before and after site −40 are highly significantly different (χ2 = 2815.84, DF = 3, P < 0.0001). A similar pattern was also observed in a previous study (Shabalina et al. 2004), which suggested that the 30-nt segment immediately upstream of the initiation codon was functionally important in eukaryotic genes, although that study suffered from the fact that sequences upstream of the initiation codon in the genome are not necessarily part of the 5′-UTR because many yeast genes have 5′-UTRs shorter than 30 nt.
Figure 1 .
The site-specific nucleotide frequencies of 150 nucleotide sites (x-axis), immediately upstream from the start codon AUG in yeast 5′-UTR sequences. Site −1 is represented by 3274 5′-UTR sequences and site −150 is represented by 747 5′-UTR sequences.
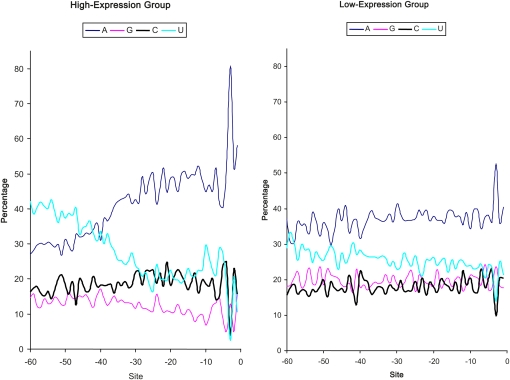
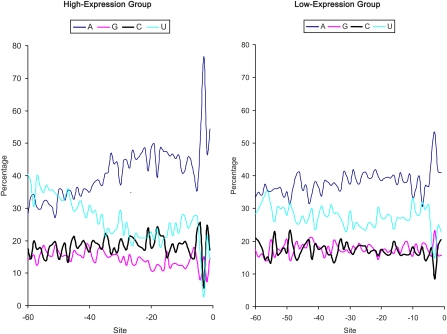
If the pattern of increasing A and decreasing U toward the initiation codon (Figure 1) is related to translation efficiency, then the pattern should be more pronounced in highly expressed genes than in lowly expressed genes. To test this prediction, we compared the nucleotide distribution between the 500 genes with the highest CAI values (high-expression group, HEG) and the 500 genes with the lowest CAI values (low-expression group, LEG). Genes in HEG exhibit the pattern much more dramatically than those in the LEG (Figure 2). The nucleotide frequency distribution between HEG and LEG are highly significantly different (χ2 = 592.74, DF = 3, P < 0.0001). The same pattern is observed when the predicted protein synthesis rate (MacKay et al. 2004) is used as a proxy of translation efficiency (Figure 3).
Figure 2 .
Contrasting site-specific nucleotide frequencies between highly expressed genes (500 genes with the highest CAI values) and lowly expressed genes (500 genes with the lowest CAI values). The increase in A usage toward the initiation AUG is visually more dramatic in highly expressed genes than lowly expressed genes. The nucleotide frequency distribution between HEG and LEG are highly significantly different (χ2 = 592.74, DF = 3, P < 0.0001).
Figure 3 .
Contrasting site-specific nucleotide frequencies between highly expressed genes (500 genes with the highest predicted protein synthesis rates) and lowly expressed genes (500 genes with the lowest predicted protein synthesis rates). Protein synthesis rates are from supplemental table II in MacKay et al. (2004).
Genes having a pre-AUG AN with N < 12 produce more proteins than those With N ≥ 12
Genes with a pre-AUG A<12 have protein abundance higher than those with a pre-AUG A≥12 (Table 1), with the two groups differing from each other significantly (F = 4.96, DFmodel = 1, DFerror = 2218, P = 0.0261). There is no significant difference either among poly(A) length groups 2, 3,…, 11 or among the poly(A) length groups with the length ≥12.
Table 1. Comparison of protein abundance among genes with different lengths (L) of pre-AUG poly(A).
| L | N | Mean | SD |
|---|---|---|---|
| 2 | 275 | 7.9081 | 1.4264 |
| 3 | 523 | 8.0553 | 1.6128 |
| 4 | 599 | 8.1663 | 1.5917 |
| 5 | 355 | 8.2830 | 1.7301 |
| 6 | 187 | 8.1552 | 1.7907 |
| 7 | 114 | 8.1861 | 1.6532 |
| 8 | 66 | 8.6858 | 1.7825 |
| 9 | 34 | 8.3668 | 1.5573 |
| 10 | 20 | 7.7982 | 1.6347 |
| 11 | 18 | 9.2160 | 1.5686 |
| 12 | 12 | 7.2013 | 1.4117 |
| 13 | 4 | 7.6616 | 0.4500 |
| 14 | 4 | 7.4732 | 1.8465 |
| 15 | 5 | 7.6702 | 1.6804 |
| 16 | 4 | 7.8449 | 1.1042 |
The differences are statistically significant (F = 2.32, DFmodel = 14, DFerror = 2205, P = 0.0036). The P-value would be reduced to 0.0004 if the genes with poly(A) length ≥12 were lumped into one group. A gene (YJR113C) with an A21 in its 5′-UTR was included into the group of genes with a A16.
One peculiar data point in Table 1 is the protein abundance for genes with a pre-AUG A10. These genes have a singularly low protein abundance and include a gene with the lowest protein abundance (YJL084C/ALY2, alpha arrestin) of only 3.8950. The A10 in this gene precedes “GA,” i.e., in the configuration of AAAAAAAAAAGA. There are two other yeast genes (YPL271W and YBL101C) sharing this AAAAAAAAAABA configuration (where B means “not A,” i.e., C, G, or U) with protein abundance values equal to 8.3610 and 6.6839 (which is also low), respectively. Also, one of the A10 genes (YMR181C) is starvation-induced (Gilbert et al. 2007) and has a relatively low protein abundance as well (= 6.5800). Without these four genes, the mean protein abundance for A10 genes is 8.1528.
