Abstract
Because neurogenesis persists in the adult mammalian brain and can be regulated by physiological and pathological events, we investigated its possible involvement in the brain's response to focal cerebral ischemia. Ischemia was induced by occlusion of the middle cerebral artery in the rat for 90 min, and proliferating cells were labeled with 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdUrd) over 2-day periods before sacrificing animals 1, 2 or 3 weeks after ischemia. Ischemia increased the incorporation of BrdUrd into cells in two neuroproliferative regions—the subgranular zone of the dentate gyrus and the rostral subventricular zone. Both effects were bilateral, but that in the subgranular zone was more prominent on the ischemic side. Cells labeled with BrdUrd coexpressed the immature neuronal markers doublecortin and proliferating cell nuclear antigen but did not express the more mature cell markers NeuN and Hu, suggesting that they were nascent neurons. These results support a role for ischemia-induced neurogenesis in what may be adaptive processes that contribute to recovery after stroke.
Diseases of the brain have singularly adverse effects on the quality and duration of life. Unlike many other tissues, the mature brain has limited regenerative capacity, and its unusual degree of cellular specialization restricts the extent to which residual healthy tissue can assume the function of damaged brain. However, cerebral neurons are derived from precursor cells that persist in the adult brain (1–5), so stimulation of endogenous neural precursors in the adult brain could have therapeutic potential (6).
Neurogenesis occurs in discrete regions of the adult brain, including the rostral subventricular zone (SVZ) of the lateral ventricles (7) and the subgranular zone (SGZ) of the dentate gyrus (DG) (8). Neurons that arise in the SVZ travel via the rostral migratory stream to the olfactory bulb (9) and also enter association neocortex (10), and new neurons leaving the SGZ migrate into the adjacent DG granule cell layer. Neurogenesis in these regions is subject to physiological regulation by glucocorticoids (11), sex hormones (12), growth factors (13–16), excitatory neurotransmission (17), learning (18), and stress (19) and can be modified pharmacologically (20).
Pathological events can also stimulate neurogenesis in the adult brain. Mechanical injury to the DG granule cell layer in the rat increases proliferation of granule neuron precursors in the adjacent SGZ, as shown by enhanced incorporation of [3H]thymidine and 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdUrd), induction of proliferating cell nuclear antigen (PCNA), and coexpression of mature neuronal markers in [3H]thymidine-labeled cells (21). Seizures also trigger neurogenesis in the SGZ, with BrdUrd-labeled cells expressing the neuronal markers TOAD-64, class IIIβ-tubulin, and microtubule-associated protein 2 (MAP-2) (22). Finally, apoptosis induced by oxidative stress in mouse corticothalamic neurons increases BrdUrd labeling in cells that go on to express the neuronal markers doublecortin (DCX), Hu, and NeuN (23).
Ischemic brain injury triggers molecular and cellular repair mechanisms that contribute to recovery and may include ischemic activation of neurogenesis in the adult brain (24). In global cerebral ischemia in the gerbil, neurogenesis increases in the SGZ (25), with enhanced BrdUrd labeling of cells coexpressing the neuronal markers NeuN, MAP-2, and calbindin. These cells migrate into the granule cell layer, where they become mature neurons. Global ischemia also enhances proliferation of BrdUrd-labeled neuronal precursors in mouse DG (26). One study of neurogenesis in focal cerebral ischemia used a photothrombotic model of stroke in the rat and showed enhanced incorporation of BrdUrd in periinfarction cortex, with some labeled cells coexpressing MAP-2 or NeuN (27). Another study found that after middle cerebral artery (MCA) occlusion in the rat, BrdUrd labeling was increased in the ipsilateral SVZ and rostral migratory stream and colocalized with the neuronal markers polysialylated neural cell adhesion molecule and neuron-specific β-tubulin (28). These findings were interpreted as evidence for directed migration of neuronal precursors toward the infarct, possibly in response to a chemical signal.
In previous studies (29, 30), we have investigated the ability of ischemia to trigger potentially neuroprotective processes such as angiogenesis. To begin examining the possibility that neurogenesis is another such process, we studied the effect of focal cerebral ischemia on neurogenesis in two main neuroproliferative regions of the adult rat brain—the SGZ and SVZ—by using BrdUrd to label proliferating cells and cell type-specific antibodies to characterize the cells. The results indicate that focal cerebral ischemia promotes neurogenesis in both SGZ and SVZ and provide a model for investigating the mechanisms that underlie this phenomenon and its functional consequences.
Materials and Methods
Focal Cerebral Ischemia.
Anesthesia was induced in male Sprague–Dawley rats (280–310 g) with 4% isoflurane/66% N2O/30% O2 and maintained with 1.5% isoflurane/68.5% N2O/30% O2. Blood pressure, blood gases, and blood glucose were monitored via the left femoral artery. Rectal temperature and temporalis muscle temperature contralateral to MCA occlusion were monitored continuously and maintained at 37.0–37.5°C with heating pads. Ischemia was induced by intraluminal MCA occlusion with a suture (31). The left external carotid artery was ligated with 6–0 silk suture and dissected distally, and the left internal carotid was isolated and separated from the vagus nerve. The extracranial branch of the left internal carotid was ligated near its origin with 6–0 silk suture. A 3–0 surgical monofilament nylon suture with rounded tip was introduced into the left internal carotid through the external carotid stump, advanced 20–21 mm past the carotid bifurcation, left in place for 90 min, and then withdrawn.
BrdUrd Administration.
