Abstract
Despite the prominent and worldwide use of Bacillus thuringiensis (Bt) insecticidal toxins in agriculture, knowledge of the mechanism by which they kill pests remains incomplete. Here we report genetic mapping of a membrane transporter (ABCC2) to a locus controlling Bt Cry1Ac toxin resistance in two lepidopterans, implying that this protein plays a critical role in Bt function.
INSECTICIDE-RESISTANT phenotypes commonly arise through parallel mutations in the same gene across multiple species. However, independent resistance mechanisms can also exist within a single species. For example, resistance to dichlorodiphenyltrichloroethane (DDT) among many arthropods is caused by amino acid substitutions in the voltage-gated sodium channel (Davies et al. 2007), yet DDT resistance can also be achieved in Drosophila melanogaster through increased expression of the detoxifying enzyme cytochrome P450 CYP6G1 (Daborn et al. 2002). Identifying a specific insecticide resistance mutation in one organism provides candidate resistance genes to test in other species and strains.
The bacterium Bacillus thuringiensis (Bt) produces insecticidal toxins used for controlling agricultural pests as foliar sprays or by expressing toxin genes in transgenic plants. Insecticidal activity of the ∼200 characterized Bt toxins varies considerably between insect orders (Schnepf et al. 1998), and they exhibit a lower impact on nontarget species than conventional pesticides do (Gatehouse et al. 2011). To kill lepidopteran pests, Bt toxins must be ingested by caterpillars, become activated by gut proteases, and then bind to midgut receptors (Soberon et al. 2009). Two mechanisms have been proposed for the subsequent steps in the toxins’ mode of action: (i) pore formation in midgut epithelial cells followed by colloid-osmotic lysis or (ii) activation of a signaling cascade after binding to a primary target in the midgut (Soberon et al. 2009).
Numerous Bt Cry1Ac-binding proteins have been identified on the midgut brush border membrane, and some have been expressed in cell lines or in Drosophila to validate their function (Vadlamudi et al. 1995; Nagamatsu et al. 1998; McNall and Adang 2003). These studies have produced a suite of candidate genes for genotype–phenotype association tests on Bt-resistant and Bt-susceptible insect strains, to attempt to identify the four separate Bt resistance mutations reported in Lepidoptera (Heckel et al. 2007). Mutations within a 12-cadherin domain protein were found to cause Cry1Ac resistance in laboratory selected strains of Heliothis virescens (Gahan et al. 2001), Pectinophora gossypiella (Morin et al. 2003), and Helicoverpa armigera (Xu et al. 2005). However, modified Bt toxins are able to kill P. gossypiella that carry cadherin mutations, suggesting the presence of other major Bt-binding targets (Soberon et al. 2007). Most recently, genetic mapping has correlated a second and independent Cry1Ac resistance mechanism in H. virescens with an inactivating mutation in ABC transporter C2, which has not previously been associated with a Bt mode of action (Gahan et al. 2010).
Resistance to Bt Cry1A spray formulations has evolved in field populations of the diamondback moth, Plutella xylostella (Tabashnik et al. 1990), and greenhouse isolates of the cabbage looper, Trichoplusia ni (Janmaat and Myers 2003). In both species, resistance to Cry1Ab and Cry1Ac toxins is brought about by a single recessive, autosomal locus with reduced toxin binding in the midgut (Tabashnik et al. 1997; Wang et al. 2007). This evidence suggests that the likely mechanism for resistance occurs through loss or alteration of a Bt toxin receptor. Genes for the 12-cadherin domain protein, as well as for all other known Bt-binding proteins, have been mapped in P. xylostella and are all unlinked to the major gene responsible for field-evolved resistance (Baxter et al. 2005, 2008). Here we perform extensive backcrosses and genetic mapping to identify this Bt Cry1Ac resistance locus in P. xylostella and T. ni.
