Abstract
The assembly of molecular hubs upon integrin and growth factor stimulation represents a preferential way to transduce signals throughout the cell. Among the intracellular kinases that are responsive to integrin and growth factor activation, Src Family Kinases (SFKs) are crucial regulators of cell migration and invasion. Increasing evidence highlight the importance of adaptor proteins in these processes, based on their ability to create signalling platforms that control downstream signals. Among these adaptors we will discuss the molecular features of p130Cas and p140Cap proteins in terms of regulation of cell migration and invasion in normal and transformed cells.
Keywords: p130Cas, p140Cap, integrins, receptor tyrosine kinases, migration, invasion
Introduction
To migrate and to invade a cell needs to detach from its neighbors, i.e. adjacent cells in an epithelium, to extend lamellipodia and filopodia from the leading edge and to create new dynamic adhesions, which form and rapidly disassemble at the base of protrusions [1, 2]. Cell invasion also requires the release or activation of proteases that degrade the extracellular matrix (ECM) and allows cells to sort out from the basal lamina invading surrounding tissues [3]. Under physiological conditions cell motility and invasion are tightly controlled by a complex interplay among cell-cell, cell matrix and growth factors receptors resulting in the maintenance of the architectural integrity of human tissues. This subtle regulation is lost in human tumours leading to uncontrolled dissemination of cancer cells into the body [4-7]. At least three major class of membrane proteins are involved in these events, namely, the integrin receptors, the Receptor tyrosine kinases (RPTKs), and the E-cadherin.
Integrins are cell surface heterodimeric receptors for the ECM formed by the non covalent association of alpha and beta subunits [8]. Integrins specifically localize to focal adhesions, which are sites of close apposition with the ECM where actin filaments are anchored to the plasma membrane. Integrins are catalytically inactive and translate positional cues into biochemical signals by direct and/or functional association with intracellular adaptors or growth factor and cytokine receptors, thus regulating integrin ability to transduce signals inside the cells, the so called “outside-in signalling” [9]. Integrin adaptor proteins, such as p130Cas, serve as multi-functional scaffolding proteins, leading to protein-protein interactions by the recruitment of kinases, phophatases and their substrates together [9]. A growing body of evidence shows that integrins, RPTKs and cytokine receptors have no longer to be considered as individual receptors, but rather as joint modules in which attachment to the matrix confers positional control to respond to soluble growth factors [6, 10-13]. In the case of the EGF receptor (EGFR), beta1 integrin is both sufficient to partially activate the receptor itself and required for the full activation of the EGFR in response to EGF [14]. This EGFR trans-activation accounts for a specific repertoire of mechanisms, namely cell survival and actin cytoskeleton organization involved in cell migration.
The cell-cell adhesion receptor E-cadherin is the major membrane protein involved in binding between neighbouring cells in adherens junctions. As a practical consequence of its adhesive functions, E-cadherin has also been shown to prevent EGFR activation and downstream signalling, leading to negative regulation of proliferation [15-18]. E-cadherin is frequently down-regulated or lost in epithelial tumours [19-21].
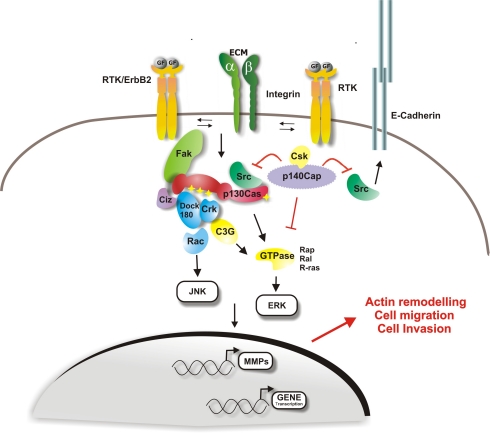
In this review we will focus on p140Cap and p130Cas as major regulators of the complex cross-talk among integrin, E-cadherin and RPTKs signalling. Both p140Cap and p130Cas regulate cell growth and cell motility, while p130Cas can also sustain cell survival [9]. Owing to their modular structure, both proteins can undergo tyrosine phosphorylation and association with effector proteins, leading to the assembly of molecular platforms that regulate a variety of signalling events (Figure 1).