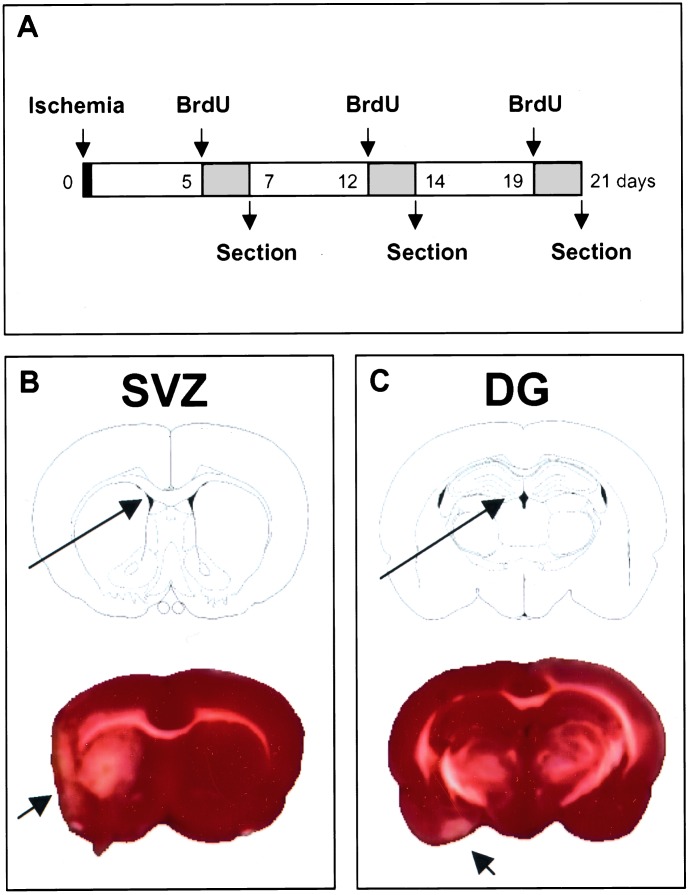
BrdUrd (Sigma), 50 mg/kg in saline i.p., was given twice daily, at 8-h intervals, on consecutive days (days 5 and 6, 12 and 13, or 19 and 20 after ischemia). Rats were killed the day after the last dose (Fig. 1). This schedule labels cells undergoing DNA replication over 2-day spans ending 1, 2, or 3 weeks after ischemia, so at each time BrdUrd-labeled cells range in age from 0 to 2 days after division.
Figure 1.
BrdUrd labeling of ischemic brain. One MCA was occluded for 90 min, followed by reperfusion. (Upper) BrdUrd was given as four injections over one of the 2-day spans indicated by shading (days 5 and 6, 12 and 13, or 19 and 20 after ischemia), and animals were killed on day 7, 14, or 21 after ischemia. (Lower) Brain regions examined for BrdUrd incorporation in SVZ and DG (long arrows) are shown with the corresponding TTC-stained brain slices 1 week after ischemia; red is viable and white is nonviable (short arrows) tissue. White matter tracts including the corpus callosum also appear white with this method. Neither SVZ nor DG is within the infarct area. TTC staining was repeated three times with similar results. BrdU, BrdUrd.
Assessment of Injury.
Rats were perfused transcardially with 200 ml of saline, then 300 ml of 4% paraformaldehyde in 0.1 M sodium phosphate (pH 7.4), and killed by decapitation. Brains were postfixed in 4% paraformaldehyde overnight and embedded in paraffin. Six-micrometer sections through dorsal hippocampus (A–P coordinate, bregma −3.0 mm) and rostral SVZ (A–P coordinate, bregma +1.0 mm) were cut on a microtome and stained with cresyl violet. DNA damage was assessed in fresh-frozen 20-μm sections by using the Klenow fragment of DNA polymerase I (32). To determine nonspecific labeling, some sections were incubated without the Klenow fragment enzyme. To demonstrate the extent of infarction, 2-mm coronal brain sections were immersed in 2% 2,3,5-triphenyltetrazolium hydrochloride (TTC) in saline for 20 min at 37°C and fixed for 30 min in 4% paraformaldehyde (33).
BrdUrd Immunohistochemisty.
Brains (four per experimental condition) were removed after perfusion with saline and 4% paraformaldehyde in PBS. Adjacent 50-μm sections [corresponding to coronal coordinates interaural 8.7–10.2 mm, bregma −0.30 to bregma −1.2 mm (SVZ) and interaural 4.48–5.86 mm, bregma −4.52 to bregma −3.14 (DG)] were cut with a cryostat and stored at −80°C. Sections were pretreated with 50% formamide/280 mM NaCl/30 mM sodium citrate at 65°C for 2 h, incubated in 2 M HCl at 37°C for 30 min, and rinsed in 0.1 M boric acid (pH 8.5) at room temperature for 10 min. Sections were incubated in 1% H2O2 in PBS for 15 min, in blocking solution (2% goat serum/0.3% Triton X-100/0.1% BSA in PBS) for 2 h at room temperature, and with mouse monoclonal anti-BrdUrd antibody (Roche; 2 μg/ml) at 4°C overnight. Sections were washed with PBS, incubated with biotinylated goat anti-mouse secondary antibody (Vector, 1:200 dilution) for 2 h at 25°C, washed, and placed in avidin-peroxidase conjugate (Vector) solution for 1 h. The horseradish peroxidase reaction was detected with 0.05% diaminobenzidine (DAB) and 0.03% H2O2. Processing was stopped with H2O; sections were dehydrated through graded alcohols, cleared in xylene, and coverslipped in permanent mounting medium (Vector). Sections were examined with a Nikon E800 epifluorescence microscope.
Double Immunolabeling.
Sections were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature, washed twice with PBS, and incubated in 2 M HCl at 37°C for 1 h. After washing again, sections were incubated with blocking solution, then with primary antibodies at 4°C overnight, and then with secondary antibodies in blocking solution at room temperature for 2 h. The anti-BrdUrd antibodies used were mouse monoclonal anti-BrdUrd (Roche; 2 μg/ml) or sheep polyclonal anti-BrdUrd (BioDesign, New York; 25 μg/ml). The other primary antibodies were mouse monoclonal anti-NeuN (Chemicon; 1:200 dilution), mouse monoclonal anti-PCNA (Chemicon; 1:100 dilution), mouse monoclonal anti-human neuronal protein HuC/HuD (Hu) (Molecular Probes; 5 μg/ml), and affinity-purified goat polyclonal anti-DCX (Santa Cruz Biotechnology; 1:100 dilution). The secondary antibodies were FITC-conjugated goat anti-mouse IgG (Vector; 1:200 dilution), FITC-conjugated pig anti-goat IgG (Jackson ImmunoResearch; 1:200 dilution), rhodamine-conjugated rat-absorbed donkey anti-mouse IgG (Jackson ImmunoResearch; 1:200 dilution), and rhodamine-conjugated rat-absorbed donkey anti-sheep IgG (Jackson ImmunoResearch; 1:200 dilution). Sections were mounted with Vectashield (Vector) and 4′,6-diamidino-2-phenylindole was used to counterstain nuclei. Fluorescence signals were detected with a Nikon E800 microscope at excitation/emission wavelengths of 535/565 nm (rhodamine, red), 470/505 nm (FITC, green), and 360/400 nm (4′,6-diamidino-2-phenylindole, blue). Results were recorded with a Magnifire digital camera (ChipCoolers, Warwick, RI).
