Abstract
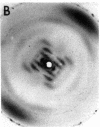
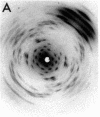
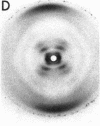
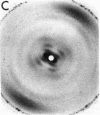
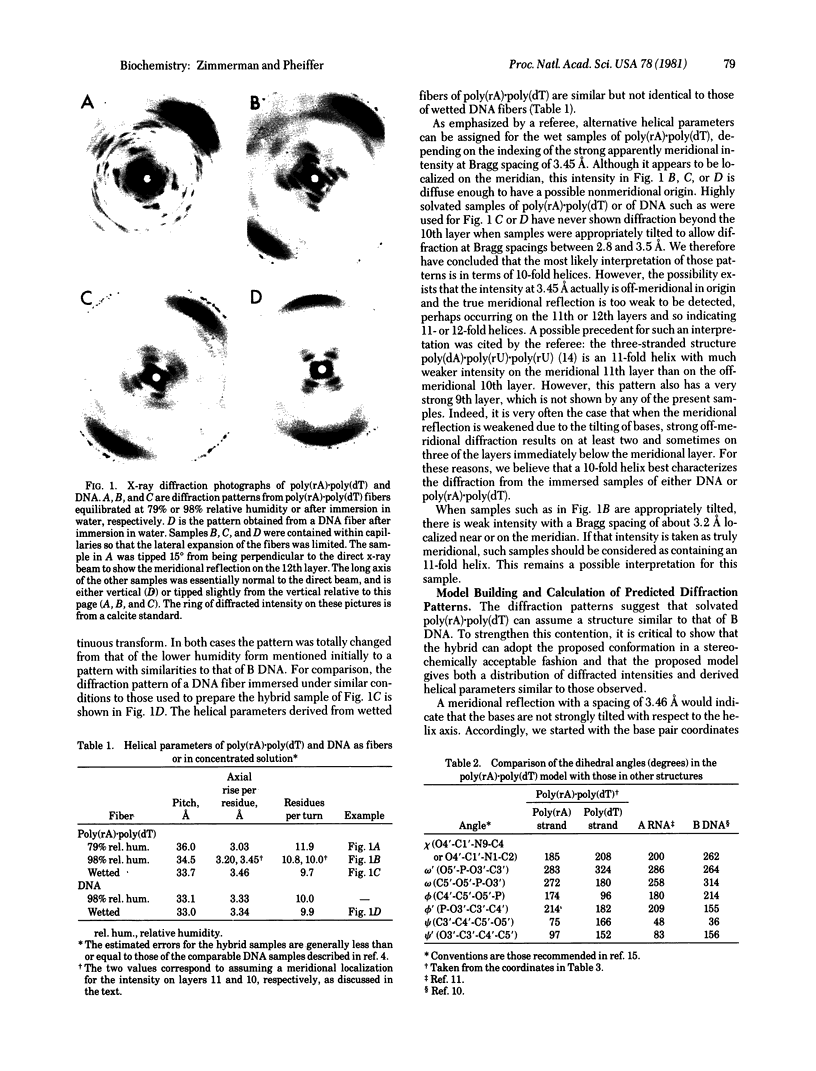
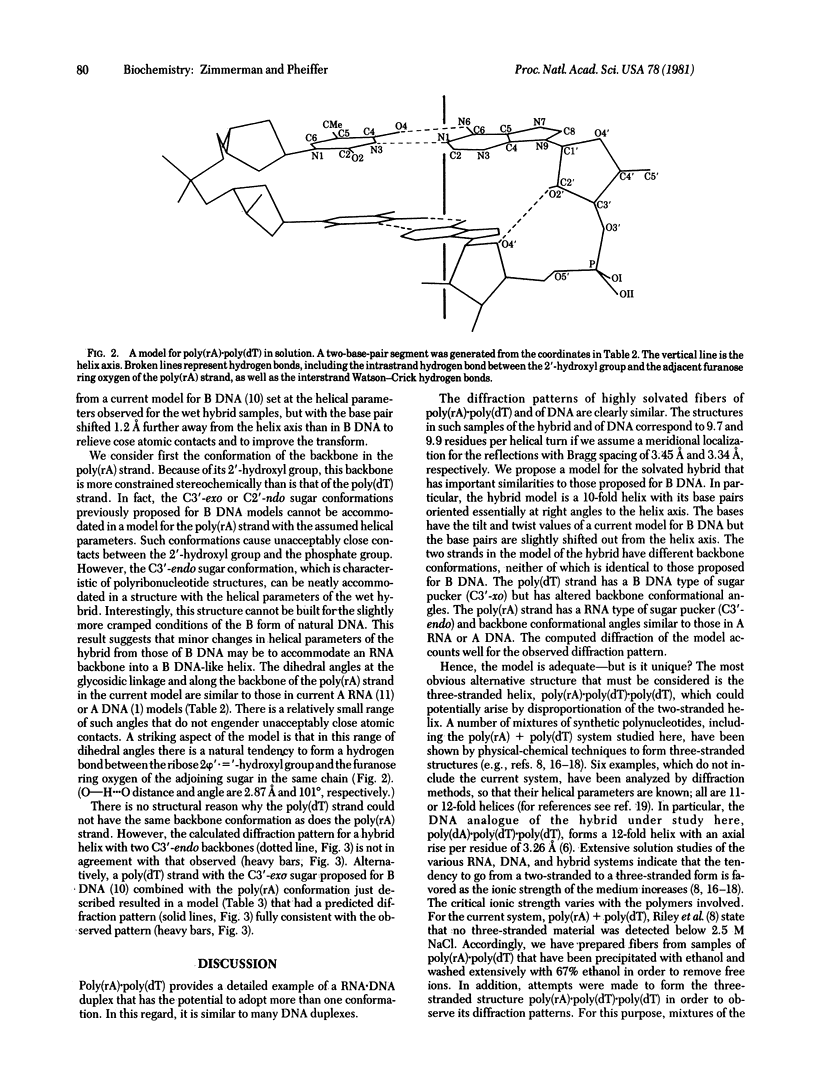
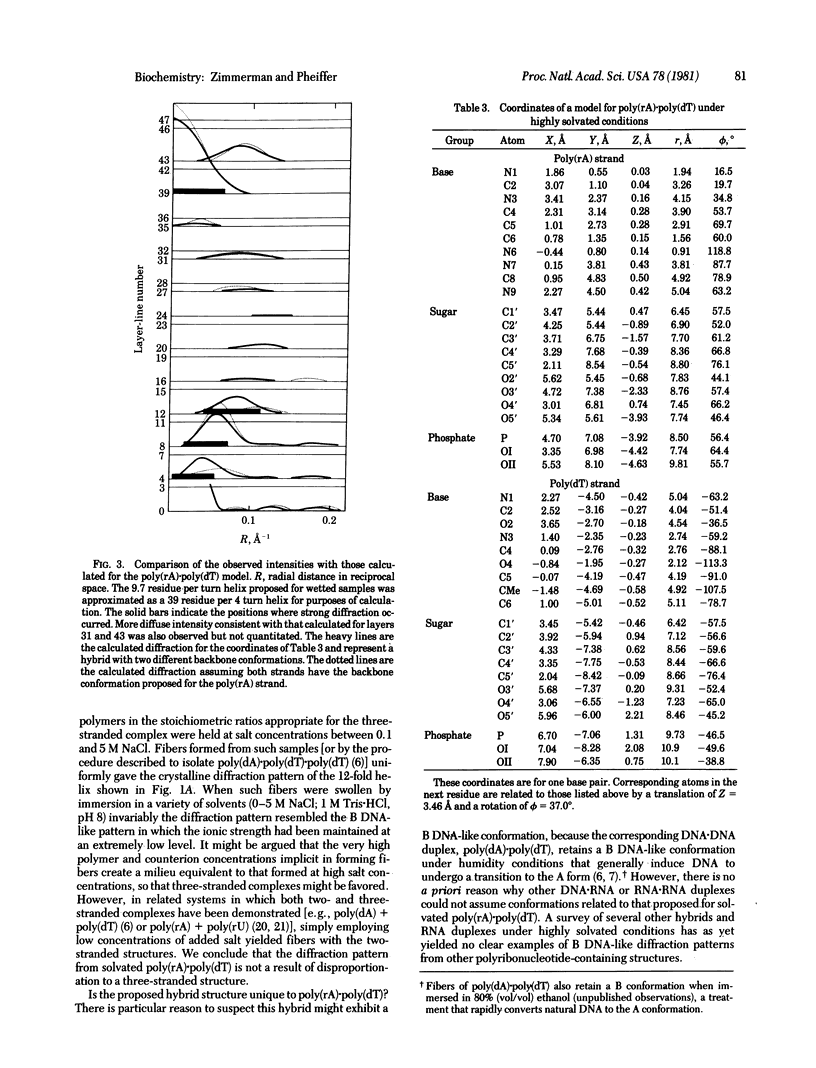
The RNA.DNA hybrid poly(rA).poly(dT) can adopt two conformations, depending upon its degree of hydration. Fibers yield a conventional A' RNA-like pattern at 79% relative humidity. In contrast, under highly solvated conditions this material yields an x-ray diffraction pattern with striking similarities to that of B DNA and clearly different from the RNA-like diffraction patterns previously obtained from fibers of other hybrids. A structural model is proposed for the solution form of the hybrid that has several similarities to models proposed for B DNA. The hybrid model accommodates the 2'-hydroxyl groups of the poly(rA) strand in intrachain hydrogen bonds to the adjacent ribose moieties.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Bond P. J., Selsing E., Smith P. J. Models of triple-stranded polynucleotides with optimised stereochemistry. Nucleic Acids Res. 1976 Oct;3(10):2459–2470. doi: 10.1093/nar/3.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Bond P. J. Structures for Poly(U)-poly(A)-poly(U)triple stranded polynucleotides. Nat New Biol. 1973 Jul 25;244(134):99–101. doi: 10.1038/newbio244099a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Fuller W., Hodgson A., Prutton I. Molecular conformations and structure transitions of RNA complementary helices and their possible biological significance. Nature. 