We used the same crossing strategy for both P. xylostella and T. ni (Heckel et al. 1999). Resistant and susceptible individuals were first crossed, producing F1 progeny. Crossing over within homologous chromosomes does not occur in female Lepidoptera, so F1 males were used in backcrosses to produce mapping families, and F1 females were used in backcrosses for associating candidate resistance genes to a specific chromosome. Backcross progeny from each cross were either reared without insecticide (untreated controls) or exposed to Cry1Ac toxin (bioassay treated). Through this strategy, survivors of Cry1Ac were expected to be homozygous for the recessive resistance mutation, while controls were expected to be either homozygous or heterozygous.
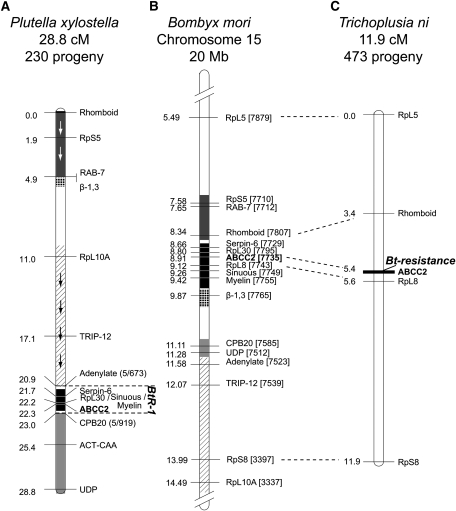
The Bt Cry1Ac resistance locus (BtR-1) in the NO-QA strain of P. xylostella from Hawaii was originally identified using anonymous AFLP markers (Heckel et al. 1999). A sequenced AFLP marker linked to BtR-1 contained coding sequence for the predicted gene Thyroid Hormone Receptor Interactor 12 (GenBank JN030496), which has an ortholog located on chromosome 15 of Bombyx mori (Baxter et al. 2005). As Lepidoptera commonly show conserved chromosomal synteny, predicted proteins from B. mori chromosome 15 were compared using BLAST against a P. xylostella transcriptome (454-ESTs) to design specific primers for linkage mapping. Sixteen genes (Supporting Information, Table S1) were mapped in most progeny in backcrosses to NO-QA (3 families, 184 bioassay survivors, 46 controls), and a linkage map was generated to identify the resistance locus. Multiple rearrangements and inversions of macro-chromosomal regions were observed when compared with B. mori, yet blocks of genes were clearly clustered (Figure 1 A and B). Five gene markers—Serpin-6, RpL30, Sinuous, Myelin Proteolipid, and the resistance candidate gene ABCC2—were in perfect association with the BtR-1 locus, suggesting that this chromosomal region may be gene dense or have a low recombination rate.
Figure 1.
Bt resistance loci in Lepidoptera. Linkage maps for (A) P. xylostella and (C) T. ni in comparison with (B) B. mori’s sequenced chromosome 15 (partial). P. xylostella and B. mori show multiple chromosomal rearrangements while maintaining genetic synteny. Blocks of common genes are shaded and arrows depict inverted orientation in P. xylostella. T. ni and B. mori show a conserved gene order in this data set. Bt Cry1Ac resistance loci are in complete linkage with the ABCC2 gene. P. xylostella showed low levels of recombination across this region, and five mapped genes are in complete linkage with BtR-1. RpL8 also maps to BtR-1 (see Table 1), but was not polymorphic in these mapping crosses. Both linkage maps were constructed using JoinMap 3.0 (grouping = LOD 10, Kosambi’s mapping function). P. xylostella were fed on transgenic Bt Cry1Ac-expressing canola, and T. ni had purified Cry1Ac toxin incorporated into their artificial diet. Centimorgan distances may be affected by linkage disequilibrium caused by selection for resistance. B. mori gene identifiers refer to the final four numbers (note underlining) of the gene ID (e.g., BGIBMGA003337-TA). Accession numbers are provided in Table S1.
Attempts were made to further resolve the BtR-1 locus by creating 36 additional backcross families between the susceptible strain Waite and Cry1Ac-resistant NO-QAGE, a descendant of NO-QA (Tabashnik et al. 2000). Despite genotyping >900 progeny, mapping resolution of the resistance locus was not improved (Table 1).