Cell Counting.
BrdUrd-positive cells in SGZ and SVZ were counted blindly in five to seven DAB-stained, 50-μm coronal sections per animal, spaced 200 μm apart. Cells were counted under high power on a Nikon E800 microscope with Magnifire digital camera, and the image was displayed on a computer monitor. Results were expressed as the average number of BrdUrd-positive cells per section and reported as the mean ± SEM. Differences between means were determined by Student's t test, with P < 0.05 considered significant.
Results
Absence of Ischemic Injury in DG and SVZ.
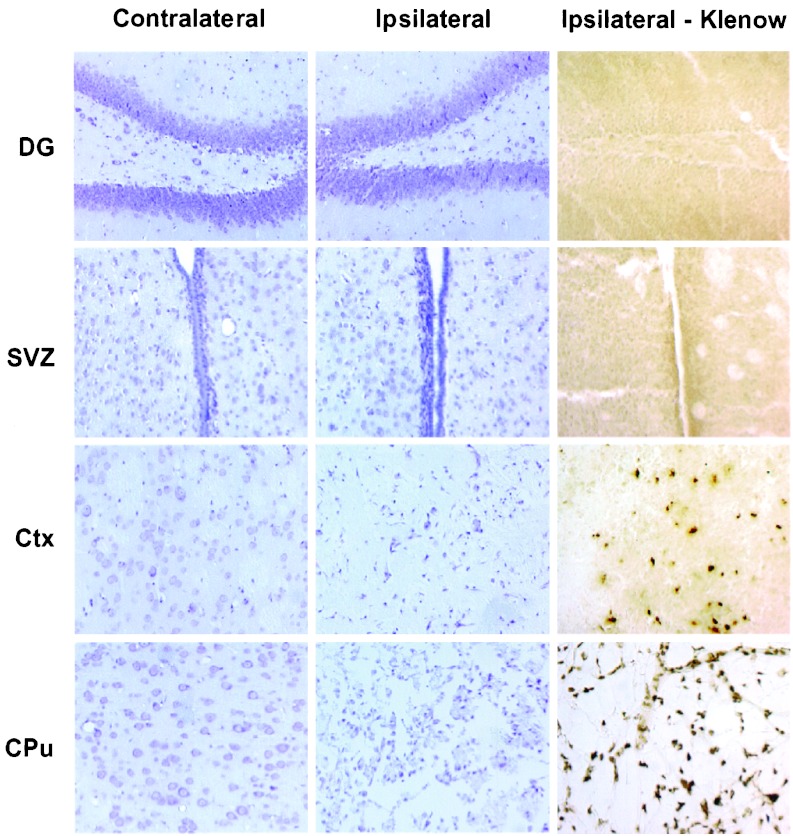
The brain regions examined for BrdUrd incorporation—SGZ and SVZ—are illustrated in Figs. 1 and 2; neither lies within the territory of the occluded MCA. As expected, therefore, neither showed histological features of neuronal injury when stained with cresyl violet nor DNA damage when assayed with the Klenow enzyme fragment. In contrast, ischemic neuronal injury and DNA damage were prominent in areas like cerebral cortex and caudate-putamen, which are supplied by the MCA and undergo infarction.
Figure 2.
Distribution of injury and DNA damage in ischemic brain. Sections were obtained from the contralateral control (Left) or ipsilateral ischemic (Center and Right) hemisphere of brains 1 week after ischemia. DG, SVZ, cerebral cortex (Ctx), and caudate-putamen (CPu) were stained with cresyl violet to detect ischemic neuronal loss (Left and Center) or with the Klenow fragment of DNA polymerase I to label DNA strand breaks (Right). Neither DG nor SVZ, which are outside the ischemic territory, showed neuronal loss or DNA damage, whereas the ipsilateral cortex and caudate-putamen, which are within this territory, showed both. The experiment was repeated three times with similar results.
BrdUrd Incorporation into DG and SVZ.
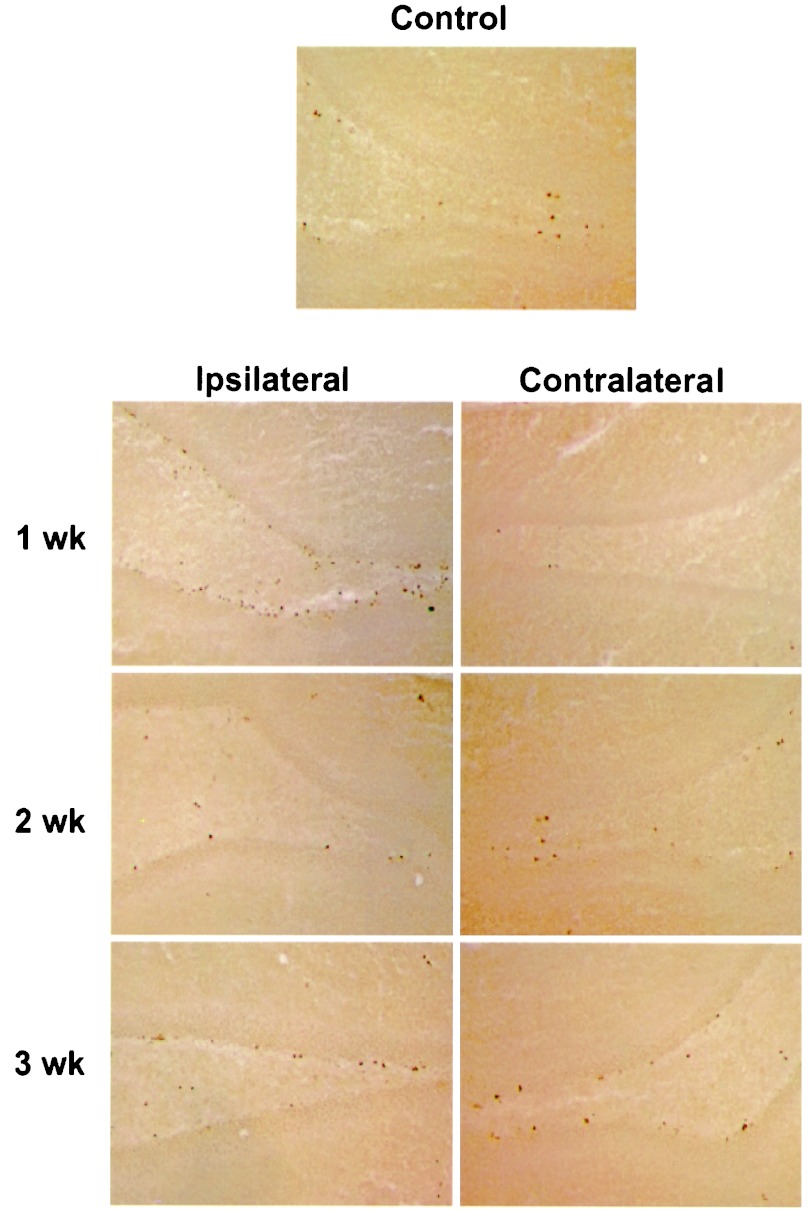
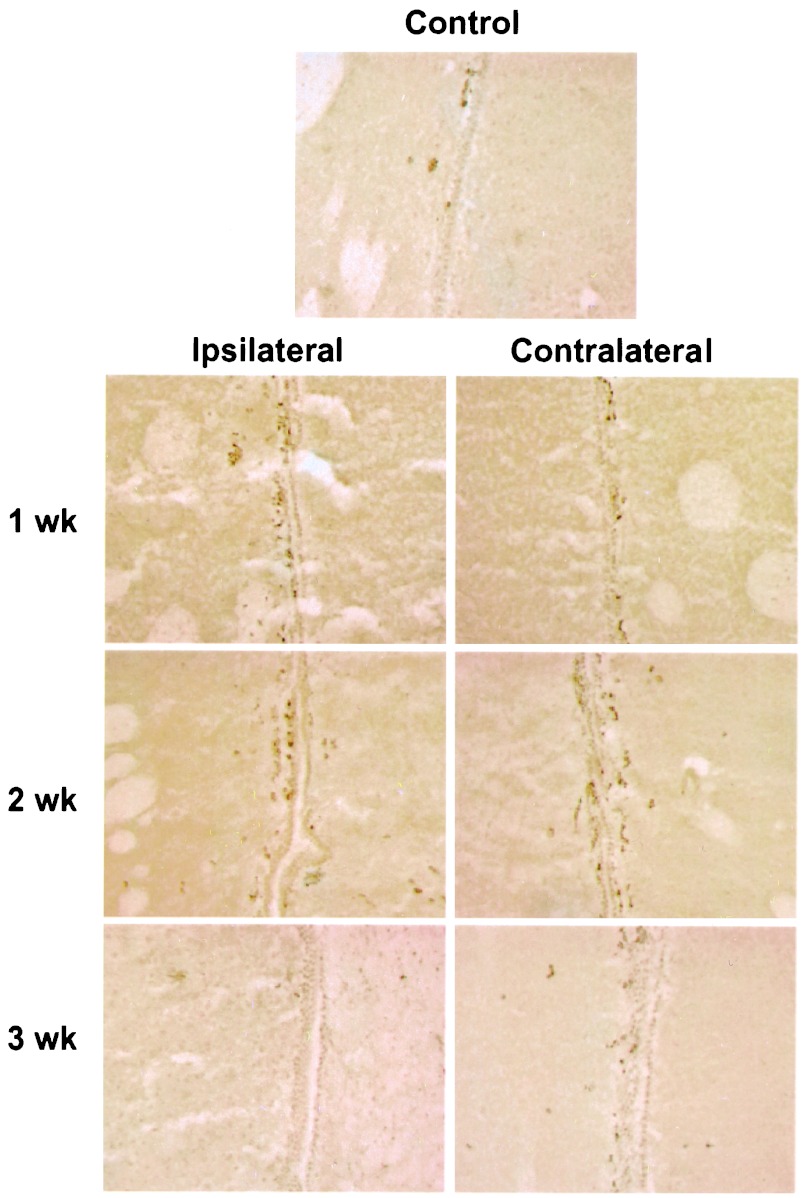