1968 Nov 9;220(5167):561–564. doi: 10.1038/220561a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W., Dover S. D. Optimised parameters for RNA double-helices. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1392–1399. doi: 10.1016/0006-291x(72)90867-4. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Arnott S., Selsing E. Structures for the polynucleotide complexes poly(dA) with poly (dT) and poly(dT) with poly(dA) with poly (dT). J Mol Biol. 1974 Sep 15;88(2):509–521. doi: 10.1016/0022-2836(74)90498-7. [DOI] [PubMed] [Google Scholar]
- Arnott S. The geometry of nucleic acids. Prog Biophys Mol Biol. 1970;21:265–319. doi: 10.1016/0079-6107(70)90027-1. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M. J., PATTERSON D. L. PHYSICAL AND CHEMICAL CHARACTERIZATION OF THE ORDERED COMPLEXES FORMED BETWEEN POLYINOSINIC ACID, POLYCYTIDYLIC ACID AND THEIR DEOXYRIBO-ANALOGUES. J Mol Biol. 1965 Jun;12:410–428. doi: 10.1016/s0022-2836(65)80264-9. [DOI] [PubMed] [Google Scholar]
- DAVIES D. R. Polyinosinic plus polycytidylic acid: a crystalline polynucleotide complex. Nature. 1960 Jun 25;186:1030–1031. doi: 10.1038/1861030a0. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Bond P. J., Peticolas W. L. Characterization of the A in equilibrium B transition of DNA in fibers and gels by laser Raman spectroscopy. Biopolymers. 1975 Jun;14(6):1245–1257. doi: 10.1002/bip.1975.360140613. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G., RICH A. Studies on the formation of two- and three-stranded polyribonucleotides. Biochim Biophys Acta. 1957 Dec;26(3):457–468. doi: 10.1016/0006-3002(57)90091-4. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism spectra of poly[d(AC):d(GT)], poly[r(AC):r(GU)], and hybrids poly[d(AC):r(GU)] and poly[r(AC):d(GT)] in the presence of ethanol. Biopolymers. 1975 Mar;14(3):487–498. doi: 10.1002/bip.1975.360140305. [DOI] [PubMed] [Google Scholar]
- Herbeck R., Zundel G. Influence of temperature and magnesium ions on the secondary and tertiary structures of tRNAPhe and 23 S RNA - infrared investigations. Biochim Biophys Acta. 1976 Jan 5;418(1):52–62. doi: 10.1016/0005-2787(76)90326-9. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Frazier J., Miles H. T. Interaction of polyribothymidylic acid with polyadenylic acid. J Biol Chem. 1971 Dec 10;246(23):7073–7086. [PubMed] [Google Scholar]
- Kölkenbeck K., Zundel G. The significance of the 2' OH group and the influence of cations on the secondary structure of the RNA backbone. Biophys Struct Mech. 1975 May 30;1(3):203–219. doi: 10.1007/BF00535757. [DOI] [PubMed] [Google Scholar]
- Milman G., Langridge R., Chamberlin M. J. The structure of a DNA-RNA hybrid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1804–1810. doi: 10.1073/pnas.57.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E. J., MacEwan A. W. Molecular and crystal structure of the polynucleotide complex: polyinosinic acid plus polydeoxycytidylic acid. J Mol Biol. 1970 Mar 14;48(2):243–261. doi: 10.1016/0022-2836(70)90159-2. [DOI] [PubMed] [Google Scholar]