Figure 1.
p130Cas and p140Cap signalling involved in migration and invasion. Upon extracellular matrix binding or growth factors stimulation, integrins and Receptor Protein Tyrosine Kinases (RPTK) represent the major upstream regulators of p130Cas and p140Cap, mainly through the regulation of Src kinase activity. Once tyrosine phosphory-lated by Src, p130Cas recruits proteins that activate downstream pathways, resulting in actin cytoskeleton reorganization, increased cell motility and migration. p130Cas by acting on metalloproteinases (MMPs) promoter is also required for the invasive program. Upon cell matrix adhesion or mitogen stimulus, p140Cap inhibits Src kinase activity and p130Cas tyrosine phosphorylation and p130Cas/Crk complex formation. As a consequence, the effect of p130Cas on actin cytoskeleton re-organization is impaired and cell migration and invasion are inhibited. Moreover, by inactivating Src, p140Cap also regulates the epidermal growth factor receptor (EGFR) pathway through E-cadherin-dependent inactivation of EGFR signalling. p140Cap by interacting with E-cadherin and EGFR at the cell membrane, immobilizes E-Cadherin at the cell membrane thus preventing cell migration and invasion.
Structure and major interactors of p140Cap
The human p140Cap (Cas-associated protein) is coded by the Srcin1 gene, which is conserved in human, mouse, rat, dog, cow, and zebrafish and in humans is localized on Chromosome 17q21.1.
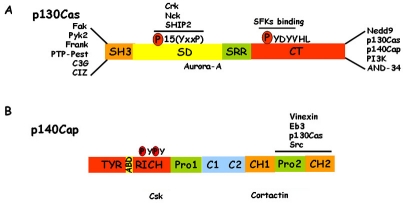
The p140Cap protein was originally identified as SNIP (SNAP-25b-Interacting Protein) in the rat brain. In this tissue p140Cap/SNIP is involved in the regulation of neurosecretion, likely by anchoring SNAP-25 to the membrane cytoskeleton [22]. p140Cap is a multi-site docking protein, composed by a putative N-terminal myrystylation site, a tyrosine-rich domain, two proline-rich regions, a coil-coiled domain, two charged amino acid rich regions and a putative actin-binding site (Figure 2) [22, 23]. p140Cap is mainly expressed in brain, testis and epithelial-rich tissues such as mammary gland, lung, colon and kidney [22-24]. The presence of many conserved sequence motifs that can undergo extensive post-translational modification, such as tyrosine and serine phosphorylation, suggests that p140Cap can elicit protein-protein interactions, leading to the formation of multi-protein complexes. Indeed p140Cap is tyrosine phosphorylated in epithelial cells upon integrin-mediated adhesion and EGF receptor activation [23] and it has been shown to associate with several proteins. In normal epithelial cells, p140Cap and p130Cas are associated in a indirect manner and this association occurs through the last 217 amino acids of p140Cap and the amino acids 544-678 of p130Cas. The same C-terminal region of p140Cap has been described to bind directly to the SH3 domain of the Src kinase in breast tumor MCF7 cells [25], suggesting that Src kinase may represent the molecular bridge between p140Cap and p130Cas. Moreover in MCF7 cells by Far Western Blotting p140Cap has been shown to bind directly the kinase C-terminal Src kinase (Csk), a potent negative regulator of Src. The domains involved in the interaction of Csk with p140Cap remain to be elucidated.
Figure 2.
p140Cap and p130Cas structure. A) p130Cas consists of an N-terminal SH3 domain, a substrate domain (SD), a serine rich region (SRR), and a C-terminal domain (CT). The main interactors are indicated. In particular, many proteins associate to the N-terminal domain and the Src family kinases (SFKs) bind the CT domain. The 15 YxxP motifs are phosphorylated by Src family kinases to mediate Crk binding. B) p140Cap consists of an N-terminal tyrosine-rich region (Tyr-rich), an actin binding domain (ABD), a proline rich domain (Pro1), a coil-coiled region (C1-C2), two domains rich in charged amino acids (CH1, CH2) and a C-terminal proline rich domain (Pro2). Src, p130Cas, EB3 and Vinexin bind to the Pro2 domain of p140Cap. The binding regions of Cortactin and Csk have yet to be defined.
By two hybrid screening in human brain, the C-terminal domain of p140Cap has also been found to associate with Vinexin SH3 domain. Vinexin belongs to a family composed of vinexin, c-Cbl associated protein/ponsin, and Arg-binding protein 2. This association has been confirmed by immunoprecipitation in epithelial cells and rat brain. In non-neuronal cells, Vinexin is localized at focal adhesions where it is involved in growth factor- and integrin-mediated signal transduction, cytoskeletal organization, cell spreading, motility, and growth [26]. The Vinexin-p140Cap interaction might affect sub-cellular localization of these proteins and be involved in the formation and/or maintenance of synapses [24].