When brains of postischemic animals were stained for BrdUrd, there was increased labeling in the SGZ and SVZ. In the SGZ (Fig. 3), more cells were labeled ipsilateral than contralateral to ischemia at 1 week. The labeled cells were irregularly distributed immediately subjacent to the DG granule cell layer. The location of labeling was similar at 2 and 3 weeks, indicating that cell division occurred at the same site and that cell migration could not be detected within the 2-day labeling period. In contrast, when SGZ cells are followed for 14–17 days after global ischemia, they migrate out of the SGZ and into the granule cell layer (25). In the SVZ (Fig. 4), the number of BrdUrd-labeled nuclei were about equal on the two sides of the brain.
Figure 3.
BrdUrd incorporation into DG. Sections through DG were stained for BrdUrd and visualized with DAB. BrdUrd labeling was increased on the ipsilateral ischemic side relative to nonischemic control brains and the contralateral side at 1 week but not at 2 or 3 weeks. The experiment was repeated three times with similar results.
Figure 4.
BrdUrd incorporation into SVZ. Sections through SVZ were stained for BrdUrd and visualized with DAB. BrdUrd labeling was increased both ipsilateral and contralateral to ischemia at 1 and 2 weeks, relative to nonischemic control brains and 3 weeks. The experiment was repeated three times with similar results.
Quantitation of Ischemia-Induced Increase in BrdUrd Incorporation.
To quantify changes in BrdUrd labeling after ischemia, we counted BrdUrd-reactive nuclei in brain sections from sham-operated rats and rats killed 1, 2, or 3 weeks after ischemia. Fig. 5 shows that in the SGZ at 1 week, the number of BrdUrd-labeled cells increased ≈8-fold ipsilateral and ≈4-fold contralateral to ischemia, compared with sham-operated controls. By 2–3 weeks, incorporation of BrdUrd declined to basal levels. The increase in BrdUrd labeling in the SVZ was similar in magnitude to that in the ipsilateral SGZ and to that produced by olfactory bulbectomy (13) or growth factors (14, 15). However, the pattern of postischemic BrdUrd labeling in the SVZ was different from that in the SGZ in that it was bilaterally symmetrical. In addition, labeling in the SVZ was maximal at 2 weeks rather than 1 week.
