Abstract
A recent study in South Africa has confirmed, for the first time, that a vaginal gel formulation of the antiretroviral drug Tenofovir, when topically applied, significantly inhibits sexual HIV transmission to women [Karim et al., Science 329, 1168 (2010)]. However, the gel for this drug and anti-HIV microbicide gels in general have not been designed using an understanding of how gel spreading and retention in the vagina govern successful drug delivery. Elastohydrodynamic lubrication theory can be applied to model spreading of microbicide gels [Szeri et al., Phys. Fluids 20, 083101 (2008)]. This should incorporate the full rheological behavior of a gel, including how rheological properties change due to contact with, and dilution by, ambient vaginal fluids. Here, we extend our initial analysis, incorporating the effects of gel dilution due to contact with vaginal fluid produced at the gel-tissue interface. Our original model is supplemented with a convective-diffusive transport equation to characterize water transport into the gel and, thus, local gel dilution. The problem is solved using a multi-step scheme in a moving domain. The association between local dilution of gel and rheological properties is obtained experimentally, delineating the way constitutive parameters of a shear-thinning gel are modified by dilution. Results show that dilution accelerates the coating flow by creating a slippery region near the vaginal wall akin to a dilution boundary layer, especially if the boundary flux exceeds a certain value. On the other hand, if the diffusion coefficient of boundary fluid is increased, the slippery region diminishes in extent and the overall rate of gel spreading decreases.
INTRODUCTION
Elastohydrodynamic flows occur in many biomedical phenomena.1, 2, 3, 4, 5 A recent example involves application of a bolus of a semi-solid microbicide vehicle to the lower female reproductive tract, to inhibit infection by sexually transmitted pathogens such as HIV.1, 6, 7, 8, 9, 10 Very recently, a vaginal gel has shown promising effectiveness in blocking HIV transmission in women.11 Women who used a formulation of the antiretroviral drug Tenofovir were 39% less likely overall to contract HIV than those who used a placebo. However, the gel in this trial was not designed using knowledge of the mechanisms of its vaginal spreading and consequent drug release. Moreover, there is widespread agreement that more effective microbicide vehicles should be developed, to improve behavioral acceptability, i.e., willingness of patients to choose to use the product,12 as well as biological functioning against HIV.
There are several active factors that govern the distribution or coating process of semi-solid or fluid microbicide vehicles.13, 14, 15, 16, 17 Among these are gravity, transvaginal pressure gradients due to contractility of the supporting viscera, and transverse squeezing forces from the distended epithelium. Salient gel rheological properties are related to its shear thinning and the possible existence of a yield stress, both of which can be altered by dilution. There have been initial fluid mechanical studies of intravaginal vehicle flows; these focused on the individual effects of gravity or epithelial squeezing.14, 15, 16 Those initial studies are instructive in developing a physical understanding of the mechanisms of intravaginal vehicle deployment flows. The second generation model developed by Szeri et al.18 involves simultaneous effects of a longitudinally directed force along the vaginal canal, e.g., gravity, and transversely directed elastic epithelial squeezing, in a lubrication flow analysis. Both Newtonian and non-Newtonian fluids (Carreau constitutive model) were considered. A single dimensionless number, independent of viscosity, was derived to characterize the relative influences of squeezing and gravitational acceleration on the shape of spreading in the Newtonian case. A second scale, involving viscosity, was used to determine the spreading rate. In the case of a non-Newtonian shear-thinning fluid, the Carreau number also played a role.
This second generation analysis did not, however, take into account the dilution of gel due to contact with ambient vaginal fluids. Such dilution begins immediately after gel insertion, prior to the deposition of semen. It can alter gel rheological properties and, consequently, the flow process itself. In general, effects of dilution on the rheology of a fluid have been investigated in terms of molecular structure.19, 20 Effects of dilution on vaginal gel rheology have specifically been investigated.21 Initial work on how vaginal gel flows are influenced by dilution involved comparing models outputs when input with rheological properties for diluted, fully mixed gels vs. undiluted gels.17 However, gel dilution in vivo involves contact of the bolus with fluid at the gel-tissue interface, not homogeneous mixing. The result is a time and space-dependent distribution of local gel dilution, as fluid from the boundary permeates the gel and alters its local properties. The goal of the present paper is to address this inhomogeneous gel dilution problem, focusing upon effects of vaginal fluid present on the surface of the epithelium.