Table 1. Percentage of Bt-susceptible alleles from 39 P. xylostella backcrosses with chromosomal crossing over.
| Adenylatea | ABCC2 | RpL8 | Myelin | CPB20 | |
|---|---|---|---|---|---|
| Bioassay | 5/673 | 0 | 0 | 0 | 5/919 |
| % | 0.74 | 0 | 0 | 0 | 0.54 |
| Controls | 95/182 | — | — | — | 170/325 |
| % | 52.2 | — | — | — | 52.3 |
P. xylostella crosses were performed using the Bt-susceptible strain Waite and Bt-resistant strains NO-QA (3 families) or NO-QAGE (36 families). Control backcross progeny inherit alleles derived from the Bt-susceptible or Bt-resistant grandparent at a 1:1 ratio.
A total of 246 bioassay survivors from 14 NO-QAGE mapping families did not carry polymorphic variation in the Adenylate gene. In these cases, progeny were genotyped for marker RpL8, and no susceptible genotypes were observed. Recombinant individuals were genotyped for ABCC2, RpL8, and Myelin Proteolipid, and all inherited Bt-resistant genotypes.
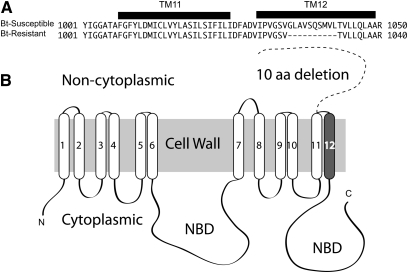
As ABCC2 is correlated with Cry1Ac resistance in H. virescens, the ortholog was cloned and sequenced using a genomic BAC library constructed with susceptible strain Geneva88 (Figure S1) (Baxter et al. 2010). The gene contains 26 exons, and these were verified through PCR amplification from Bt-susceptible (Geneva88) and Bt-resistant (NO-QAGE × Waite backcross progeny) midgut cDNA. The resistant strain NO-QAGE contains a 30-bp deletion in exon 20, which is predicted to remove the 12th and final transmembrane domain and aberrantly position the carboxyl-terminal outside the cell. If this gene is translated and inserted into the midgut membrane, a core ATP-binding loop is expected to be nonfunctional (Figure 2).
Figure 2.
P. xylostella ABCC2 schematic. A P. xylostella BAC library was screened with partial gene sequence from ABCC2, which was identified from an EST library, and two clones were sequenced (25L19 and 11G15). P. xylostella ABCC2 genomic sequence was predicted on the basis of similarity with the H. virescens homolog and then confirmed through amplification from midgut cDNA, using backcross progeny that survived a Cry1Ac bioassay (Bt-resistant) or from the control strain Geneva88 (Bt-susceptible). (A) Partial amino acid alignment of ABCC2 of Bt-susceptible (JN030490) and Bt-resistant (JN030491) P. xylostella. A 30-bp, 10-amino-acid deletion is predicted within transmembrane (TM) domain 12. (B) Schematic of ABCC2 displaying 12 transmembrane domains predicted using Phobius (http://phobius.sbc.su.se/) and two nucleotide-binding domains (NBD). The 10-amino-acid deletion in Bt-resistant individuals is predicted to remove TM12, leaving the carboxyl terminus in a noncytoplasmic region (dashed line).
Like P. xylostella, Bt resistance in T. ni is autosomal, recessive, and predicted to be a single major gene. First, we performed a series of backcrosses using F1 females to associate Bt resistance candidate genes with chromosomes. The cadherin-like protein (Zhang 2007), aminopeptidase N5 (APN5), and alkaline phosphatase (ALP) all mapped to separate chromosomes and were all unlinked to Cry1Ac resistance. In both B. mori and P. xylostella, APN5 is located on the same chromosome as other known APN genes, suggesting that none of these carry the resistance mutation (Crava et al. 2010). The gene ribosomal protein L8, however, was located on the same chromosome as the mutation causing Cry1Ac resistance, as in P. xylostella (Table 2).