In the brain, p140Cap also directly associates with all members of the microtubule (MT) plus-end tracking protein EB family, through a short 92 amino acid C-terminal region (aa 1124-1216), likely via a positively charged S/P-rich region [25]. Finally, p140Cap has also been shown to pull-down with Cortactin, a Src kinase substrate and F-actin binding protein, capable of promoting actin stability and nucleation in non-neuronal cells [27], and dendritic spines of cultured hippocampal neurons [28]. In hippocampal neurons, depletion of either EB3 or p140Cap causes defective spine morphology, indicating that the two proteins are involved in stabilization of mature mushroom-like spines. The interactions of p140Cap with EB3-positive MT plus-end and Cortactin may therefore represent a link between the local signalling of MTs and the actin cytoskeleton within the dendritic spines [25].
Overall, the p140Cap binding partners are mainly implicated in membrane fusion and actin cytoskeleton remodelling. p140Cap association to p130Cas, Src, Cortactin and the presence in the p140Cap sequence of a putative actin-binding domain in the p140Cap sequence homologous to the yeast protein AIP3 [29] suggest that p140Cap may also be a potential actin-binding protein.
p140Cap negatively regulates Src-dependent migration and invasion
So far, one of the major functions described for the p140Cap is to inhibit the activation of Src kinase. The mechanism through which p140Cap acts on Src activation has been recently defined. In breast cancer cells, upon cell-matrix adhesion or EGF stimulation, p140Cap activates Csk kinase that by phosphorylating the negative regulatory tyrosine on the C-terminal domain of c-Src induces a conformational change in the c-Src structure that prevents its activity. Consistently, silencing of p140Cap increases Src activation, leading to a fine tuning of integrin and growth factor receptor signalling (Figure 1) [30, 31]. As a consequence, in cells expressing high levels of p140Cap, upon integrin-mediated adhesion, the association between Src and Fak is impaired. p140Cap over-expression does not modify phosphorylation of Fak on autocatalytic tyrosine 397, but rather inhibits Src-dependent phosphorylation of Fak tyrosine 925. Moreover, elevated p140Cap expression also impairs integrin-dependent p130Cas phosphorylation, leading to inhibition of Rac1 [31].
As expected for the major role of Src and downstream signalling in actin cytoskeleton dynamics and cell migration, high levels of p140Cap impair spreading and extension of lamellipodia and filopodia on ECM proteins of breast cancer cells. In addition, p140Cap over-expression also inhibits migration on fibronectin-coated tran-swells and invasion in Matrigel. Consistently, p140Cap silencing induces an increase in cell spreading in the early phases of cell adhesion, a fibroblastic-like shape and increased motility and invasion. Cells expressing a truncated form of p140Cap, lacking the Src-binding domain, restores integrin-dependent Src and Rac activation and are capable of migrating and invading properly [31].
In addition, p140Cap specifically interferes with invasive and migratory properties of cancer cells inhibiting EGFR-dependent cell scatter of breast and colon cancer. This occurs by reinforcing E-cadherin inhibition of EGFR activity in tumor cells. Indeed, it has been demonstrated that p140Cap plays a crucial role in the regulation of E-cadherin-dependent cell-cell adhesion. In particular, in MCF7 breast cancer cells, p140Cap controls cell-cell contact dynamics by increasing the amount of immobilized E-cadherin at the cell surface (Figure 3), thereby regulating the strength of cell-cell adhesion. This mechanism depends on Src kinase activity, as shown by the recovery of E-cadherin mobility at the cell surface through the expression of a kinase-defective Csk mutant, or by Csk silencing. In the same conditions, EGFR phosphorylation is restored, as well as the ability to scatter in response to EGF. Thus p140Cap impairs cancer cell scatter through Src inactivation which results in decreased E-cadherin mobility at the cell membrane, thereby affecting EGFR signalling leading to cell motility (Figure 4) [30].
Figure 3.
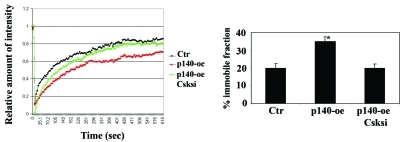
p140Cap stabilizes E-Cadherin molecules at the cell membrane. E-cadherin stability and recycling at the cell membrane is crucial for cell-contact growth inhibition and inhibition of cell scatter. Our recent results show that p140Cap stabilizes E-cadherin at the cell membrane through the inhibition of Src kinase. p140Cap over-expressing (p140-oe) and Ctr breast cancer MCF-7 cells were co-transfected with E-Cadherin-GFP and Csk-RNAi. E-Cadherin dynamics were analyzed by fluorescence recovery after photobleaching (FRAP). The Fluorescence recovery curves are shown on the left, and the histogram of the % of immobile E-Cadherin fraction on the right (*p<0.05). The data show that p140 over-expressing cells have a higher amount of the immobile fraction (35%) compared to control cells (20%), confirming that p140Cap stabilizes E-Cadherin molecules at the cell membrane. Moreover, E-cadherin membrane dynamics were restored when Src was rescued by Csk knock-down. siRNA oligos decrease the immobile fraction of p140 over-expressing cells to the level of Ctr cells. The figure is modified from [31].