In the method introduced here, we supplement our elastohydrodynamic lubrication model18 with a convective-diffusive transport equation to account for boundary dilution that is inhomogeneous throughout the diluted, non-Newtonian fluid. In this process, flow of the fluid—in our focus, a gel—is driven by a transversal force (e.g., wall compliance). This flow influences transport of the fluid from the boundary, that is permeating the gel. Consequently, gel dilution by boundary fluid can be interpreted as a convective-diffusive phenomenon. The present work begins by applying our initial results to spreading of a homogenously diluted microbicide gel, over a range of dilutions. Then, an associated convection-diffusion equation for boundary fluid dilution is formulated. This is suitable for inhomogeneous dilution along the boundary, but we illustrate its application by considering a fluid flux at the boundary that is constant in time and space; values of this flux derive from data on production of human vaginal fluid. The governing Reynolds lubrication equation and convective-diffusive transport equation are solved simultaneously using an implicit multi-step scheme in a moving domain.
The theory developed here improves our understanding of the biophysics of microbicide gel flows in vivo, which can lead to improved understanding of drug delivery by those gels. In addition, the theory here may find application in other elastohydrodynamic problems of interest.
PROBLEM FORMULATION
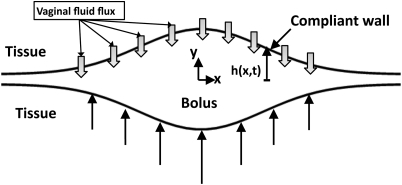
We first present the Reynolds lubrication equation as an evolution equation for the shape of a bolus of non-Newtonian fluid, and then derive a governing equation for the distribution of vaginal fluid exuded from epithelial surfaces. The equations are developed in the symmetric domain –h(x, t) ≤ y ≤ h(x, t). The physical problem and computational domain are sketched in Fig. 1. The model is formulated in a 2 D Cartesian domain. The simplification of two dimensional flow is quite relevant anatomically; the cross section of the undistended human vaginal canal is “H” shaped, with the transverse dimension large compared to the vertical openings on its two sides.22, 32 For the constitutive model, we take the form,
| (1) |
here, is the shear rate and τxy is the shear stress. One can choose F(τ) = 1/m0 for a Newtonian fluid or, as we do below,
| (2) |
for a Carreau-like fluid which exhibits shear thinning and a finite viscosity at zero-shear rate, m0. m is the viscosity of the Carreau-like model and n is the power index. The original Carreau model can be written as . Here, η is the viscosity of the Carreau model, η0 is the zero shear viscosity, and λ is the relaxation time of the fluid. The parameters of the Carreau model can be converted into those of Carreau-like model asymptotically18 in the relationship m0 = η0 and m = η0/λ1–n. The two models may be matched at small and large strain rates although they are not precisely equivalent.
Figure 1.
(Color online) Definition sketch of the vaginal canal. The introitus is to the right. Vaginal fluid fluxes and squeezing forces are effective for both of the vaginal surfaces.
Next, we present Reynolds lubrication equation into which our constitutive model is substituted,
| (3) |
where,
| (4) |
Here, we made use of the one dimensional constrained continuum model23 approximation. The idea is to relate the fluid pressure near a compliant wall to the local deformation of that wall. In general, for a deformation h, the fluid pressure is given by p = (E/T)h ≡ Mh. Here, E is the elastic (Young) modulus of the compliant layer, T is its thickness, and M is the compliance of the elastic wall.