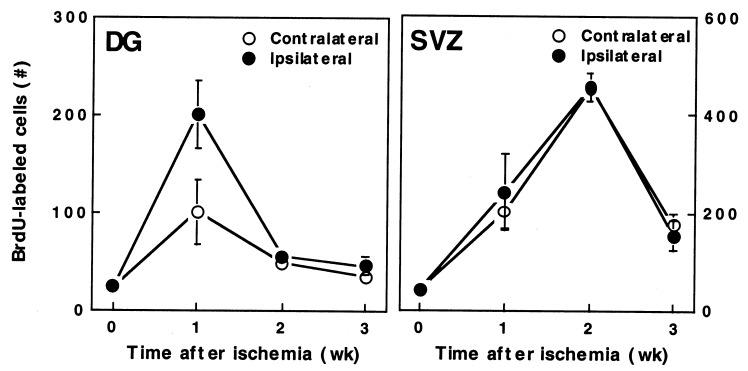
Figure 5.
BrdUrd-immunoreactive cell counts in DG and SVZ. BrdUrd-reactive cells were counted in nonischemic brains (time = 0) and in ischemic brains on the ischemic side (ipsilateral, ●) and nonischemic side (contralateral, ○) at 1–3 weeks. Data are the mean ± SEM (n = 8–15). In DG, cell counts were increased at 1 week relative to time = 0 on both the ischemic (P < 0.001, Student's t test) and the nonischemic (P < 0.05, Student's t test) side, and at 1 week on the ischemic relative to the nonischemic side (P < 0.001, paired Student's t test). In SVZ, cell counts were increased relative to time = 0 at 1 week on the ischemic (P < 0.05, Student's t test) and nonischemic (P < 0.005, Student's t test) sides and at 2 weeks on the ischemic (P < 0.005, Student's t test) and nonischemic (P < 0.001, Student's t test) sides. BrdU, BrdUrd.
Relationship Between BrdUrd Labeling and NeuN Immunoreactivity.
To characterize the cells labeled by BrdUrd after ischemia, we first performed double-labeling with antibodies against BrdUrd and NeuN, a neuron-specific developmentally regulated nuclear protein associated with withdrawal from the cell cycle and terminal differentiation (34). In DG, NeuN is first expressed after SGZ neuronal precursors migrate into the granule cell layer (35). Fig. 6 shows that BrdUrd and NeuN were seen in distinct nonoverlapping cell populations, indicating that BrdUrd was not incorporated into mature neurons. Instead, BrdUrd labeling in DG was localized to the SGZ immediately underlying the NeuN-positive granule cell layer, where neuronal precursors reside and where global ischemia in gerbils stimulates neurogenesis (25). BrdUrd labeling in the SVZ was also discretely localized to a narrow band of NeuN-negative cells adjacent to the ventricle.
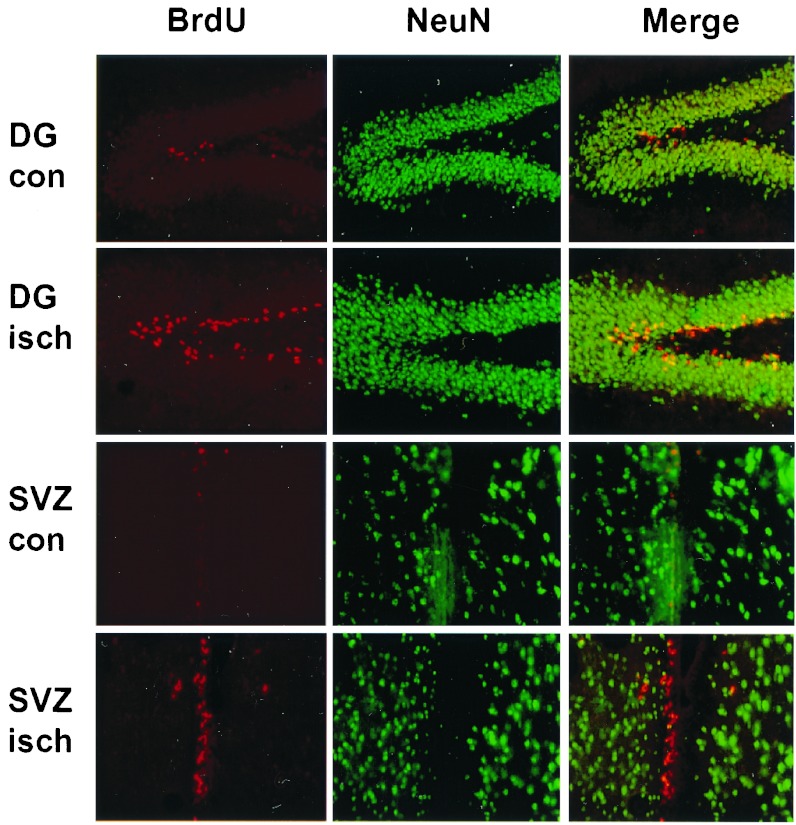
Figure 6.
Relationship between BrdUrd incorporation and NeuN expression. Double immunolabeling for BrdUrd (Left, red) and NeuN (Center, green) was performed in DG and SVZ from control brains (con) and the ischemic side of brains 1 week after MCA occlusion (isch). Merged images show lack of coexpression of BrdUrd and NeuN and localize BrdUrd labeling to the SGZ of DG and the SVZ. The experiment was repeated three times with similar results. BrdU, BrdUrd.
Relationship Between BrdUrd Labeling and Hu, DCX, and PCNA Immunoreactivity.
The absence of BrdUrd labeling in NeuN expressing cells and the confinement of BrdUrd labeling to known zones of neurogenesis after ischemia is consistent with labeling of neuronal precursors. To test this hypothesis, we double-labeled brain sections with antibodies against BrdUrd and three developmentally regulated marker proteins: Hu, a nuclear and cytoplasmic protein expressed early in neuronal maturation about the time precursors exit the cell cycle (36, 37); DCX, a microtubule-associated protein found in the soma and processes of migrating neurons (38, 39) and after lesion-induced neurogenesis (23); and PCNA, which is involved in DNA replication and repair and expressed in proliferating cells (40). In the ipsilateral SGZ 1 week after ischemia, there was little or no BrdUrd labeling in cells expressing Hu (which was expressed mainly in the DG hilus), but almost completely overlapping of BrdUrd labeling and DCX and PCNA expression (Fig. 7). In the SVZ, there was also little or no overlap between BrdUrd labeling and Hu expression and essentially complete overlap between BrdUrd labeling and DCX expression. However, in contrast to the SGZ, only a few cells that were labeled with BrdUrd expressed PCNA (Fig. 8).