Figure 4.
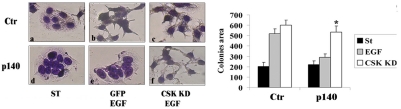
p140Cap impairs EGF-dependent cell scatter through inhibition of Src activity. Adherens junction dynamics are important for cell scatter. p140Cap by increasing the amount of immobilized E-cadherin at the cell surface, regulates the ability of cells to scatter. Left panels: p140Cap over-expressing (p140-oe) and Ctr breast cancer MCF-7 cells infected with GFP or kinase-negative Csk mutant (Csk-KD) recombinant adenoviruses were grown in small islets, serum deprived for 12 h, untreated (a,d) or treated with 50ng/ml EGF for 12 h (b, c, e, and f), fixed, stained with Diff Quick kit, and photographed at 20x magnification (bar is 10 mm). Right panel: the mean area of the colonies was measured with Metamorph software in 20 random fields on six independent experiments (*p<0.05). The data show that p140Cap impairs the EGF-dependent cell scatter ability (panel e) and that the rescue of Src kinase activity is sufficient to restore cell scatter (panel f). The figure is modified from [31].
Structure and major interactors of p130Cas
The p130Cas protein is characterized by the presence of multiple conserved sequence motifs and extensive post-translational modification, mainly consisting of tyrosine and serine phosphorylation. These structural features allow the assembly of specific multi-protein complexes that result in the regulation of cell adhesion, migration and survival. In particular, p130Cas consists of an N-terminal Src homology 3 (SH3) domain, a substrate domain, a serine rich region, and a C-terminal domain (Figure 2). The SH3 domain interacts with polyproline-rich sequences present in several proteins including Fak, PYK2/RAFTK, phosphatases like PTP-PEST, PTP1B, and effectors as C3G and CIZ [32, 33] The substrate domain, characterized by a sequence of 15 YxxP motifs, upon Src family kinases phosphorylation, is tyrosine phosphorylated and exposes additional binding sites for SH2 containing proteins such as the Crk adaptors [34, 35], while the serine rich region represents a docking site for other partners such as 14-3-3 and GRB2. Lastly, the C-terminus contains a polyproline-rich region responsible for the binding of the Src family kinase, PI3K, BCAR3/AND-34, CHAT-H and ubiquitin ligases such as AIP4, APC/C and CDH1, as well as a binding site for the adaptor protein p140Cap [23, 36, 37].
p130Cas tyrosine phosphorylation and cell migration
p130Cas represents a nodal signalling platform on which integrin and RPTKs signalling convey. Integrins, RPTKs and oestrogen receptor (ER) are major upstream regulators of p130Cas, mainly through the activation of Src and Fak kinases, leading to p130Cas tyrosine phosphorylation on the C-terminal binding site YDYVHL (Figure 1) [9]. Moreover, physical stretching of p130Cas induces a conformational change that enables Src-family kinase-dependent p130Cas tyrosine phosphorylation. These findings point out a function for p130Cas as a sensor that integrates mechanical forces coming from the extracellular environment into intracellular signals leading to actin cytoskeleton reorganization [38, 39].
The role of p130Cas in cell migration was initially inferred by studies performed in mouse embryo fibroblasts (MEFs) derived from p130Cas knock-out mice. p130Cas null MEFs show defects in stress fibre formation and cell spreading, impaired actin bundling and cell migration [40], that were restored by full-length p130Cas expression. The tyrosine phosphorylation of the substrate domain of p130Cas provides binding sites for Crk proteins that in turn associates with DOCK180, a guanine nucleotide exchange factor that switches the small GTPase Rac1 from a GDP-bound inactive to a GTP-bound active state at lamellipodia and filopodia adhesion sites [41, 42]. This drives localized Rac activation, membrane ruffling and actin cytoskeleton remodelling, focal adhesion turnover, pseudopodia formation and extension. In addition, ARP2/3 and PAK kinase activation enhance cell migration [43].
It has been reported that expression of Bmx/Etk, a member of the Tec/Btk family of nonreceptor kinases, enhances tyrosine phosphorylation of p130Cas and the formation of p130Cas/Crk complex. Moreover, the co-expression of Bmx with p130Cas results in an enhanced membrane ruffling and haptotactic cell migration. The expression of a mutant form of p130Cas, unable to interact with Crk, inhibits Bmx-induced membrane ruffling and cell migration, indicating that p130Cas and the adaptor protein Crk complex formation is instrumental for connecting several stimuli to the regulation of actin cytoskeleton and cell motility [44]. Un-coupling of p130Cas/Crk negatively regulates cell migration. Indeed, the non-receptor tyrosine kinase Abl phosphorylates Crk-II on tyrosine 221, inducing intramolecular folding that prevents binding of the C-terminal Crk-II SH2 domain to the phosphorylated p130Cas substrate domain, leading to decreased cell movement [45, 46].