In our problem, inhomogeneous dilution by vaginal fluids being exuded from the boundaries changes the rheological properties of the overlying microbicide gel. Therefore, rheological parameters—zero-shear viscosity, power-law exponent, and relaxation time scale—must be updated at each time step and at each location in the fluid. The relationship between the rheological properties and the volume fraction is estimated experimentally. That is, we use data for rheometry of gels that have been serially diluted, and thoroughly mixed with a test fluid, here, vaginal fluid stimulant.24 The assumption is that local rheological properties of a spreading, diluting gel are well approximated by those properties of homogeneously diluted gel, in equilibrium at each degree of dilution. Now, we introduce two-dimensional convection-diffusion equation for coating flow of a microbicide gel,
| (5) |
here, u1 and u2 are the velocities of the gel in the x- and the y-direction. and are unit vectors in the x- and the y-direction. ν is the concentration of absorbed water. Generally, the diffusion coefficient, D, varies with concentration.25 In this study, we utilized a dilution dependent diffusion coefficient D, which has the form,
| (6) |
here, D0 is the diffusion coefficient in the dry state, i.e., ν is equal to zero. The parameter, ad, defines the interaction of the network with water.26
A boundary condition for the transient dilution of a microbicide gel is given in terms of the fluid flux at the wall,
| (7) |
Boundary flux may alter based on the time of day or the phase of the ovulation cycle. It may be affected by osmotic effects or by hydrodynamic pressure in the vaginal lumen. Here, we assume that this flux is constant. Clearly, experimental work is a necessary prerequisite to a more sophisticated theoretical treatment. Usually, the direction of the flux is normal to the wall. Strictly, the flux direction must be updated for each time step because the shape of the bolus is varying through time. Here, we assume that the flux direction is normal to the x -axis. The assumption is reasonable when the gradient of the height profile in the x -direction, ∂h/∂x, has a small value, i.e., ∂h∕∂x≪1. During spreading of a microbicide gel, the physical domain changes shape. The computational domain in the x-direction must be chosen to be of sufficient length. The upper and lower limits in the y-direction are taken as ±h(x, t). Hence, special attention should be paid to redefining the grid points in the y-direction at each time step. Although the grid points in the y-direction are changing, the Reynolds lubrication equation, Eq. 3 is solvable because only the integrated value over the grid points in the y-direction, not the individual value, is involved in the equation. On the other hand, in the case of the convection-diffusion equation, Eq. 5, the grid points in the y-direction should be fixed in order to solve the equation. This is because the dependent variable must be found at individual values of the grid points in the y-direction.
In order to make progress on this problem, the straightforward domain mapping (x, y) → (x, ζ) is applied. Here, y = h(x, t)ζ. The domain (x, ζ) has the shape of a rectangle instead of the shape of the bolus and the grid points in the ζ-direction are fixed for every time step. The shape of each domain is shown in Fig. 2. With this domain mapping, Eq. 5 takes the form
| (8) |
Here, h = h(x, t), u1 = u1(x, y, t), u2 = u2(x, y, t), and V = V (x, ζ, t) =ν (x, y, t). The boundary condition has the form in the (x, ζ) domain.
Figure 2.
Shape of domains. Left domain represents the physical domain. Right domain represents mapped domain by y = h(x, t)ζ.
The initial condition for the shape of the bolus is taken as,
| (9) |
The volume contained in such a bolus (above h∞) is , where c is the vaginal width, i.e., 2 cm. The volume of the bolus, Vb, is 3 mL. As a scale for the height H, we choose 0.5 cm; this scale is of the order of magnitude of the maximum height of the bolus at time zero. Here, we choose h∞ = 0.05 H and b = 0.45 H, and one obtains .
Basically, two governing transport equations and one constitutive equation, which vary through time and space, are required to define transient inhomogeneous boundary dilution of a flowing microbicide gel. Here, elastohydrodynamic flow is driven by initial distention of elastic surfaces and the resultant squeezing of the fluid bolus, which is initially at rest. This distention leads to pressure gradients and flow, eventually. For the convective-diffusive transport equation of vaginal fluid, the concentration of added vaginal fluid is initially zero everywhere. We first integrate the x-momentum equation in the y-direction to obtain the shear stresses. The x-momentum equation is the x component of Navier-Stokes equation with the lubrication approximation (∂p/∂x = ∂τ/∂y) with gravity neglected. Here, shear stress can be obtained as, τ = y∂p/∂x = yM∂h/∂x. Note that at y = 0, τ = 0 due to symmetry. Then, by substituting shear stresses into Eq. 2 and integrating the x-momentum equation in the y-direction twice, u1 values are calculated. Velocities in the y-direction (u2) are easily extracted from the incompressibility condition. Thus far, equations are solved in (x, y) domain. We then solve the convection-diffusion equation 8 in (x, ζ) domain, by substituting velocities. After finding the local volume fraction distribution of vaginal fluid, the constitutive equation 2, which is a function of ν, is updated at each point. Iteration now proceeds and the Reynolds lubrication equation is solved with the updated parameters. We have developed the governing equations accounting for the gravitational forces. However, there is not a standard way of placing these microbicide vehicles. Gravitational effects can be in any direction of the spreading, as determined by the patients posture during and after insertion of the microbicide. In the present manuscript, gravity is set to zero for simplicity.