The observation above led us to ask whether the pre-AUG AN needs to be contiguous for its effect on protein abundance. We compiled genes with a pre-AUG 12mer that has 11 A residues but with a non-A breaking the contiguity, i.e., in the configuration of ABAAAAAAAAAA, AABAAAAAAAAA, AAABAAAAAAAA,……, AAAAAAAAAABA and compared their protein abundance with those of genes containing a pre-AUG A12. Protein abundance is significantly higher in the former (mean = 8.5970, n = 43) than in the latter (mean = 7.2013, n = 12), with t = 2.3453, DF = 53, and P = 0.0228 (two-tailed test). Thus, contiguity in poly(A) appears important for decreasing protein production. However, protein abundance is higher for genes with a non-A close to the 5′ end than with a non-A at the 3′ end, i.e., ABAAAAAAAAAA and AABAAAAAAAAA genes produce more proteins (mean = 8.6399, n = 9) than AAAAAAAAAABA and AAAAAAAAABAA genes (mean = 6.9207, n = 6), with t = 2.2314, DF = 13, P = 0.0439 (two-tailed test). This suggests that where contiguity is broken may be important.
Genes with a pre-AUG A11 produce more proteins than those with a pre-AUG A12
The binding affinity of Pab1p approaches maximum when the poly(A) length reaches 12 but decreases quickly when the poly(A) length is 11 or shorter, with hardly any affinity when the poly(A) length is 8 (Sachs et al. 1987). So we examined whether genes with a pre-AUG A11, on average, produce more proteins than genes with a pre-AUG A12.
Among the yeast genes with characterized protein abundance (Ghaemmaghami et al. 2003), 12 have a pre-AUG A12 and 18 have a pre-AUG A11 (including the Pab1/YER165W mRNA, Table 2). Genes with a pre-AUG A11 have higher protein abundance than genes with a pre-AUG A12 (t = 3.5827, DF = 28, P = 0.0013, two-tailed test, Table 2). This is consistent with the interpretation that mRNAs with a pre-AUG A<12 may enhance translation, but mRNAs with a pre-AUG A≥12 may be subject to translation repression mediated by PABP binding to the pre-AUG poly(A) and interfering with the ribosomal scanning. The mean protein abundance values are 7.2013 for genes with a pre-AUG A12 and 9.2160 for genes with a pre-AUG A11 (Table 2). Converted back to the original scale, the former is 1341.12 molecules/cell and the latter is 10056.42 molecules/cell.
Table 2. Protein abundance for genes with a poly(A) tract of length 11 or 12 (A11 or A12) in their 5′-UTR.
| Gene | L5′-UTRa | MaxLPoly(A)b | lnProtc | CAI |
|---|---|---|---|---|
| YHR082C | 867 | 12 | 7.1448 | 0.4409 |
| YBR077C | 68 | 12 | 8.1566 | 0.3919 |
| YDR295C | 266 | 12 | 7.3121 | 0.3714 |
| YFR047C | 43 | 12 | 7.8878 | 0.4590 |
| YGL006W | 282 | 12 | 4.5945 | 0.4082 |
| YGR238C | 133 | 12 | 6.1493 | 0.3607 |
| YHR043C | 112 | 12 | 7.7817 | 0.3977 |
| YKR097W | 175 | 12 | 10.2470 | 0.5721 |
| YLR190W | 461 | 12 | 6.9747 | 0.3952 |
| YLR192C | 109 | 12 | 9.7912 | 0.5625 |
| YMR016C | 684 | 12 | 5.7506 | 0.3615 |
| YMR251W-A | 142 | 12 | 8.7225 | 0.6739 |
| YNL051W | 203 | 12 | 6.1493 | 0.4228 |
| YNL128W | 265 | 12 | 6.9881 | 0.3633 |
| YBR056W-A | 47 | 11 | 7.5809 | 0.4077 |
| YDL140C | 519 | 11 | 10.1271 | 0.4864 |
| YDL246C | 321 | 11 | 7.1664 | 0.4695 |
| YDR033W | 555 | 11 | 12.1143 | 0.7725 |
| YDR055W | 82 | 11 | 9.3654 | 0.5406 |
| YER115C | 294 | 11 | 8.1442 | 0.3465 |
| YER159C | 180 | 11 | 8.5424 | 0.2948 |
| YER165W | 145 | 11 | 12.1955 | 0.7079 |
| YGL037C | 373 | 11 | 8.9517 | 0.5606 |
| YHL012W | 77 | 11 | 7.4276 | 0.3696 |
| YJR070C | 72 | 11 | 10.5931 | 0.6736 |
| YKL080W | 130 | 11 | 9.9577 | 0.4954 |
| YKL084W | 52 | 11 | 11.5340 | 0.4050 |
| YKL186C | 377 | 11 | 7.4939 | 0.3297 |
| YKR092C | 318 | 11 | 9.4679 | 0.5404 |
| YLL013C | 298 | 11 | 6.7403 | 0.4263 |
| YLL029W | 155 | 11 | 8.0823 | 0.3828 |
| YLR328W | 90 | 11 | 8.5424 | 0.4664 |
| YLR341W | 98 | 11 | 7.9929 | 0.4145 |
| YML091C | 316 | 11 | 7.0521 | 0.4622 |
| YMR070W | 213 | 11 | 7.4353 | 0.3715 |
| YMR291W | 735 | 11 | 9.2261 | 0.4517 |
| YMR296C | 211 | 11 | 10.0159 | 0.4094 |
| YOL155W-A | 195 | 11 | 8.6049 | 0.3095 |
| YPL154C | 279 | 11 | 7.7964 | 0.6100 |
| YPL204W | 189 | 11 | 9.2396 | 0.4254 |
| YPR041W | 121 | 11 | 10.7845 | 0.5831 |
The protein abundance is significantly higher in A11 genes (mean = 8.9694) than in A12 genes (mean = 7.4036), on the basis of a two-sample t-test (t = 3.0879, DF = 39, P = 0.0037, two-tailed test). Codon adaptation index (CAI) is included as a covariate for analysis of covariance (see text for details).