Additional molecules that play important roles in modulating tyrosine phosphorylation of p130Cas leading to cell migration are the zyxin/Ajuba family of LIM proteins. These proteins bind to actin cytoskeleton and are implicated in cell motility. Ajuba allows p130Cas localization to nascent adhesive sites in migrating cells thereby leading to the activation of the small GTPase Rac, whereas Zyxin interacts with the SH3 domain of p130Cas and with a nucleocyto-plasmic transcription factor, CIZ/NMP4/ZNF384 [47].
Recent data also show that p130Cas activates several GTPases other than Rac. The association between p130Cas and AND-34, an NSP family member, which acts as a GTP exchange factor for Ral, Rap1 and R-Ras enhances Src activation and cell migration, likely through a Rap1-dependent mechanism [48].
p130Cas and cell invasion
p130Cas tyrosine phosphorylation upon integrin or growth factor receptor activation has also been linked to cell invasion and it has been reported that the SH3 domain of p130Cas is also required for this process. Indeed, Fak-null cells are not invasive when transformed by v-Src, but they acquire invasive properties upon over-expression of p130Cas SH3 domain, indicating that this domain is required for rescue of v-Src cell invasion. In this context, the formation of Src/p130Cas/Crk/DOCK180 complex increases Rac1 and JNK activities and MMP-9 expression, leading to an invasive cell phenotype [49].
Another effector that associates to p130Cas SH3 domain and mediates invasion is CIZ (Cas-Interacting Zinc finger protein). This nucleocyto-plasmic shuttling protein, by binding to a consensus-like sequence in the matrix metallo proteinase (MMPs) promoter, up-regulates the transcription of MMPs [50], thus acting as a downstream element connecting p130Cas to the invasive program. Negative regulators of cell invasion have also been shown to impact on p130Cas. One of this is p140Cap which when over-expressed in breast carcinoma cells inhibits Src kinase activity resulting in down-regulation of p130Cas tyrosine phosphorylation and consistently of cell invasion [31]. In addition, the KAI1/CD82 protein, a member of the tetraspanin superfamily, has been recently rediscovered as a cancer metastasis suppressor and suppresses prostate carcinoma cell migration by decreasing p130Cas protein levels and its coupling with Crk [51, 52].
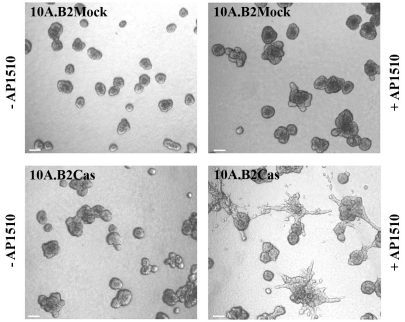
In breast carcinoma cells, the ErbB2 oncogene potentiates the migration and invasion machinery facilitating p130Cas/Crk coupling and ERK activation [53]. Recently, we have reported that in p130Cas null MEFs transformed by ErbB2, the absence of p130Cas result in the inability to migrate and invade, that is rescued by re-expression of full lenght p130Cas [54]. Moreover, p130Cas silencing in ErbB2-dependent breast carcinoma impairs in vitro migration and invasion as well as in vivo lung metastasis formation. The human mammary MCF10A.B2 cells that express a chimeric form of ErbB2, which can homodimerise in the presence of the synthetic ligand AP1510 [55], are a suitable in vitro model for the study of the ErbB2-dependent transformation in three-dimensional (3D) matrix instead of the classical monolayer culture. Interestingly, p130Cas over-expression in MCF10A.B2 cells confers 3D invasive capacity to the acinar structures with degradation of the basement membrane and the formation of 3D matrix adhesions (Figure 5). The 3D invasion of p130Cas over-expressing (Figure 6)/ErbB2 transformed epithelial cells is dependent on activation of both Erk1/2 MAPK and PI3K Akt signaling pathways with subsequent hyper activation of mTOR/p70S6K and Rac1, as two distinct downstream effectors [56] (Figure 7).
Figure 5.
p130Cas triggers acina invasion of ErbB2 transformed MCF10 cells. p130Cas over-expressing or Mock ErbB2 transformed MCF10 cells were plated on a Matrigel/collagen 1:1 matrix and left un-stimulated or activated for ErbB2 by treating with the small molecule AP1510. 3D invasive protrusions are present only in p130Cas over-expressing and ErbB2 activated acinar structures. The figure is modified from [57].