We solved the Reynolds lubrication equation explicitly. On the other hand, the convection-diffusion equation for the exuded vaginal fluid was solved implicitly. We split the convective-diffusive transport finite difference equation into two, one with the x-derivative taken implicitly and the next with the y-derivative taken implicitly, following alternating direction implicit (ADI) method. The resulting tri-diagonal matrices were solved by the Thomas algorithm. Central differences were used for the first and second order spatial derivatives.
RESULTS
Preliminary results for spread of a homogeneously diluted microbicide gel
The volume of native vaginal fluid present in the vagina is estimated at approximately 0.5–0.75 mL.24 The production of fluid is measured as 1–10 cm3/day, depending on the phase of the menstrual cycle.27 Taking the vaginal surface area as about 100 cm2, this corresponds to a flux of q ≈ 10–9–10–8 m/s. Hence, for a typical gel volume of 3 mL the range of dilution by vaginal secretions is 10% to 30%.17 As a preliminary analysis, the effect of dilution by vaginal secretions on a prototype gel will be considered. This is an aqueous gel with 3% hydroxyethylcellulose (known as Azopharma Orange) that is similar to the placebo gel used in the successful microbicide gel trial.11 As an initial approach to effects of gel dilution on flow, we assume that the mixing ratio of gel and vaginal secretions is constant and that dilution is homogeneous during the spreading process, as in the initial studies of dilution.17 Gel rheological properties at body temperature (37 °C) were obtained for serial dilutions of the gel with vaginal fluid stimulant,24 using a constant stress protocol on a TA Instruments model AR 1500ex rheometer, with a 4° cone and 20 cm plate configuration. Shear rates ranged from ∼10–4–42 s–1 and data were fitted to the Carreau-like model, cf. Table TABLE I..
Table 1.
Parameters of Carreau-like model for homogeneously diluted AO gel by viscometric experiments.
| Case | Dilution | n | m0(Pa.s) | m(Pa.sn) |
|---|---|---|---|---|
| 1 | 0% | 0.640693 | 738.965 | 78.5395 |
| 2 | 10% | 0.609243 | 509.617 | 53.2084 |
| 3 | 20% | 0.58495 | 229.597 | 27.7059 |
| 4 | 30% | 0.474114 | 146.972 | 16.4701 |
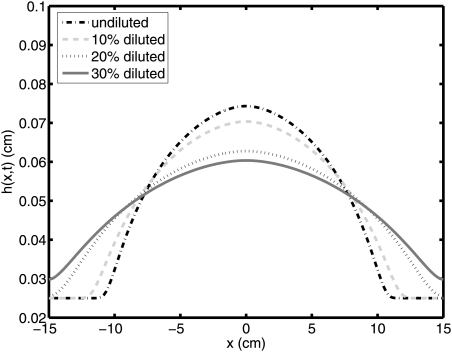
Results are illustrated in Fig. 3 for the profile of the bolus at 30 min after the onset of flow. The height profiles are shown for 0%, 10%, 20%, and 30% dilutions. Plots show only the upper half of the bolus because flow is symmetrical about the x-axis. The spreading length of the bolus increases with the extent of gel dilution. This effect was also observed in Ref. 17. Table TABLE I. shows that the zero shear viscosity m0 decreases by about 80% and the shear thinning exponent n decreases by about 25% as dilution increases to 30%. Qualitatively, dilution enhances the spread of the gel. In order to quantify the effect of dilution clearly, the coated area is shown in Fig. 4. The length of spreading of the bolus, which is proportional to the effective surface area coated, we define as four standard deviations of the bolus height profile. Then, this is multiplied with the physical width of the vagina, i.e., 2 cm. The effect is clearly significant; for example, a 30% diluted gel coats in 2 min the same area coated in 10 min by an undiluted gel.
Figure 3.
(Color online) Height profile of boluses of homogenously diluted gel, at 30 min.
Figure 4.
(Color online) Coated area of homogenously diluted gel vs. time.