5′-UTR length.
Maximum length of poly(A) tracts in 5′-UTR.
Log-transformed protein abundance.
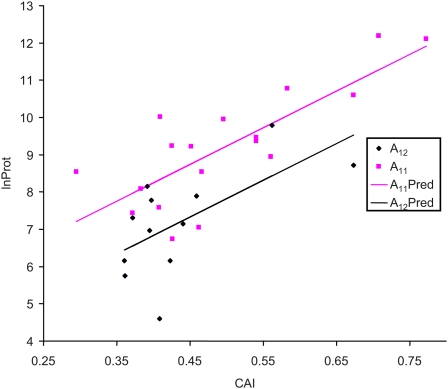
The analysis above based on protein abundance has the problem that protein abundance is affected by a number of other factors such as translation elongation and mRNA abundance. For example, the protein abundance is highly correlated with CAI, which is a measure of translation elongation efficiency (Figure 4). It is therefore possible that the difference in protein abundance between the A11 genes and the A12 genes may simply be due to the mRNAs from the A11 genes having higher translation elongation efficiency than those from the A12 genes. It is known that different genes can differ much in translation elongation efficiency because of their differential codon adaptation to the tRNA pool (Xia 1998, 2005; van Weringh et al. 2011).
Figure 4 .
Visualization of the difference in protein abundance between A11 and A12 genes, when codon adaptation index (CAI) is use as a covariate to control for differences in translation elongation efficiency. A11Pred and A12Pred are fitted regression lines from an analysis of covariance, and lnProt is the log-transformed protein abundance from Ghaemmaghami et al. (2003). The difference in intercept is 1.4108 (P = 0.0015).
To control for the potential difference in translation elongation efficiency between the A11 and A12 genes, we have performed an analysis of covariance (ANCOVA) to test the difference in protein abundance between the A11 and A12 genes with CAI as a covariate. The relationship between protein production and CAI (Figure 4) is highly significant (slope = 9.8479, P < 0.0001), and the intercept for A11 genes is highly significantly greater than that for A12 genes by 1.4108 (P = 0.0015). Thus, the A11 genes still have significantly higher protein abundance than the A12 genes when translation elongation efficiency has been controlled for by using CAI as a covariate.
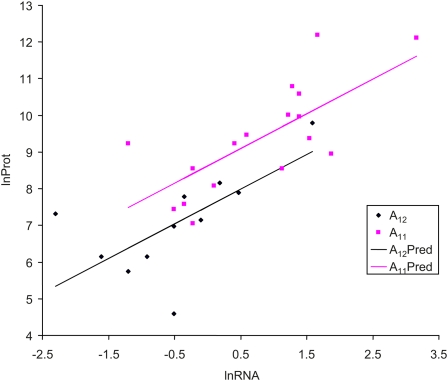
We have also performed a similar ANCOVA by using characterized mRNA abundance (Holstege et al. 1998) as a covariate. Protein abundance is significantly correlated with the mRNA abundance (Figure 5) for yeast genes with either an A11 or an A12 in their 5′-UTR, with a slope of 0.9455 (P < 0.0001). The intercept for A11 genes is significantly greater than that for A12 genes by 1.1096 (P = 0.0274). Thus, the A11 genes produce more proteins than the A12 genes, when mRNA abundance has been controlled for. A similar pattern is observed when the mRNA abundance characterized by Miura et al. (2008) is used.
Figure 5 .
Visualization of the difference in protein abundance between the A11 and the A12 genes, when mRNA abundance (lnRNA) is use as a covariate to control for the potential difference in mRNA abundance between the A11 and the A12 genes. A11Pred and A12Pred are fitted regression lines from an analysis of covariance. lnProt is the log-transformed protein abundance from Ghaemmaghami et al. (2003). The difference in intercept is 1.1096 (P = 0.0274). lnRNA is the log-transformed mRNA abundance from Holstege et al. (1998). A similar pattern is observed when mRNA abundance from Miura et al. (2008) is used.
Protein synthesis rate and ribosomal density depend on the length of pre-AUG AN
One problem with the analysis above is that protein abundance depends on both protein production and protein degradation. For example, cyclins typically have a half-life in minutes (Aviram et al. 2008) and are consequently expected to have relatively low abundance even if translation initiation and elongation of their mRNAs are efficient. Thus, the low protein abundance in the A12 genes relative to the A11 genes could be due to more rapid degradation of proteins from the A12 genes than those from the A11 genes.