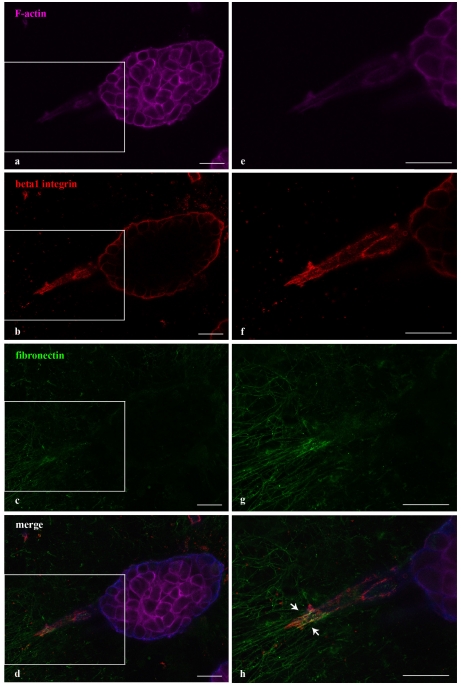
Figure 6.
3D matrix adhesions in invasive acinar structures of ErbB2 transformed MCF10 cells over-expressing p130Cas. p130Cas over-expressing ErbB2 transformed MCF10 cells were plated on a Matrigel/collagen 1:1 matrix and activated for ErbB2. Confocal images representing 3D matrix adhesions characterizing invasive protrusions of p130Cas over-expressing and ErbB2-activated acini. F-actin (purple, panel a), beta-1 integrin (red, panel b) and fibronectin (green, panel c) staining are shown together with merged images (panel d). Magnifications of the insets are shown in panels e, f, g and h. White arrows in panel h indicate co-localization between beta1 integrin and fibronectin. Scale bars: 25 μm. The figure is modified from [57].
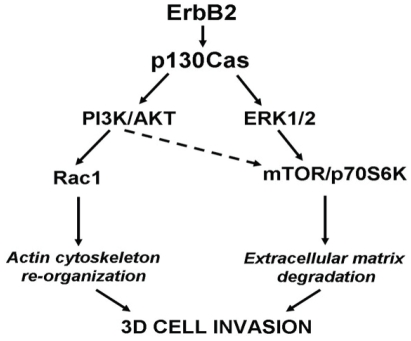
Figure 7.
Signaling pathways leading to 3D invasion of ErbB2 transformed MCF10 cells over-expressing p130Cas. PI3K/Akt and Erk1/2 pathways are both activated during invasion triggered by ErbB2 transformation of p130Cas over-expressing mammary epithelial cells. ErbB2/p130Cas/Erk1/2 MAPK signalling pathway preferentially targets mTOR/p70S6K, whereas the ErbB2/p130Cas/PI3K/Akt cascade triggers Rac1 activation. Both signaling pathways are required for mammary epithelia invasion in 3D suggesting that they cooperate in the regulation of different processes that ultimately lead to cell invasion. The figure is modified from [57].
Conclusions
As outlined in this review, p140Cap and p130Cas adaptor proteins represent key elements in the control of cell migration and invasion in normal and pathological conditions. Their expression pattern in tissues and cells do not completely overlaps, since, while p130Cas is ubiquitous, p140Cap is preferentially expressed in neurons and in epithelial cells. In breast cancers, p130Cas is frequently over-expressed, while p140Cap is not expressed in more aggressive human breast cancers [56]. Interestingly, Src kinase is a common target of these two proteins. Even though both p130Cas and p140Cap have been described to bind to Src, they exert opposite roles on Src activity. Indeed p140Cap is a negative regulator of Src kinase, while p130Cas enhances and sustains Src activity. Therefore, it is likely that Src activity is finely tuned by p140Cap and p130Cas relative expression in tissues in which they are co-expressed. Interestingly, their reciprocal levels of expression might influence the ability of cancer cells to migrate and to invade. Therefore, in a next future it would be very important to evaluate how the balance between these two proteins results in different degrees of cell migration and invasion during normal development or in pathological conditions.
Acknowledgments
This work was supported by grants of the AIRC, AICR, MIUR (PRIN and FIRB, Futuro in Ricerca), Compagnia di San Paolo, Regione Piemonte (Oncoprot, Druidi, PiStem and BioTher), Regione Piemonte Sanità. MP. Camacho Leal is supported by the AICR.