In order to understand the basis for increased spreading with dilution an effective viscosity of the gel is estimated, in relation to the shear stress on the wall. The shear stress can be written as
| (10) |
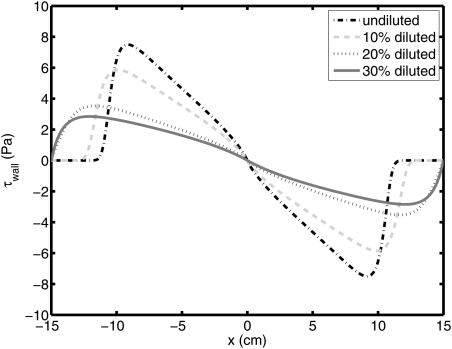
We show the shear stress on the wall, or y = h(x, t), for each case at 30 min in Fig. 5. Effective viscosity can be defined as inverse of F(τxy). Fig. 6 shows the effective viscosity on the wall at 30 min. The larger effective viscosity leads to less coated area. Therefore, the coated area of more diluted gel is increasing because of the smaller effective viscosity.
Figure 5.
(Color online) Shear stress on the wall for homogenously diluted gel at 30 min.
Figure 6.
(Color online) Effective viscosity of homogenously diluted gel on the wall at 30 min.
Boundary dilution of a microbicide gel by vaginal fluid
In order to conduct analysis of the consequences of inhomogeneous boundary dilution, the parameters of the constitutive equation and the volume fraction of vaginal fluid are linked to data from experiments, as described above. From curve fitting of experimental data obtained for the AO gel diluted in 5% increments from 0% to 75%, the parameters of the Carreau-like constitutive model take the form,
| (11) |
Here, ν is the volume fraction of vaginal fluid and the parameter, m, can be obtained from the relationship, m = m0λn–1. The volume of the bolus is 3 mL, the compliance of the wall is 104/0.5 Pa/cm.18 The factor D0 in the diffusion coefficient is set to either 10–6 cm2/s or 10–5 cm2/s. These values were chosen in relation to the diffusivity of water molecules in water (2 × 10–5 cm2/s).28 Because the gel is initially quite hydrated (>90%), we expect relatively little restriction in diffusion of water molecules into and within it.
For simplicity, a constant flux of vaginal fluid exuded from the walls is assumed here. Although this assumption may not correspond exactly to in vivo conditions, the results provide conceptual insight for the transient dilution phenomenon. Volume fraction at the boundary is found iteratively because both ν and time-derivative of ν at the boundary are used in the boundary condition, . Additionally, the physical properties of the vaginal fluid are equal to those of water.24
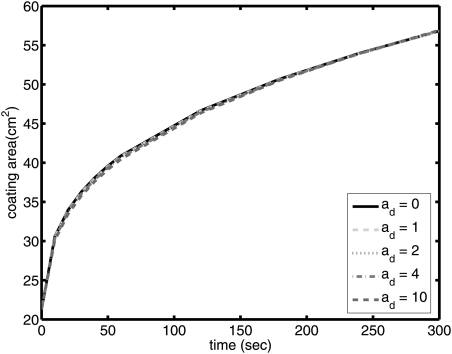
Coated area is plotted for different ad values in Fig. 7. Here, q is kept constant at 1 × 10–8 m/s (∼8 cm3/day): if effects of non-zero ad are not manifest at this value, then for lower values of flux, we can simply equate ad to zero. As seen in Fig. 7, coated area is insensitive to the value of ad. This can be partly explained, as noted above, by the relatively low rate of dilution and the fact that the gels, as prepared, are generally already quite dilute. In the analyses that follow, ad will be set equal to 0.
Figure 7.
(Color online) Coating area of the gel on the surface for ad = 0, 1, 2, 4, and 10 (q = 1 × 10–8 m/s and D = 10–6 cm2/s).
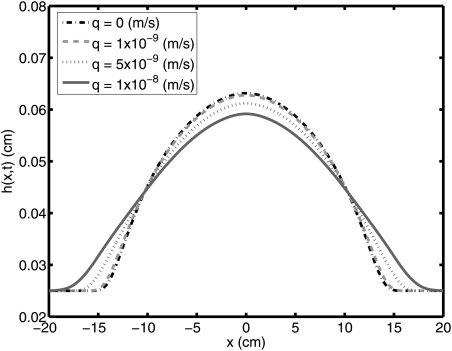
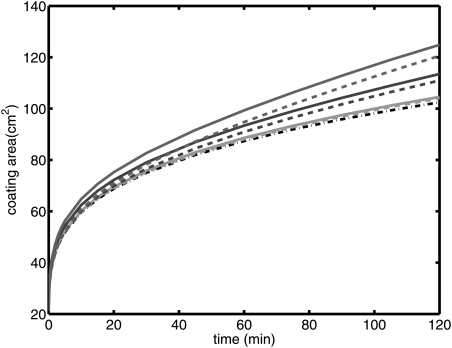
We now present results for different q values, cf. Table TABLE II.. The height profile of the bolus for each case is shown in Fig. 8. In Fig. 9, the coated area is plotted. As can be seen from the figure, coated area increases as the flux of the exuded vaginal fluid increases. In Figures 1011, the diffusion coefficient is increased from 10–6 cm2/s to 10–5 cm2/s. In Fig. 10, the height profiles of the bolus for D0 = 10–5 cm2/s are shown. Coated area for each case is given in Fig. 11. Here, coated area again increases as the flux of the exuded vaginal fluid increases.