Table 2. Percentage of Bt-susceptible alleles from two T. ni backcrosses with no chromosomal crossing over.
| Individuals genotyped | % progeny that inherit a chromosome from the susceptible strain | ||||
|---|---|---|---|---|---|
| RpL8 | Cadherin | APN5 | ALP | ||
| Chromosomea | 15 | 6 | 9 | 3 | |
| Cry1Ac bioassay | 57 | 0.0 | 38.6 | 47.4 | 64.9 |
| Untreated control | 65 | 50.8 | 43.1 | 43.1 | 46.2 |
| χ-Squared | 39.67 | 0.25 | 0.23 | 4.31 | |
| P-value | <<0.0001 | 0.62 | 0.63 | 0.037 | |
T. ni crosses were performed using Bt-resistant strain GLEN-Cry1Ac-BCS (Wang et al. 2007) and a Bt-susceptible strain purchased from Benzon Research (Carlisle, PA). F1 females inherited one chromosome set from the susceptible grandmother and one chromosome set from the resistant grandfather. As there is no crossing over in females, backcross progeny inherit one complete maternal chromosome derived from a susceptible or resistant origin. Data set combines two related families: cross A (31 bioassay survivors, 35 untreated controls) and cross B (35 bioassay survivors, 30 untreated controls). Bioassay survivors did not inherit any RpL8 alleles derived from the susceptible grandmother, indicating that this gene is on the same chromosome as the Bt resistance locus. The ALP allele from the Bt-susceptible strain shows a significant overrepresentation in bioassay survivors; however, this is probably due to small sample sizes.
Indicates the B. mori chromosome containing this gene ortholog.
Eight male informative backcrosses produced 326 bioassay survivors and 147 untreated controls to resolve the precise Bt Cry1Ac resistance locus in T. ni. Gene fragments from Rhomboid and Ribosomal protein genes L5, L8, and S8, as well as the candidate resistance gene ABCC2, were PCR amplified and mapped in backcross progeny. ABCC2 was in complete linkage with the resistance mutation (Table 3), demonstrating that this chromosomal region contains a genetic mutation that ultimately causes Bt Cry1Ac resistance in H. virescens, P. xylostella, and T. ni (Figure 1C).
Table 3. Percentage of Bt-susceptible alleles inherited from eight T. ni backcrosses crosses with chromosomal crossing over.
| RpL5 | Rhomboid | ABCC2 | RpL8 | RpS8 | |
|---|---|---|---|---|---|
| Bioassay | 15/307 | 9/323 | 0/325 | 1/322 | 18/323 |
| % | 4.9 | 2.8 | 0.0 | 0.3 | 5.6 |
| Controls | 65/142 | 70/146 | 72/147 | 72/147 | 78/147 |
| % | 45.8 | 47.9 | 49.0 | 49.0 | 53.1 |
Eight backcrosses between F1 males and GLEN-Cry1Ac-BCS-resistant females produced 326 bioassay progeny and 147 untreated controls for linkage mapping. Approximately 50% of control progeny inherited paternal alleles from the Bt-susceptible strain, confirming Mendelian segregation. ABCC2 genotypes from bioassay survivors show a perfect association with the Bt resistance locus in T. ni.
Here we have demonstrated that a single homologous locus controls recessive resistance to Bt Cry1Ac toxins in widely divergent Lepidoptera, suggesting independent, parallel evolutionary responses to this strong selective agent. Although functional evidence is still required to confirm that a mutation in ABCC2 directly causes Bt resistance, the frameshifting 22-bp deletion in H. virescens (Gahan et al. 2010) and the 30-bp deletion in the NO-QAGE strain of P. xylostella provide strong circumstantial evidence. Complementation tests showing a common genetic basis of resistance in P. xylostella strains from Hawaii, Pennsylvania, South Carolina, and the Philippines implicate the ABCC2 gene (Tabashnik et al. 1997; Baxter et al. 2005); however, additional unlinked resistance genes are evident in the Philippine strain and in some populations from Malaysia (Sayyed et al. 2000). The association of ABCC2 with Bt resistance in a third species, T. ni, further supports the hypothesis that this gene is functionally implicated in resistance. It remains to be seen whether resistant strains of T. ni from Mexico (Tamez-Guerra et al. 2006) and Canada (Estada and Ferre 1994; Janmaat and Myers 2003) also have ABCC2 mutations. Like the previous work with H. virescens, the results here provide evidence of resistance-conferring mutations in an ABC transporter gene that has not previously been associated with Bt toxin interaction. Future functional analysis of ABCC2, and sequencing of the corresponding genome regions of T. ni and P. xylostella, will be needed to fully elucidate the role of ABCC2 in field-evolved resistance to Bt Cry1Ac toxins and how ABCC2 interacts with the other genes affecting the complex genetic basis of Bt resistance in Lepidoptera (Tabashnik et al. 1998; Heckel et al. 2007).