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 3.Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berx G, Raspe E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin Exp Metastasis. 2007;24:587–597. doi: 10.1007/s10585-007-9114-6. [DOI] [PubMed] [Google Scholar]
- 5.Giancotti FG. A structural view of integrin activation and signaling. Dev Cell. 2003;4:149–151. doi: 10.1016/s1534-5807(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 6.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 7.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. The emergence of integrins: a personal and historical perspective. Matrix Biol. 2004;23:333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 10:858–870. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 10.Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streuli CH. Integrins and cell-fate determination. J Cell Sci. 2009;122:171–177. doi: 10.1242/jcs.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uberti B, Dentelli P, Rosso A, Defilippi P, Brizzi MF. Inhibition of beta1 integrin and IL-3Rbeta common subunit interaction hinders tumour angiogenesis. Oncogene. 29:6581–6590. doi: 10.1038/onc.2010.384. [DOI] [PubMed] [Google Scholar]
- 13.Cabodi S, Di Stefano P, Leal Mdel P, Tinnirello A, Bisaro B, Morello V, Damiano L, Aramu S, Repetto D, Tornillo G, Defilippi P. Integrins and signal transduction. Adv Exp Med Biol. 674:43–54. doi: 10.1007/978-1-4419-6066-5_5. [DOI] [PubMed] [Google Scholar]
- 14.Cabodi S, Morello V, Masi A, Cicchi R, Broggio C, Distefano P, Brunelli E, Silengo L, Pavone F, Arcangeli A, Turco E, Tarone G, Moro L, Defilippi P. Convergence of integrins and EGF receptor signaling via PI3K/Akt/FoxO pathway in early gene Egr-1 expression. J Cell Physiol. 2008 doi: 10.1002/jcp.21603. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Suzuki K. Density-dependent inhibition of growth involves prevention of EGF receptor activation by E-cadherin-mediated cell-cell adhesion. Exp Cell Res. 1996;226:214–222. doi: 10.1006/excr.1996.0221. [DOI] [PubMed] [Google Scholar]
- 16.Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein -coupled receptors. Sci STKE. 2000;2000:RE1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- 17.Perrais M, Chen X, Perez-Moreno M, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18:2013–2025. doi: 10.1091/mbc.E06-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. Embo J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berx G, Van Roy F. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 2001;3:289–293. doi: 10.1186/bcr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin Cell Dev Biol. 2004;15:657–663. doi: 10.1016/j.semcdb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Chin LS, Nugent RD, Raynor MC, Vavalle JP, Li L. SNIP, a novel SNAP-25-interacting protein implicated in regulated exocytosis. J Biol Chem. 2000;275:1191–1200. doi: 10.1074/jbc.275.2.1191. [DOI] [PubMed] [Google Scholar]
- 23.Di Stefano P, Cabodi S, Boeri Erba E, Margaria V, Bergatto E, Giuffrida MG, Silengo L, Tarone G, Turco E, Defilippi P. P130Casassociated protein (p140Cap) as a new tyrosine-phosphorylated protein involved in cell spreading. Mol Biol Cell. 2004;15:787–800. doi: 10.1091/mbc.E03-09-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito H, Atsuzawa K, Sudo K, Di Stefano P, Iwamoto I, Morishita R, Takei S, Semba R, Defilippi P, Asano T, Usuda N, Nagata K. Characterization of a multidomain adaptor protein, p140Cap, as part of a pre-synaptic complex. J Neurochem. 2008;107:61–72. doi: 10.1111/j.1471-4159.2008.05585.x. [DOI] [PubMed] [Google Scholar]
- 25.Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Defilippi P, Akhmanova A, Hoogenraad CC. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Kioka N, Ueda K, Amachi T. Vinexin, CAP/ponsin, ArgBP2: a novel adaptor protein family regulating cytoskeletal organization and signal transduction. Cell Struct Funct. 2002;27:1–7. doi: 10.1247/csf.27.1. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hering H, Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J Neurosci. 2003;23:11759–11769. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amberg DC, Zahner JE, Mulholland JW, Pringle JR, Botstein D. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol Biol Cell. 1997;8:729–753. doi: 10.1091/mbc.8.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damiano L, Di Stefano P, Camacho Leal MP, Barba M, Mainiero F, Cabodi S, Tordella L, Sapino A, Castellano I, Canel M, Frame M, Turco E, Defilippi P. p140Cap dual regulation of E-cadherin/EGFR cross-talk and Ras signalling in tumour cell scatter and proliferation. Oncogene. 29:3677–3690. doi: 10.1038/onc.2010.128. [DOI] [PubMed] [Google Scholar]