Table 2.
(D0 = 10–6 cm2/s) The constant wall flux of vaginal fluid. Mean volume fractions are obtained at 1 min. MVF1 is obtained from numerical solution of Eq. 8. MVF2 is calculated by Eq. 12.
| Case | q(m/s) | MVF1 | MVF2 |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 5 | 1 × 10–9 | 0.000140 | 0.000137 |
| 6 | 5 × 10–9 | 0.000698 | 0.000686 |
| 7 | 1 × 10–8 | 0.001424 | 0.001371 |
Figure 8.
(Color online) Height profile of bolus at 2 h for q = 0 m/s, 1 × 10–9 m/s, 5 × 10–9 m/s, and 1 × 10–8 m/s (D = 10–6 cm2/s).
Figure 9.
(Color online) Coating area of the gel on the surface for q = 0 m/s, 1 × 10–9 m/s, 5 × 10–9 m/s, and 1 × 10–8 m/s (D = 10–6 cm2/s).
Figure 10.
(Color online) Height profile of bolus at 2 h for q = 0 m/s, 1 × 10–9 m/s, 5 × 10–9 m/s, and 1 × 10–8 m/s (D = 10–5 cm2/s).
Figure 11.
(Color online) Coating area of the gel on the surface for q = 0 m/s, 1 × 10–9 m/s, 5 × 10–9 m/s, and 1 × 10–8 m/s (D = 10–5 cm2/s).
Table TABLE II. gives the mean volume fraction of the vaginal fluid for each case. In addition, the value of the volume fraction, as calculated based on the volume of a microbicide gel and the exuded vaginal fluid, MVF2, is shown to help verify the accuracy of the numerical analysis. This latter volume fraction can be obtained from
| (12) |
DISCUSSION
Dilution of a fluid from its boundary during the course of flow within an enclosed channel is a time and space dependent process. At each instant, the spatial distribution of local rheological properties, which depend upon the local dilution, has a profound effect upon the velocity profile. This effect is exacerbated for a non-Newtonian, shear thinning gel, as was considered here. Analyses of the vaginal coating flows of microbicide drug delivery gels, the application of primary interest here, should, therefore, account for the boundary dilution process. There are many factors, (e.g., the diluent fluid flux at the boundary and the diffusion coefficient of diluent within the gel), which influence the coupled dilution-gel flow processes—in this model and, presumably, in vivo. More accurate results can be obtained when all of these factors are considered, as discussed in the following paragraphs.
Distribution of volume fraction and the diffusion coefficient
The coating area of the gel increases due to dilution by fluid from the boundary. In this model and presumably for microbicide gel flow in vivo, the local distribution of gel dilution (expressible as the volume fraction of fluid penetrant added to the gel) is not uniform. In Figs. 1213, we show the volume fraction of diluent fluid for D = 10–6 cm2/s and D = 10–5 cm2/s, respectively. Results are plotted for case 7 in Table TABLE II. at 1 min, 5 min, 30 min, and 2 h. Here, we note the high volume fraction in the vicinity of the wall, giving rise to what we term a slippery region. In addition, at the leading edge, the volume fraction of diluent fluid is also increased. This can be interpreted as due to the gel flow from the center to the leading edge. The presence of such a slippery region near the vaginal wall increases the flow rate and coating area even though the size of the slippery region is relatively small, appearing as a dilution boundary layer. For the case of the higher diffusion coefficient, the contours of increased dilution near the boundaries extend further into the gel, cf. Fig. 13. That is, the diluent fluid is diffusing more easily from the slippery regions to inner regions of the gel bolus, thereby decreasing the spreading rate. This is illustrated in Figure 14.
Figure 12.
(D = 10–6 cm2/s). Contour plot for volume fraction of vaginal fluid at 1 min, 5 min, 30 min, and 2 h (top to bottom). Note different contour levels in different panels.