The ultimate solution to avoid the shortcomings above is to use a more direct measure of translation efficiency than protein abundance to avoid the confounding effect of mRNA abundance or protein degradation. Such measures, expressed as translation efficiency on the basis of ribosomal density, have become available recently for yeast genes (Arava et al. 2003; Serikawa et al. 2003; MacKay et al. 2004; Ingolia et al. 2009).
Among the 3916 genes in MacKayData, 2139 genes have their transcription start sites experimentally determined by Miura et al. (2006). The predicted protein synthesis rate was measured under two experimental conditions (control yeast cells and those treated with a mating pheromone). The two measured rates, however, are similar, with the Pearson correlation being 0.9317 for log-transformed data. We simply took the average of the two rates, referred to hereafter as MeanRate (of protein synthesis), and studied its relationship to the length of pre-AUG AN.
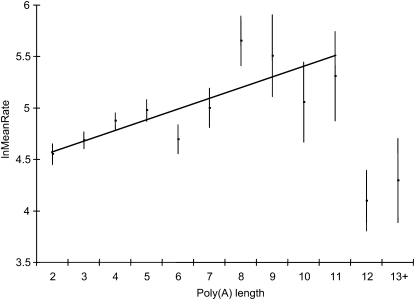
MeanRate increases with the length of pre-AUG AN, but decreases dramatically when poly(A) length reaches 12 (Figure 6). The regression line in Figure 6 is highly significant (P < 0.0001) for genes whose pre-AUG AN is no longer than 11. Thus, a pre-AUG AN, up to 11, tends to increase the protein synthesis rate, but a long (≥12) pre-AUG AN tends to decrease the protein synthesis rate.
Figure 6 .
Protein synthesis rate (lnMeanRate) increases with the length of pre-AUG poly(A), but decreases dramatically when the poly(A) length reaches 12. The straight line indicates the regression line for genes with poly(A) length shorter than 12. The vertical bars show one standard error above and below the mean for each poly(A) length category.
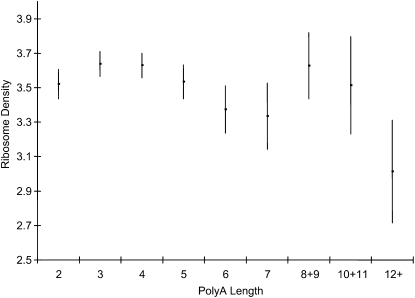
We have also examined the relationship between the experimentally measured ribosomal density and the length of the pre-AUG AN. Ingolia et al. (2009) characterized ribosomal density for yeast genes in both rich and starvation conditions. Here we use their ribosomal density for 5311 yeast genes characterized under two rich conditions, log transformed to stabilize variance. While ribosomal density fluctuate for genes with the pre-AUG AN length (Figure 7), the mean ribosomal density for genes with a pre-AUG A<12 (= 3.6879) is significantly greater than that (=3.0136) for genes with a pre-AUG A≥12 (t = 2.1932, DF = 2911, P = 0.0284, two-tailed test).
Figure 7 .
Relationship between ribosomal density (log-transformed normalized read density) and poly(A) length. The vertical bars show one standard error (SE) above and below the mean for each poly(A) length category. Some neighboring poly(A) length groups were lumped to reduce SE. Ribosomal density for genes in the “12+” group is significantly lower (t = 2.1932, DF = 2911, P = 0.0284, two-tailed test) than that of all other categories treated as one group.
Is low protein abundance in cyclin due to rapid degradation?
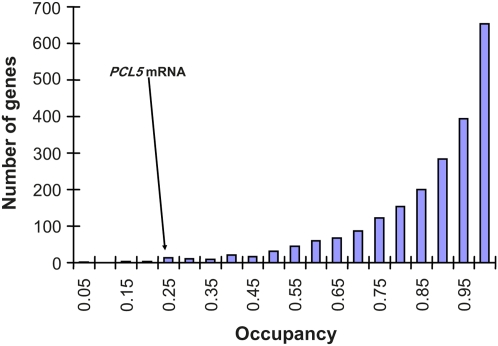
The cyclin gene PCL5/YHR071W has a pre-AUG A29, which is the longest among all yeast genes. The Pcl5 protein has a half-life of only 2–3 min, and its low abundance is usually attributed to its rapid degradation (Aviram et al. 2008). Our results suggest that the low abundance may be partially attributable to inefficient translation initiation presumably mediated by the binding of Pab1p to the long pre-AUG A29. The ribosomal occupancy is extremely low for PCL5 mRNA relative to other yeast genes (Figure 8).
Figure 8 .
Frequency distribution of occupancy (proportion of mRNA associated with polysomes) of 2175 yeast genes (MacKay et al. 2004) with 5′-UTR characterized by Miura et al. (2006). The mean occupancy of PCL5 mRNA from two measurements is 0.2360.
Discussion
Interaction between pre-AUG poly(A) and Pab1p
Our finding that yeast genes with a pre-AUG A≥12 have a much reduced predicted protein synthesis rate (Figures 6) and ribosomal density (Figure 7) is consistent with the hypothesis that, while a pre-AUG AN may enhance translation by binding to translation initiation factors (Gallie and Tanguay 1994; Shirokikh and Spirin 2008), a long pre-AUG AN, by binding tightly to PABP, may repress translation by interfering with ribosomal scanning (Sachs et al. 1987; Wu and Bag 1998; Bag 2001; Melo et al. 2003a,b; Patel et al. 2005; Ma et al. 2006; Patel and Bag 2006). This hypothesis would predict that removing PABP would remove its inhibitory effect on mRNA with long pre-AUG AN. This is exactly what has been observed in a previous in vitro experiment (Shirokikh and Spirin 2008) without PABP, where the translation-enhancing effect is greater for longer poly(A) than for shorter poly(A), i.e., the ranking order of translation initiation efficiency is A25 > A12 > A5.