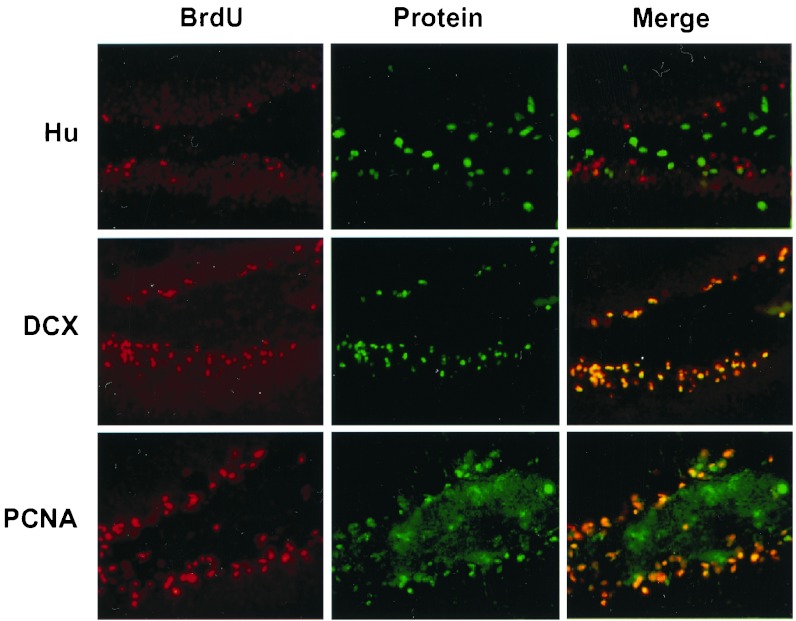
Figure 7.
Relationship between BrdUrd incorporation and expression of Hu, DCX, and PCNA in DG. Double labeling for BrdUrd (Left, red) and Hu, DCX, and PCNA (Center; top to bottom, green) was performed in sections through DG ipsilateral to MCA occlusion, taken 1 week after ischemia. Merged images show no coexpression of BrdUrd with Hu, but extensive coexpression of BrdUrd with DCX and PCNA. The experiment was repeated three times with similar results. BrdU, BrdUrd.
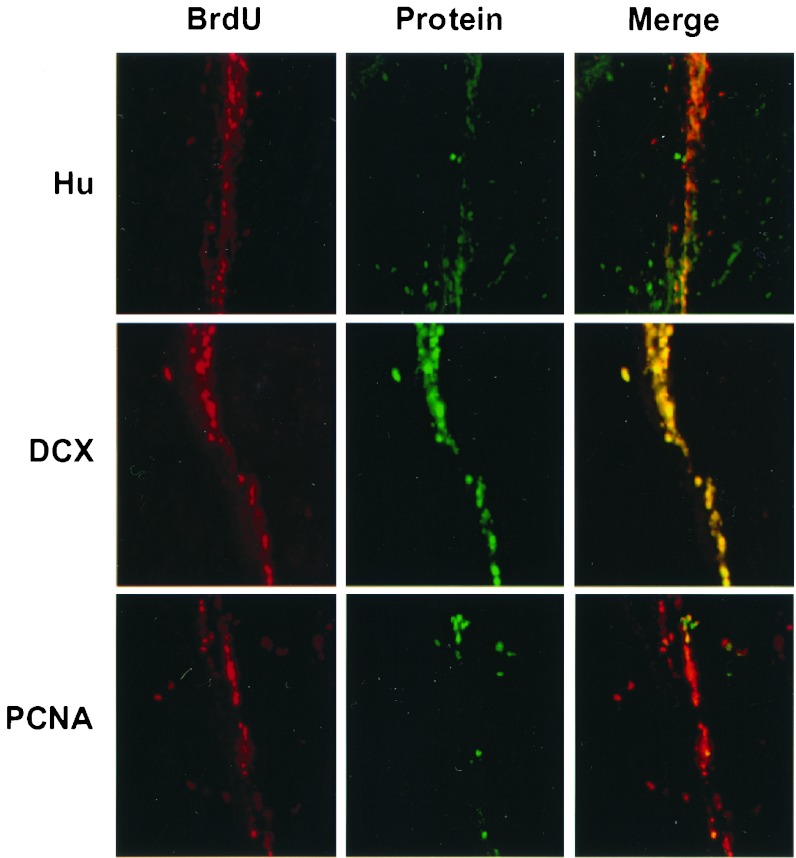
Figure 8.
Relationship between BrdUrd incorporation and expression of Hu, DCX, and PCNA in SVZ. Double labeling for BrdUrd (Left, red) and Hu, DCX, and PCNA (Center; top to bottom, green) was performed in sections through SVZ ipsilateral to MCA occlusion, taken 1 week after ischemia. Merged images show no coexpression of BrdUrd with Hu, extensive coexpression of BrdUrd with DCX, and expression of PCNA in only a small percentage of BrdUrd-labeled cells. The experiment was repeated three times with similar results. BrdU, BrdUrd.
Discussion
Recovery from stroke is thought to involve reactivation of molecular and cellular processes quiescent since development (24). These processes may include not only mechanisms to promote cell survival or enhance cell function but also the regeneration of lethally injured cell populations. The finding that neurons can arise in adult mammalian brain from resident, or even circulating, precursors (41, 42) suggests an endogenous capacity for neuronal replacement that could enhance recovery in diseases such as stroke.
We addressed whether focal ischemia stimulates neurogenesis in brain regions where neurogenesis is known to occur in the adult—the SGZ and SVZ. Our major finding is that focal ischemia increases incorporation of BrdUrd, a marker for DNA replication and a surrogate for cell division, in both regions. Moreover, this occurs both ipsilateral and contralateral to ischemia and involves cells that coexpress marker proteins associated with immature neurons during development.