Figure 13.
(D = 10–5 cm2/s). Contour plot for volume fraction of vaginal fluid at 1 min, 5 min, 30 min, and 2 h (top to bottom). Note different contour levels in different panels.
Figure 14.
(Color online) Coating area of the gel on the surface for q = 0 m/s (dashed-dot), 1 × 10–9 m/s, 5 × 10–9 m/s, 1 × 10–8 m/s (D = 10–5 cm2/s (dashed), and 10–6 cm2/s (solid)).
In the context of intravaginal flow of a microbicide gel, we have very little information about variability, spatial, and temporal, of vaginal fluid flux at the wall. This flux is due to a transudation process rather than active secretion.24 Such transudation is influenced by vaginal wall fluid permeability which has been shown to vary.29, 30 The current model, by assuming a temporally and spatially constant wall flux, is an approximation, strictly speaking. Still, results in this study provide insight about the importance of transient dilution for the coating flow of the gel.
Coating area and velocity profile
Obviously, the coating area increases when the magnitudes across the velocity profile increase. The mean values of the magnitude of the velocity are shown in Tables 3, TABLE IV.. As expected, for larger coating areas (e.g., case 7 in Fig. 9), the velocity reaches higher values.
Table 3.
(D0 = 10–6 cm2/s) Averages of velocity profiles at x = 5 cm and averages of effective viscosities at 1 min and final coating areas.
| Case | mean of |u|(mm/s) | mean of 1/F(τxy)(Pa.s) | Coating area (cm2) |
|---|---|---|---|
| 1 | 0.0145 | 622.58 | 102.48 |
| 5 | 0.0262 | 618.04 | 104.57 |
| 6 | 0.0761 | 601.57 | 113.48 |
| 7 | 0.0827 | 583.74 | 124.86 |
Table 4.
(D0 = 10–5 cm2/s) Averages of velocity profiles at x = 5 cm and averages of effective viscosities at 1 min and final coating areas.
| Case | mean of |u|(mm/s) | mean of 1/F(τxy)(Pa.s) | coating area (cm2) |
|---|---|---|---|
| 1 | 0.0145 | 622.58 | 102.48 |
| 5 | 0.0156 | 621.47 | 104.04 |
| 6 | 0.0207 | 617.06 | 110.97 |
| 7 | 0.0288 | 611.61 | 120.57 |
Velocity profile and effective viscosity
From Reynolds lubrication equation, the velocity profile has the form,
| (13) |
The magnitude of the velocity is proportional to the function F(τxy), which is the inverse of the effective viscosity and the gradient of the height profile in the x-direction. Clearly, the gradient of the height profile in the x-direction has similar values for all cases in Table TABLE II. (see Figs. 810). The mean values of the effective viscosity, or the inverse of the function F(τxy), are shown in Tables 3, TABLE IV.. The magnitude of the velocity increases when the effective viscosity decreases. Note that the increase in velocity is higher for the lower D0 = 10–6 cm2/s due to larger decrease in effective viscosity near the wall.
Effective viscosity and volume fraction of vaginal fluid
The parameters of the function F(τxy) are determined by the volume fraction of vaginal fluid (see Eq. 11). As noted above, in the more diluted gel, in which the volume fraction of diluent is higher, the effective viscosity is relatively small. From Table TABLE II., we see that the mean volume fraction is higher in the case of the larger wall flux. Hence, the larger wall flux corresponds to the smaller effective viscosity in Tables 3, TABLE IV..
In conclusion, we have presented a new model of an internal elastohydrodynamic flow of a non-Newtonian fluid that is being continuously diluted due to flux of another fluid at its boundary. Inhomogeneous boundary dilution clearly provides the potential for significant acceleration of the coating flow; for example, from Fig. 9, one can observe a decrease by nearly 50% in the time required to coat an area of 100 cm2 at the highest boundary flux rates. Thus, understanding boundary dilution is critical to understanding the physical processes underlying the pharmacokinetics. This may have applications in a number of biomedical flow problems of interest, e.g., in the respiratory and musculo-skeletal systems. Our primary focus is upon vaginal coating flows of anti-HIV microbicide gels. Results here improve the accuracy with which those flows can be modeled. Vaginal fluid production can vary due to a number of factors, including progression of the menstrual cycle and age. This new model incorporates such fluid production and is amenable to input of spatial (along the vaginal canal) and temporal variations in that production. It can be used in methodology that provides objective evaluations of microbicide gel performance and in the design of new, improved gels (e.g., Ref. 31).