Our results suggest an alternative to the dominant hypothesis concerning the function of PABP on translation initiation. Several studies have shown that exogenous poly(A) can inhibit translation initiation (Lodish and Nathan 1972; Jacobson and Favreau 1983; Grossi De Sa et al. 1988), and the inhibitive effect can be eliminated by the addition of PABP (Grossi De Sa et al. 1988; Gilbert et al. 2007). Similarly, noncoding poly(A) sequences, such as BC1 and BC200 RNA expressed in neurons, are known to bind PABP (Muddashetty et al. 2002) and to inhibit translation initiation when highly expressed (Wang et al. 2002, 2005; Kondrashov et al. 2005). The dominant hypothesis is that PABP, in addition to its function in mRNA stabilization and circularization, also serves as a translation initiation factor (Kahvejian et al. 2005; Khanam et al. 2006) that functions by binding to pre-AUG AN. Thus, either exogenous poly(A) RNA or intrinsically produced poly(A) RNA such as BC1 and BC200 RNA that sequesters PABP would reduce translation initiation (Khanam et al. 2006; Gilbert et al. 2007). Consistent with this hypothesis, adding PABP eliminated the inhibitive effect of the exogenous poly(A) RNA (Grossi De Sa et al. 1988; Gilbert et al. 2007). The hypothesis also explains why poly(A) is over-represented in 5′-UTR in yeast genes, especially those highly expressed ones because such pre-AUG AN would gain enhanced translation initiation by interacting with PABP. However, this hypothesis has three difficulties. First, it cannot explain why genes with a long pre-AUG AN have a reduced protein synthesis rate as well as a reduced ribosomal density shown in this article. Second, it cannot explain why, in the complete absence of PABP, pre-AUG AN can still enhance translation initiation for both capped and uncapped mRNA (Shirokikh and Spirin 2008). Third, it cannot explain why adding translation initiation factors eIF-4B and eIF-4F (including eIF-4A) in combination also eliminated the inhibitive effect of exogenous poly(A) RNA on translation initiation (Gallie and Tanguay 1994). Our new hypothesis is that pre-AUG AN binds to translation initiation factors such as eIF-4B and eIF-4F to facilitate translation initiation. Exogenous or intrinsic poly(A) RNAs can inhibit translation initiation not only because they compete for PABP but also because they would sequester the translation initiation factors eIF-4B and eIF-4F. Adding PABP can eliminate the inhibitive effect of exogenous poly(A) RNA because PABP would bind to the poly(A) and free the translation initiation factors sequestered by these poly(A) RNAs. This new hypothesis, which was implicitly proposed in a previous study (Gallie and Tanguay 1994) demonstrating the binding of poly(A) RNA to eIF-4B and eIF-4F, eliminates all three difficulties plaguing the other hypothesis.
Presence of a pre-AUG AN appears to be a key feature in a set of internal ribosomal entry sites (IRESs) empirically verified in a recent study on yeast translation (Gilbert et al. 2007). All those poly(A) tracts are shorter than 12 consecutive A’s. These include not only the genes involved in the invasive growth in the yeast, but also transcripts that are routinely transcribed and translated, such as eIF-4G and Pab1 transcripts. The IRES activity mediated by the pre-AUG AN does seem to require Pab1p (Gilbert et al. 2007). A recent study using mRNAs without a poly(A) tail (Kahvejian et al. 2005) suggests that PABP may serve as a translation initiation factor independent of its binding to the poly(A) tail. It is possible that the multiple PABP functions may depend on how strong it binds to pre-AUG AN, with strong binding inhibiting translation and weak binding enhancing translation. It is also possible that the association between the IRES activity and the pre-AUG AN is coincidental. A recent study on IRESs from both the yeast and Drosophila melanogaster shows that IRES activity increases consistently with decreasing stability of secondary structure (Xia and Holcik 2009). A pre-AUG poly(A) would contribute to a weak RNA secondary structure when nucleotide U usage is dramatically reduced (Figures 1–3).
While there is empirical evidence that mammalian PABP expression may be autoregulated by PABP binding to the pre-AUG AN of its own mRNA (Wu and Bag 1998; Bag 2001; Ma et al. 2003a,b, 2006; Patel et al. 2005; Patel and Bag 2006; Bag and Bhattacharjee 2010), there is no evidence that Pab1p abundance in yeast is autoregulated. Pab1p abundance is high, being the top 39th among the 3841 yeast genes with characterized protein abundance (Ghaemmaghami et al. 2003). Its mRNA ranked the top 114th in ribosomal density among the 5164 yeast genes with ribosomal density characterized by Ingolia et al. (2009). Such a high protein abundance and a high ribosomal density is strong evidence that the high protein abundance in Pab1p does not interfere with the translation of its mRNA. If the autoregulation requires a pre-AUG A12, then Pab1 mRNA would escape autoregulation because it has only a pre-AUG A11. The mammalian PAPB seems to be less strict on contiguity of poly(A), especially its RNA-recognition motif (RRM) 3+4 (Khanam et al. 2006).