Four previous reports relate directly to our study. Liu et al. (25) and Takagi et al. (26) used a different model—global cerebral ischemia—to show enhanced BrdUrd labeling of SGZ cells in the gerbil and mouse. In animals followed for weeks to months, BrdUrd-labeled cells coexpressed mature neuronal marker proteins and migrated into the DG granule cell layer (25). Global ischemia affects both cerebral hemispheres, and neurogenesis increased bilaterally. Two reports of neurogenesis in focal cerebral ischemia have also appeared. Gu et al. (27) used photochemically induced, cortical microvascular occlusion and showed that BrdUrd labeling colocalized with the expression of MAP-2 and NeuN in a small percentage of cells near the infarct. Parent et al. (28) used a focal ischemia model similar to ours and, in a preliminary report, found increased BrdUrd labeling of cells expressing neuronal markers in the ipsilateral SVZ and rostral migratory stream.
Our findings differ from those reported previously in that we observed bilaterally increased neurogenesis after unilateral ischemia and involvement of both the SGZ and SVZ. In the absence of a known mechanism for ischemia-induced neurogenesis and because possible mechanisms include trans-synaptic and diffusible chemical effects, it is unclear if either finding should be surprising. Contralateral electrical, chemical, and vascular effects of hemispheric ischemia are well described, and bilateral induction of neurogenesis by a unilateral stimulus has been reported (22, 43). Unilateral stimulation of the perforant path, leading to focal seizure discharges without neuronal injury, increases neurogenesis in the SGZ both ipsilateral and contralateral to the stimulated side (22), and traumatic injury to the cerebral cortex increases BrdUrd labeling in the SVZ bilaterally (43). Because transplanted neural stem cells can migrate into tumors from the contralateral hemisphere (44), endogenous neuronal precursors might be expected to proliferate in response to contralateral ischemia as well. The observation that ischemia stimulated neurogenesis in both the SGZ and the SVZ contrasts with the differential effects of certain growth factors in these regions (14, 15). However, there is no reason to believe that only a single factor is responsible for stimulating neurogenesis after ischemia or that no factor exists that is capable of stimulating neurogenesis at several sites.
Injury to the DG granule cell layer is known to stimulate neurogenesis in the SGZ (21), but we could detect no evidence of ischemic neuronal morphology or DNA damage in the SGZ or SVZ in our model. This is consistent with previous reports that the ability of global ischemia (25) or seizures (22) to promote neurogenesis in the SGZ does not depend on local cell damage. BrdUrd may be incorporated into DNA (45) and PCNA may be induced (46) not only during cell division but also during DNA repair. However, the absence of detectable DNA damage in the neuroproliferative regions under study and the colocalization of BrdUrd incorporation with markers such as DCX, which is not associated with DNA repair, suggest that the BrdUrd labeling that we observed reflects cell division. This observation is supported further by the finding that BrdUrd incorporation was observed only in known neuroproliferative zones.
In attempting to characterize neuronal precursors based on the expression of marker proteins, limitations must be recognized. First, unambiguous stage-specific markers have not been identified (3). Second, the functional significance of individual proteins inferred from studies of development or transplantation may not apply to neurogenesis from endogenous precursors in the adult brain. Moreover, the sequence of marker protein expression may differ in different neuroproliferative regions. Finally, even markers associated with a discrete function, such as neuronal migration, may be permissive rather than determinative, so expression may not always have the same functional correlate.
The BrdUrd administration schedule that we used labeled cells that incorporated BrdUrd over 2 days before sacrifice. Thus, whether animals were killed 1, 2, or 3 weeks after ischemia, the labeled cells were ≤2 days old. Because we saw no labeling outside the neuroproliferative zones, the labeled cells appear not to have had sufficient time to migrate. This observation is consistent with the finding that in global ischemia, only cells at least 14–17 days old had migrated from the SGZ into the granule cell layer (25). Most cells that incorporated BrdUrd in our study also expressed PCNA and DCX. PCNA is a cell-cycle-dependent nuclear protein that serves as a cofactor for DNA polymerase δ and has a role in ensuring the fidelity of DNA replication. PCNA is widely distributed in the developing central nervous system, including neuroproliferative zones (40). Its expression is also modified in vulnerable CA1 hippocampal neurons after global ischemia in the adult rat, suggesting it may be regulated by neuronal injury (46). Nevertheless, the distribution of PCNA in our model and the absence of neuronal injury in PCNA-expressing regions suggests involvement in DNA replication rather than repair. DCX is a neuron-specific microtubule-associated protein expressed in newly mitotic neurons (39). Although DCX is associated with migrating neurons (23, 38, 39), it is also expressed in cells that have not yet left the SVZ (39), in nonmigrating neurons in culture (38, 39), and in neurons that have completed migration (38). Thus, the coexpression of PCNA and DCX in BrdUrd-labeled cells strongly implicates these cells as newborn neurons. Why PCNA expression was coextensive with BrdUrd immunoreactivity in the SGZ but more limited in the SVZ is unclear. However, the expression of other neuronal proteins shows regional variations during development that may be related to differences in phenotypic fate (47), and such variations could serve a similar function during neurogenesis in the adult.
An important issue raised by this study is how a neurogenic signal is transmitted from a site of stimulation or injury to neuroproliferative zones of the brain. The distances involved are considerable—for example, neuronal precursors in the SVZ of adult mice migrate to the olfactory bulb as much as 0.5 cm away (9). Ablation of the olfactory bulb does not abolish proliferation or migration of these cells (13), suggesting at least a partially cell-autonomous process. But in other instances, especially where the trigger to neurogenesis is fortuitous rather than predetermined, an external signal seems necessary. Such intercellular signaling is known to underlie the ability of ischemic tissue to direct the proliferation and migration of other cell types, such as endothelial cells involved in angiogenesis. In addition, the ability of a brain lesion such as a tumor to attract transplanted neuronal precursors from the opposite hemisphere (44) seems to require target-derived signals. Growth factors, hormones, and neurotransmitters can all regulate the proliferation of neuronal precursors, and other secreted factors can control stem-cell microenvironments over long distances (48). However, whether any of these factors transmits a proliferative signal from sites of brain pathology is unknown. A related possibility is that induction of neurogenesis results from removal of a normal inhibitory influence by denervation or loss of a secreted factor.