- 31.Di Stefano P, Damiano L, Cabodi S, Aramu S, Tordella L, Praduroux A, Piva R, Cavallo F, Forni G, Silengo L, Tarone G, Turco E, Defilippi P. p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity. EMBO J. 2007;26:2843–2855. doi: 10.1038/sj.emboj.7601724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 67:1025–1048. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai R, Iwamatsu A, Hirano N, Ogawa S, Tanaka T, Mano H, Yazaki Y, Hirai H. A novel signaling molecule, p130, forms stable complexes in vivo with v- Crk and v-Src in a tyrosine phosphorylation-dependent manner. Embo J. 1994;13:3748–3756. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salgia R, Pisick E, Sattler M, Li JL, Uemura N, Wong WK, Burky SA, Hirai H, Chen LB, Griffin JD. p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene. J Biol Chem. 1996;271:25198–25203. doi: 10.1074/jbc.271.41.25198. [DOI] [PubMed] [Google Scholar]
- 36.Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 37.O'Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 38.Kostic A, Sheetz MP. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol Biol Cell. 2006;17:2684–2695. doi: 10.1091/mbc.E05-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 41.Kiyokawa E, Hashimoto Y, Kobayashi S, Sugimura H, Kurata T, Matsuda M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–3336. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 44.Abassi YA, Rehn M, Ekman N, Alitalo K, Vuori K. p130Cas Couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskele-ton and cell migration. J Biol Chem. 2003;278:35636–35643. doi: 10.1074/jbc.M306438200. [DOI] [PubMed] [Google Scholar]
- 45.Holcomb M, Rufini A, Barila D, Klemke RL. Deregulation of proteasome function induces Abl-mediated cell death by uncoupling p130CAS and c-CrkII. J Biol Chem. 2006;281:2430–2440. doi: 10.1074/jbc.M508454200. [DOI] [PubMed] [Google Scholar]
- 46.Kobashigawa Y, Sakai M, Naito M, Yokochi M, Kumeta H, Makino Y, Ogura K, Tanaka S, Inagaki F. Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK. Nat Struct Mol Biol. 2007;14:503–510. doi: 10.1038/nsmb1241. [DOI] [PubMed] [Google Scholar]
- 47.Janssen H, Marynen P. Interaction partners for human ZNF384/CIZ/NMP4–zyxin as a mediator for p130CAS signaling? Exp Cell Res. 2006;312:1194–1204. doi: 10.1016/j.yexcr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Riggins RB, DeBerry RM, Toosarvandani MD, Bouton AH. Src-dependent association of Cas and p85 phosphatidylinositol 3'-kinase in v-crk-transformed cells. Mol Cancer Res. 2003;1:428–437. [PubMed] [Google Scholar]
- 49.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, Spencer KS, Cheresh DA, Schlaepfer DD. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160:753–767. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamoto T, Yamagata T, Sakai R, Ogawa S, Honda H, Ueno H, Hirano N, Yazaki Y, Hirai H. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol Cell Biol. 2000;20:1649–1658. doi: 10.1128/mcb.20.5.1649-1658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang XA, He B, Zhou B, Liu L. Requirement of the p130CAS-Crk coupling for metastasis suppressor KAI1/CD82-mediated inhibition of cell migration. J Biol Chem. 2003;278:27319–27328. doi: 10.1074/jbc.M303039200. [DOI] [PubMed] [Google Scholar]
- 52.Zhou B, Liu L, Reddivari M, Zhang XA. The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility- and inva-siveness-inhibitory activity. Cancer Res. 2004;64:7455–7463. doi: 10.1158/0008-5472.CAN-04-1574. [DOI] [PubMed] [Google Scholar]
- 53.Spencer KS, Graus-Porta D, Leng J, Hynes NE, Klemke RL. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabodi S, Tinnirello A, Bisaro B, Tornillo G, del Pilar Camacho-Leal M, Forni G, Cojoca R, Iezzi M, Amici A, Montani M, Eva A, Di Stefano P, Muthuswamy SK, Tarone G, Turco E, Defilippi P. p130Cas is an essential transducer element in ErbB2 transformation. FASEB J. 24:3796–3808. doi: 10.1096/fj.10-157347. [DOI] [PubMed] [Google Scholar]
- 55.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tornillo G, Bisaro B, Camacho-Leal Mdel P, Galie M, Provero P, Di Stefano P, Turco E, Defilippi P, Cabodi S. p130Cas promotes invasiveness of three-dimensional ErbB2-transformed mammary acinar structures by enhanced activation of mTOR/p70S6K and Rac1. Eur J Cell Biol. 90:237–248. doi: 10.1016/j.ejcb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Tornillo G, Bisaro B, Camacho-Leal MD, Galie M, Provero P, Di Stefano P, Turco E, Defilippi P, Cabodi S. p130Cas promotes invasiveness of three-dimensional ErbB2-transformed mammary acinar structures by enhanced activation of mTOR/p70S6K and Rac1. Eur J Cell Biol Eur J Cell Biol. 2011;90:237–48. doi: 10.1016/j.ejcb.2010.09.002. [DOI] [PubMed] [Google Scholar]