Relevance to the translation of early and late genes in vaccinia virus
The finding that the length of pre-AUG AN is strongly associated with ribosomal loading and protein synthesis sheds light on the evolutionary significance of the difference in the length of pre-AUG AN between early and late genes in vaccinia virus. The early vaccinia viral genes have a pre-AUG AN with 4–14 A residues (Ahn et al. 1990; Ink and Pickup 1990), but the poly(A) tracts in late genes are often around 35 A residues (Bertholet et al. 1987; Schwer et al. 1987; Schwer and Stunnenberg 1988). The early viral genes are translated in the presence of abundant PABP, which would repress the translation of mRNAs with a long pre-AUG AN. This implies that the transcripts of the viral early genes should have only short poly(A) to avoid repression. In contrast, late viral genes are translated when the cellular protein production has been much reduced, i.e., when PABP is expected to be less abundant. So mRNAs from late viral genes can have long pre-AUG AN without suffering from translation repression mediated by PABP. It has been experimentally demonstrated that, in the absence of PABP, the translation enhancing effect of pre-AUG AN increases with its length (Shirokikh and Spirin 2008).
There is some controversy concerning whether the PABP level is reduced during the infection cycle of vaccinia virus. The degradation of host mRNA appears nearly complete 6 hr after the viral infection as no host poly(A) mRNA is detectable at/after this time (Katsafanas and Moss 2007). Furthermore, a large-scale characterization of mRNA of HeLa cells infected with vaccinia virus (Yang et al. 2010) showed that PABP mRNA was reduced to 50% by 4 hr. Although no mRNA characterization is done after this time, intuition would suggest continued reduction, and such a suggestion is consistent with the finding that no host mRNA is detectable after 6 hr after the viral infection (Katsafanas and Moss 2007).
The study by Katsafanas and Moss (2007) also showed that the viral mRNAs are located in the cavities of viral factories (VFs), where they are transcribed and translated. A number of translation initiation factors such as eIF4E and eIF4G are also localized in these cavities (Katsafanas and Moss 2007; Walsh et al. 2008). In contrast, PABP is localized on the periphery of a VF (Walsh et al. 2008), which suggests that PABP does not participate in translation of the viral genes. It is known that vaccinia virus produces poly(A) nontranslated small RNA sequences that selectively inhibit cap-dependent translation of host messages (Bablanian and Banerjee 1986; Bablanian et al. 1986, 1987, 1993; Lu and Bablanian 1996), presumably by binding to PABP and preventing it from interacting with other translation initiation factors. Both Rubella virus and Bunyamwera virus inhibit translation of host genes by producing a capsid protein that binds to PABP and prevents it from binding to other translation initiation factors (Ilkow et al. 2008; Blakqori et al. 2009).
Walsh et al. (2008) found a persistent level of PABP during the infection cycle of vaccinia virus, but did not provide any evidence that PABP is in fact produced during the viral infection cycle. However, a subsequent paper (Perez et al. 2011) from the same laboratory found that PABP is continuously synthesized in cytomegalovirus-infected cells. They suggested that this selective translation of PABP is through the mTOR+4E-BP pathway. However, this suggestion does not seem coherent. Preventing 4E-BP from binding to eIF-4E would lead to a general increase of cap-dependent translation, not selective translation of the PABP mRNA.
In summary, multiple lines of empirical evidence suggest that a pre-AUG AN shorter than 12 may enhance translation in the yeast. However, yeast genes with a pre-AUG A≥12 tend to be translated inefficiently with a low ribosomal density and output a reduced amount of protein, consistent with the interpretation that such long poly(A) tracts may bind to Pab1p, resulting in repression of translation.
Acknowledgments
We thank Y. Fang, M. Ragonnet, A. van Weringh, and S. Zhao for discussion, comments, and suggestions. B. Moss provided references on PABP and vaccinia virus. This study is supported by National Sciences and Engineering Research Council’s Discovery, Research Tools and Instrument, and Strategic Research Grants to X.X.
Literature Cited
- Ahn B. Y., Jones E. V., Moss B., 1990. Identification of the vaccinia virus gene encoding an 18-kilodalton subunit of RNA polymerase and demonstration of a 5′ poly(A) leader on its early transcript. J. Virol. 64: 3019–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arava Y., Wang Y., Storey J. D., Liu C. L., Brown P. O., et al. , 2003. Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 100: 3889–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram S., Simon E., Gildor T., Glaser F., Kornitzer D., 2008. Autophosphorylation-induced degradation of the Pho85 cyclin Pcl5 is essential for response to amino acid limitation. Mol. Cell. Biol. 28: 6858–6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Banerjee A. K., 1986. Poly(riboadenylic acid) preferentially inhibits in vitro translation of cellular mRNAs compared with vaccinia virus mRNAs: possible role in vaccinia virus cytopathology. Proc. Natl. Acad. Sci. USA 83: 1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Masters P. S., Banerjee A. K., 1986. Characterization of vaccinia virus transcripts involved in selective inhibition of host protein synthesis. Virology 148: 375–380 [DOI] [PubMed] [Google Scholar]