Acknowledgments
We thank Bruce Tabashnik and an anonymous reviewer for helpful comments on this manuscript. This work was funded by the Biotechnology and Biological Sciences Research Council (grant 021107), the Max-Planck-Gesellschaft, and the U.S. Department of Agriculture (grant 2008-35302-18806).
Literature Cited
- Baxter S. W., Zhao J. Z., Gahan L. J., Shelton A. M., Tabashnik B. E., et al. , 2005. Novel genetic basis of field-evolved resistance to Bt toxins in Plutella xylostella. Insect Mol. Biol. 14: 327–334 [DOI] [PubMed] [Google Scholar]
- Baxter S. W., Zhao J. Z., Shelton A. M., Vogel H., Heckel D. G., 2008. Genetic mapping of Bt-toxin binding proteins in a Cry1A-toxin resistant strain of diamondback moth, Plutella xylostella. Insect Biochem. Mol. 38: 125–135 [DOI] [PubMed] [Google Scholar]
- Baxter S. W., Chen M., Dawson A., Zhao J. Z., Vogel H., et al. , 2010. Mis-spliced transcripts of nicotinic acetylcholine receptor α6 are associated with field evolved spinosad resistance in Plutella xylostella (L.). PLoS Genet. 6: e1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crava C. M., Bel Y., Lee S. F., Manachini B., Heckel D. G., et al. , 2010. Study of the aminopeptidase N gene family in the lepidopterans Ostrinia nubilalis (Hubner) and Bombyx mori (L.): sequences, mapping and expression. Insect Biochem. Mol. 40: 506–515 [DOI] [PubMed] [Google Scholar]
- Daborn P. J., Yen J. L., Bogwitz M. R., Le Goff G., Feil E., et al. , 2002. A single P450 allele associated with insecticide resistance in Drosophila. Science 297: 2253–2256 [DOI] [PubMed] [Google Scholar]
- Davies T. G. E., Field L. M., Usherwood P. N. R., Williamson M. S., 2007. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 59: 151–162 [DOI] [PubMed] [Google Scholar]
- Estada U., Ferre J., 1994. Binding of insecticidal crystal proteins of Bacillus thuringiensis to the midgut brush border of the cabbage looper, Trichoplusia ni (Hubner) (Lepidoptera: Noctuidae), and selection for resistance to one of the crystal proteins. Appl. Environ. Microbiol. 60: 3840–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahan L. J., Gould F., Heckel D. G., 2001. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 293: 857–860 [DOI] [PubMed] [Google Scholar]
- Gahan L. J., Pauchet Y., Vogel H., Heckel D. G., 2010. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 6: e1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatehouse A. M. R., Ferry N., Edwards M. G., Bell H. A., 2011. Insect-resistant biotech crops and their impacts on beneficial arthropods. Philos. Trans. R. Soc. B Biol. Sci. 366: 1438–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckel D. G., Gahan L. J., Liu Y. B., Tabashnik B. E., 1999. Genetic mapping of resistance to Bacillus thuringiensis toxins in diamondback moth using biphasic linkage analysis. Proc. Natl. Acad. Sci. USA 96: 8373–8377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckel D. G., Gahan L. J., Baxter S. W., Zhao J. Z., Shelton A. M., et al. , 2007. The diversity of Bt resistance genes in species of Lepidoptera. J. Invertebr. Pathol. 95: 192–197 [DOI] [PubMed] [Google Scholar]
- Janmaat A. F., Myers J., 2003. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Proc. Biol. Sci. 270: 2263–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNall R. J., Adang M. J., 2003. Identification of novel Bacillus thuringiensis Cry1Ac binding proteins in Manduca sexta midgut through proteomic analysis. Insect Biochem. Mol. 33: 999–1010 [DOI] [PubMed] [Google Scholar]