- Pilet J., Blicharski J., Brahms J. Conformations and structural transitions in polydeoxynucleotides. Biochemistry. 1975 May 6;14(9):1869–1876. doi: 10.1021/bi00680a011. [DOI] [PubMed] [Google Scholar]
- RICH A., DAVIES D. R., CRICK F. H., WATSON J. D. The molecular structure of polyadenylic acid. J Mol Biol. 1961 Feb;3:71–86. doi: 10.1016/s0022-2836(61)80009-0. [DOI] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- SASISEKHAREN V., SIGLER P. B. AN X-RAY DIFFRACTION STUDY OF POLY (A+U). J Mol Biol. 1965 May;12:296–298. doi: 10.1016/s0022-2836(65)80304-7. [DOI] [PubMed] [Google Scholar]
- Sasisekharan V., Zimmerman S., Davies D. R. The structure of helical 5'-guanosine monophosphate. J Mol Biol. 1975 Feb 25;92(2):171–179. doi: 10.1016/0022-2836(75)90221-1. [DOI] [PubMed] [Google Scholar]
- Selsing E., Wells R. D., Alden C. J., Arnott S. Bent DNA: visualization of a base-paired and stacked A-B conformational junction. J Biol Chem. 1979 Jun 25;254(12):5417–5422. [PubMed] [Google Scholar]
- Selsing E., Wells R. D., Early T. A., Kearns D. R. Two contiguous conformations in a nucleic acid duplex. Nature. 1978 Sep 21;275(5677):249–250. doi: 10.1038/275249a0. [DOI] [PubMed] [Google Scholar]
- Tunis M. J., Hearst J. E. Optical rotatory dispersion of DNA in concentrated salt solutions. Biopolymers. 1968;6(8):1218–1223. doi: 10.1002/bip.1968.360060816. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. Helical parameters of DNA do not change when DNA fibers are wetted: X-ray diffraction study. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2703–2707. doi: 10.1073/pnas.76.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]