- Bablanian R., Goswami S. K., Esteban M., Banerjee A. K., 1987. Selective inhibition of protein synthesis by synthetic and vaccinia virus-core synthesized poly(riboadenylic acids). Virology 161: 366–373 [DOI] [PubMed] [Google Scholar]
- Bablanian R., Scribani S., Esteban M., 1993. Amplification of polyadenylated nontranslated small RNA sequences (POLADS) during superinfection correlates with the inhibition of viral and cellular protein synthesis. Cell. Mol. Biol. Res. 39: 243–255 [PubMed] [Google Scholar]
- Bag J., 2001. Feedback inhibition of poly(A)-binding protein mRNA translation. A possible mechanism of translation arrest by stalled 40 S ribosomal subunits. J. Biol. Chem. 276: 47352–47360 [DOI] [PubMed] [Google Scholar]
- Bag J., Bhattacharjee R. B., 2010. Multiple levels of post-transcriptional control of expression of the poy (A)-binding protein. RNA Biol. 7: 5–12 [DOI] [PubMed] [Google Scholar]
- Bertholet C., Van Meir E., ten Heggeler-Bordier B., Wittek R., 1987. Vaccinia virus produces late mRNAs by discontinuous synthesis. Cell 50: 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakqori G., van Knippenberg I., Elliott R. M., 2009. Bunyamwera orthobunyavirus S-segment untranslated regions mediate poly(A) tail-independent translation. J. Virol. 83: 3637–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan A., Wolfe K. H., 2000. Relationship of codon bias to mRNA concentration and protein length in Saccharomyces cerevisiae. Yeast 16: 1131–1145 [DOI] [PubMed] [Google Scholar]
- Duret L., Mouchiroud D., 1999. Expression pattern and, surprisingly, gene length shape codon usage in Caenorhabditis, Drosophila, and Arabidopsis. Proc. Natl. Acad. Sci. USA 96: 4482–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Tanguay R., 1994. Poly(A) binds to initiation factors and increases cap-dependent translation in vitro. J. Biol. Chem. 269: 17166–17173 [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W. K., Bower K., Howson R. W., Belle A., et al. , 2003. Global analysis of protein expression in yeast. Nature 425: 737–741 [DOI] [PubMed] [Google Scholar]
- Gilbert W. V., Zhou K., Butler T. K., Doudna J. A., 2007. Cap-independent translation Is required for starvation-induced differentiation in yeast. Science 317: 1224–1227 [DOI] [PubMed] [Google Scholar]
- Grossi de Sa M. F., Standart N., Martins de Sa C., Akhayat O., Huesca M., et al. , 1988. The poly(A)-binding protein facilitates in vitro translation of poly(A)-rich mRNA. Eur. J. Biochem. 176: 521–526 [DOI] [PubMed] [Google Scholar]
- Holstege F. C., Jennings E. G., Wyrick J. J., Lee T. I., Hengartner C. J., et al. , 1998. Dissecting the regulatory circuitry of a eukaryotic genome. (Transcriptomic data at http://web.wi.mit.edu/young/pub/data/orf_transcriptome.txt). Cell 95: 717–728 [DOI] [PubMed] [Google Scholar]
- Ilkow C. S., Mancinelli V., Beatch M. D., Hobman T. C., 2008. Rubella virus capsid protein interacts with poly(a)-binding protein and inhibits translation. J. Virol. 82: 4284–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia N. T., Ghaemmaghami S., Newman J. R. S., Weissman J. S., 2009. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324: 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ink B. S., Pickup D. J., 1990. Vaccinia virus directs the synthesis of early mRNAs containing 5′ poly(A) sequences. Proc. Natl. Acad. Sci. USA 87: 1536–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Favreau M., 1983. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 11: 6353–6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahvejian A., Svitkin Y. V., Sukarieh R., M'Boutchou M. N., Sonenberg N., 2005. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 19: 104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsafanas G. C., Moss B., 2007. Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe 2: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanam T., Muddashetty R. S., Kahvejian A., Sonenberg N., Brosius J., 2006. Poly(A)-binding protein binds to A-rich sequences via RNA-binding domains 1+2 and 3+4. RNA Biol. 3: 170–177 [DOI] [PubMed] [Google Scholar]
- Kondrashov A. V., Kiefmann M., Ebnet K., Khanam T., Muddashetty R. S., et al. , 2005. Inhibitory effect of naked neural BC1 RNA or BC200 RNA on eukaryotic in vitro translation systems is reversed by poly(A)-binding protein (PABP). J. Mol. Biol. 353: 88–103 [DOI] [PubMed] [Google Scholar]
- Lawless C., Pearson R., Selley J., Smirnova J., Grant C., et al. , 2009. Upstream sequence elements direct post-transcriptional regulation of gene expression under stress conditions in yeast. BMC Genomics 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Nathan D. G., 1972. Regulation of hemoglobin synthesis. Preferential inhibition of and globin synthesis. J. Biol. Chem. 247: 7822–7829 [PubMed] [Google Scholar]
- Lu C., Bablanian R., 1996. Characterization of small nontranslated polyadenylylated RNAs in vaccinia virus-infected cells. Proc. Natl. Acad. Sci. USA 93: 2037–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Musa T., Bag J., 2006. Reduced stability of mitogen-activated protein kinase kinase-2 mRNA and phosphorylation of poly(A)-binding protein (PABP) in cells overexpressing PABP. J. Biol. Chem. 281: 3145–3156 [DOI] [PubMed] [Google Scholar]
- MacKay V. L., Li X., Flory M. R., Turcott E., Law G. L., et al. , 2004. Gene expression analyzed by high-resolution state array analysis and quantitative proteomics: response of yeast to mating pheromone. Mol. Cell. Proteomics 3: 478–489 [DOI] [PubMed] [Google Scholar]
- Melo E. O., de Melo Neto O. P., Martins de Sa C., 2003a. Adenosine-rich elements present in the 5′-untranslated region of PABP mRNA can selectively reduce the abundance and translation of CAT mRNAs in vivo. FEBS Lett. 546: 329–334 [DOI] [PubMed] [Google Scholar]