- Morin S., Biggs R. W., Sisterson M. S., Shriver L., Ellers-Kirk C., et al. , 2003. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Natl. Acad. Sci. USA 100: 5004–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu Y., Toda S., Koike T., Miyoshi Y., Shigematsu S., et al. , 1998. Cloning, sequencing, and expression of the Bombyx mori receptor for Bacillus thuringiensis insecticidal CryIA(a) toxin. Biosci. Biotechnol. Biochem. 62: 727–734 [DOI] [PubMed] [Google Scholar]
- Sayyed A. H., Haward R., Herrero S., Ferre J., Wright D. J., 2000. Genetic and biochemical approach for characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in a field population of the diamondback moth, Plutella xylostella. Appl. Environ. Microbiol. 66: 1509–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf E., Crickmore N., Van Rie J., Lereclus D., Baum J., et al. , 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62: 775–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon M., Pardo-Lopez L., Lopez I., Gomez I., Tabashnik B. E., et al. , 2007. Engineering modified Bt toxins to counter insect resistance. Science 318: 1640–1642 [DOI] [PubMed] [Google Scholar]
- Soberon M., Gill S. S., Bravo A., 2009. Signaling vs. punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells? Cell. Mol. Life Sci. 66: 1337–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik B. E., Cushing N. L., Finson N., Johson M. W., 1990. Field development of resistance to Bacillus thuringiensis in Diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 83: 1671–1676 [Google Scholar]
- Tabashnik B. E., Liu Y. B., Malvar T., Heckel D. G., Masson L., et al. , 1997. Global variation in the genetic and biochemical basis of diamondback moth resistance to Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 94: 12780–12785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabashnik B. E., Liu Y. B., Malvar T., Heckel D. G., Masson L., et al. , 1998. Insect resistance to Bacillus thuringiensis: Uniform or diverse? Philos. Trans. R. Soc. Lond. B Biol. Sci. 353: 1751–1756 [Google Scholar]
- Tabashnik B. E., Johnson K. W., Engleman J. T., Baum J. A., 2000. Cross-resistance to Bacillus thuringiensis toxin Cry1Ja in a strain of diamondback moth adapted to artificial diet. J. Invertebr. Pathol. 76: 81–83 [DOI] [PubMed] [Google Scholar]
- Tamez-Guerra P., Damas G., Iracheta M. M., Oppert B., Gomez-Flores R., et al. , 2006. Differences in susceptibility and physiological fitness of Mexican field Trichoplusia ni strains exposed to Bacillus thuringiensis. J. Econ. Entomol. 99: 937–945 [DOI] [PubMed] [Google Scholar]
- Vadlamudi R. K., Weber E., Ji I., Ji T. H., Bulla Lee A., Jr. 1995. Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J. Biol. Chem. 270: 5490–5494 [DOI] [PubMed] [Google Scholar]
- Wang P., Zhao J. Z., Rodrigo-Simon A., Kain W., Janmaat A. F., et al. , 2007. Mechanism of resistance to Bacillus thuringiensis toxin Cry1Ac in a greenhouse population of the cabbage looper, Trichoplusia ni. Appl. Environ. Microbiol. 73: 1199–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yu L., Wu Y., 2005. Disruption of a cadherin gene associated with resistance to Cry1Ac delta-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl. Environ. Microbiol. 71: 948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., 2007. Sequence variation in cadherin alleles from the cabbage looper, Trichoplusia ni. MSc Thesis, Cornell University, Ithaca, NY [Google Scholar]