What prospects exist for mobilizing endogenous neuronal precursors to treat brain disorders such as stroke? It is uncertain whether adequate numbers of cells can be generated to repopulate a large destructive lesion such as an infarct, especially because many newly generated neurons undergo programmed cell death (49). However, it may be possible to augment neurogenesis with pharmacological measures. In one study, lithium increased BrdUrd labeling in the DG by 25%, and 65% of new cells developed into neurons (20). To participate in repair, new neurons must be able to reach the site of ischemia. The demonstration that neuronal precursors can track a tumor from the opposite hemisphere (44) is encouraging, as is evidence that neurons may be generated locally in the ischemic adult cerebral cortex (27). The ability of new neurons to integrate into cerebral circuitry is likewise critical, and both transplanted (50) and endogenous (23) neuronal precursors have been shown capable of achieving this integration. The ultimate goal, restoration of function, will be feasible only if all these preconditions can be met. The demonstration that focal cerebral ischemia can stimulate neurogenesis is a first step in that regard.
Acknowledgments
This work was supported by U.S. Public Health Service Grant NS35965.
Abbreviations
- BrdUrd
5-bromo-2′-deoxyuridine-5′-monophosphate
- DAB
diaminobenzidine
- DCX
doublecortin
- DG
dentate gyrus
- MCA
middle cerebral artery
- PCNA
proliferating-cell nuclear antigen
- SGZ
subgranular zone
- SVZ
subventricular zone
- TTC
2,3,5-triphenyltetrazolium hydrochloride
- MAP-2
microtubule-associated protein 2
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.McKay R. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Serrano A, Bjorklund A. Trends Neurosci. 1997;20:530–538. doi: 10.1016/s0166-2236(97)01119-3. [DOI] [PubMed] [Google Scholar]
- 3.Scheffler B, Horn M, Blumcke I, Laywell E D, Coomes D, Kukekov V G, Steindler D A. Trends Neurosci. 1999;22:348–357. doi: 10.1016/s0166-2236(99)01416-2. [DOI] [PubMed] [Google Scholar]
- 4.van der Kooy D, Weiss S. Science. 2000;287:1439–1441. doi: 10.1126/science.287.5457.1439. [DOI] [PubMed] [Google Scholar]
- 5.Gage F H. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 6.Horner P J, Gage F H. Nature (London) 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 7.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan M S, Hinds J W. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 9.Lois C, Garcia-Verdugo J M, Alvarez-Buylla A. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 10.Gould E, Reeves A J, Graziano M S, Gross C G. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 11.Cameron H A, McKay R D. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 12.Tanapat P, Hastings N B, Reeves A J, Gould E. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirschenbaum B, Doetsch F, Lois C, Alvarez-Buylla A. J Neurosci. 1999;19:2171–2180. doi: 10.1523/JNEUROSCI.19-06-02171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn H G, Winkler J, Kempermann G, Thal L J, Gage F H. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner J P, Black I B, DiCicco-Bloom E. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Kusky J R, Ye P, D'Ercole A J. J Neurosci. 2000;20:8435–8442. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron H A, Tanapat P, Gould E. Neuroscience. 1998;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- 18.Gould E, Beylin A, Tanapat P, Reeves A, Shors T J. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 19.Gould E, Tanapat P. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji H K. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 21.Gould E, Tanapat P. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- 22.Parent J M, Yu T W, Leibowitz R T, Geschwind D H, Sloviter R S, Lowenstein D H. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magavi S S, Leavitt B R, Macklis J D. Nature (London) 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 24.Cramer S C, Chopp M. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Solway K, Messing R O, Sharp F R. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takagi Y, Nozaki K, Takahashi J, Yodoi J, Ishikawa M, Hashimoto N. Brain Res. 1999;831:283–287. doi: 10.1016/s0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- 27.Gu W, Brannstrom T, Wester P. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Parent J M, Vexler Z S, Derugin N, Valentin V V, Ferriero D M. Soc Neurosci Abstr. 2000;26:304. [Google Scholar]
- 29.Jin K L, Mao X O, Nagayama T, Goldsmith P C, Greenberg D A. Neuroscience. 2000;100:713–717. doi: 10.1016/s0306-4522(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 30.Jin K L, Mao X O, Goldsmith P C, Nagayama T, Greenberg D A. Neuroscience. 2000;99:577–585. doi: 10.1016/s0306-4522(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 31.Longa E Z, Weinstein P R, Carlson S, Cummins R. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 32.Jin K, Chen J, Nagayama T, Chen M, Sinclair J, Graham S H, Simon R P. J Neurochem. 1999;72:1204–1214. doi: 10.1046/j.1471-4159.1999.0721204.x. [DOI] [PubMed] [Google Scholar]
- 33.Isayama K, Pitts L H, Nishimura M C. Stroke. 1991;22:1394–1398. doi: 10.1161/01.str.22.11.1394. [DOI] [PubMed] [Google Scholar]
- 34.Mullen R J, Buck C R, Smith A M. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marusich M F, Furneaux H M, Henion P D, Weston J A. J Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- 37.Barami K, Iversen K, Furneaux H, Goldman S A. J Neurobiol. 1995;28:82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- 38.Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet M C, Friocourt G, McDonnell N, Reiner O, Kahn A, et al. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 39.Gleeson J G, Lin P T, Flanagan L A, Walsh C A. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 40.Ino H, Chiba T. Brain Res Mol Brain Res. 2000;78:163–174. doi: 10.1016/s0169-328x(00)00092-9. [DOI] [PubMed] [Google Scholar]
- 41.Brazelton T R, Rossi F M, Keshet G I, Blau H M. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 42.Mezey E, Chandross K J, Harta G, Maki R A, McKercher S R. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 43.Tzeng S F, Wu J P. Neuroreport. 1999;10:2287–2292. doi: 10.1097/00001756-199908020-00012. [DOI] [PubMed] [Google Scholar]
- 44.Aboody K S, Brown A, Rainov N G, Bower K A, Liu S, Yang W, Small J E, Herrlinger U, Ourednik V, Black P M, et al. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolbeare F. Histochem J. 1996;28:531–575. doi: 10.1007/BF02331377. [DOI] [PubMed] [Google Scholar]
- 46.Tomasevic G, Kamme F, Wieloch T. Brain Res Mol Brain Res. 1998;60:168–176. doi: 10.1016/s0169-328x(98)00173-9. [DOI] [PubMed] [Google Scholar]
- 47.Pleasure S J, Collins A E, Lowenstein D H. J Neurosci. 2000;20:6095–6105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watt F M, Hogan B L. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 49.Biebl M, Cooper C M, Winkler J, Kuhn H G. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 50.Snyder E Y, Yoon C, Flax J D, Macklis J D. Proc Natl Acad Sci USA. 1997;94:11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]