- Melo E. O., Dhalia R., Martins de Sa C., Standart N., de Melo Neto O. P., 2003b. Identification of a C-terminal poly(A)-binding protein (PABP)-PABP interaction domain: role in cooperative binding to poly (A) and efficient cap distal translational repression. J. Biol. Chem. 278: 46357–46368 [DOI] [PubMed] [Google Scholar]
- Miura F., Kawaguchi N., Sese J., Toyoda A., Hattori M., et al. , 2006. A large-scale full-length cDNA analysis to explore the budding yeast transcriptome. Proc. Natl. Acad. Sci. USA 103: 17846–17851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura F., Kawaguchi N., Yoshida M., Uematsu C., Kito K., et al. , 2008. Absolute quantification of the budding yeast transcriptome by means of competitive PCR between genomic and complementary DNAs. BMC Genomics 9: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty R., Khanam T., Kondrashov A., Bundman M., Iacoangeli A., et al. , 2002. Poly(A)-binding protein is associated with neuronal BC1 and BC200 ribonucleoprotein particles. J. Mol. Biol. 321: 433–445 [DOI] [PubMed] [Google Scholar]
- Patel G. P., Bag J., 2006. IMP1 interacts with poly(A)-binding protein (PABP) and the autoregulatory translational control element of PABP-mRNA through the KH III–IV domain. FEBS J. 273: 5678–5690 [DOI] [PubMed] [Google Scholar]
- Patel G. P., Ma S., Bag J., 2005. The autoregulatory translational control element of poly(A)-binding protein mRNA forms a heteromeric ribonucleoprotein complex. Nucleic Acids Res. 33: 7074–7089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C., McKinney C., Chulunbaatar U., Mohr I., 2011. Translational control of the abundance of cytoplasmic poly(A) binding protein in human cytomegalovirus-infected cells. J. Virol. 85: 156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W., Kornberg R. D., 1987. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol. Cell. Biol. 7: 3268–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B., Stunnenberg H. G., 1988. Vaccinia virus late transcripts generated in vitro have a poly(A) head. EMBO J. 7: 1183–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B., Visca P., Vos J. C., Stunnenberg H. G., 1987. Discontinuous transcription or RNA processing of vaccinia virus late messengers results in a 5′ poly(A) leader. Cell 50: 163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serikawa K. A., Xu X. L., MacKay V. L., Law G. L., Zong Q., et al. , 2003. The transcriptome and its translation during recovery from cell cycle arrest in Saccharomyces cerevisiae. Mol. Cell. Proteomics 2: 191–204 [DOI] [PubMed] [Google Scholar]
- Shabalina S. A., Ogurtsov A. Y., Rogozin I. B., Koonin E. V., Lipman D. J., 2004. Comparative analysis of orthologous eukaryotic mRNAs: potential hidden functional signals. Nucleic Acids Res. 32: 1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H., 1987. The codon adaptation index–a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 15: 1281–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokikh N. E., Spirin A. S., 2008. Poly(A) leader of eukaryotic mRNA bypasses the dependence of translation on initiation factors. Proc. Natl. Acad. Sci. USA 105: 10738–10743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weringh A., Ragonnet-Cronin M., Pranckeviciene E., Pavon-Eternod M., Kleiman L., et al. , 2011. HIV-1 modulates the tRNA pool to improve translation efficiency. Mol. Biol. Evol. 28: 1827–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Arias C., Perez C., Halladin D., Escandon M., et al. , 2008. Eukaryotic translation initiation factor 4F architectural alterations accompany translation initiation factor redistribution in poxvirus-infected cells. Mol. Cell. Biol. 28: 2648–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Iacoangeli A., Popp S., Muslimov I. A., Imataka H., et al. , 2002. Dendritic BC1 RNA: functional role in regulation of translation initiation. J. Neurosci. 22: 10232–10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Iacoangeli A., Lin D., Williams K., Denman R. B., et al. , 2005. Dendritic BC1 RNA in translational control mechanisms. J. Cell Biol. 171: 811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Bag J., 1998. Negative control of the poly(A)-binding protein mRNA translation is mediated by the adenine-rich region of its 5′-untranslated region. J. Biol. Chem. 273: 34535–34542 [DOI] [PubMed] [Google Scholar]
- Xia X., 1998. How optimized is the translational machinery in Escherichia coli, Salmonella typhimurium and Saccharomyces cerevisiae? Genetics 149: 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., 2001. Data Analysis in Molecular Biology and Evolution. Kluwer Academic Publishers, Boston [Google Scholar]
- Xia X., 2005. Mutation and selection on the anticodon of tRNA genes in vertebrate mitochondrial genomes. Gene 345: 13–20 [DOI] [PubMed] [Google Scholar]
- Xia X., 2007. An improved implementation of codon adaptation index. Evol. Bioinform. 3: 53–58 [PMC free article] [PubMed] [Google Scholar]
- Xia X., Holcik M., 2009. Strong eukaryotic IRESs have weak secondary structure. PLoS ONE 4: e4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Xie Z., 2001. DAMBE: software package for data analysis in molecular biology and evolution. J. Hered. 92: 371–373 [DOI] [PubMed] [Google Scholar]
- Yang Z., Bruno D. P., Martens C. A., Porcella S. F., Moss B., 2010. Simultaneous high-resolution analysis of vaccinia virus and host cell transcriptomes by deep RNA sequencing. Proc. Natl. Acad. Sci. USA 107: 11513–11518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Dietrich F. S., 2006. Mapping of transcription start sites in Saccharomyces cerevisiae using 5′ SAGE. Nucleic Acids Res. 33: 2838–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]