ACKNOWLEDGMENTS
The authors acknowledge support from NIH Grant No. U19 AI 077289 (D.F.K.), and from California HIV/AIDS Research Program Grant No. ID07-B-135 (A.J.S.).
References
- Jin Z. M. and Dowson D., “Elastohydrodynamic lubrication in biological systems,” Proc. Inst. Mech. Eng., Part J: J. Eng. Tribol. 219, 367 (2005). 10.1243/135065005X33982 [DOI] [PubMed] [Google Scholar]
- Lighthill M. J., “Pressure-forcing of tightly fitting pellets along fluid-filled elastic tubes,” J. Fluid Mech. 34, 113 (1968). 10.1017/S0022112068001795 [DOI] [Google Scholar]
- Holly F. J. and Holly T. F., in Lacrimal Gland, Tear Film, and Dry Eye Syndromes, edited by Sullivan D. A.Plenum, New York, 1994). [Google Scholar]
- Jones M. B., Fulford G. R., Please C. P., McElwain D. L. S., and Collins M. J., “Elastohydrodynamics of the eyelid wiper,” Bull. Math. Biol. 70, 323 (2008). 10.1007/s11538-007-9252-7 [DOI] [PubMed] [Google Scholar]
- Gouldstone A., Brown R. E., Butler J. P., and Loring S. H., “Elastohydrodynamic separation of pleural surfaces during breathing,” Respir. Physiol. Neurbiol. 137, 97 (2003). 10.1016/S1569-9048(03)00138-1 [DOI] [PubMed] [Google Scholar]
- Stone A., “Microbicides: A new approach to preventing HIV and other sexually transmitted infections,” Nature Rev. Drug Discovery 1, 977 (2002). 10.1038/nrd959 [DOI] [PubMed] [Google Scholar]
- McGowan I., “Microbicides: A new frontier in HIV prevention,” Biologicals 34, 241 (2006). 10.1016/j.biologicals.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Vermani K. and Garg S., “The scope and potential of vaginal drug delivery,” Pharm. Sci. Technol. Today 3, 359 (2000). 10.1016/S1461-5347(00)00296-0 [DOI] [PubMed] [Google Scholar]
- Harrison P. F., Rosenberg Z., and Bowcut J., “Topical microbicides for disease prevention: Status and challenges,” Clin. Infect. Dis. 36, 1290 (2003). 10.1086/374834 [DOI] [PubMed] [Google Scholar]
- D’Cruz O. J. and Uckun F. M., “Clinical development of microbicides for the prevention of HIV infection,” Curr. Pharm. Des. 10, 315 (2004). 10.2174/1381612043386374 [DOI] [PubMed] [Google Scholar]
- Karim Q. A., Karim S. S. A., Frohlich J. A., Grobler A. C., Baxter C., Mansoor L. E., Kharsany A. B. M., Sibeko S., Mlisana K. P., Omar Z., Gengiah T. N., Maarschalk S., Arulappan N., Mlotshwa M., Morris L., and Taylor D., “Effectiveness and safety of Tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women,” Science 329, 1168 (2010). 10.1126/science.1193748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verguet S., Holt B. Y., and Szeri A. J., “Increasing the effectiveness of vaginal microbicides: A biophysical framework to rethink behavioral acceptability,” PLoS ONE 5, 11 (2010). 10.1371/journal.pone.0015501.g001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. F., Henderson M., Owen D. H., Plenys A. M., and Walmer D. K., “What is needed to advance vaginal formulation technology?,” in Vaginal Microbicide Formulations Workshop Lippencott-Raven, Philadelphia, 1998).
- Kieweg S. L., Geonnotti A. R., and Katz D. F., “Gravity-induced coating flows of vaginal gel formulations: In vitro experimental analysis,” J. Pharm. Sci. 93, 2941 (2004). 10.1002/jps.v93:12 [DOI] [PubMed] [Google Scholar]
- Kieweg S. L. and Katz D. F., “Squeezing flows of vaginal gel formulations relevant to microbicide drug delivery,” J. Biomech. Eng. 128, 540 (2006). 10.1115/1.2206198 [DOI] [PubMed] [Google Scholar]
- Kieweg S. L. and Katz D. F., “Interpreting properties of microbicide drug delivery gels: Analyzing deployment due to squeezing,” J. Pharm. Sci. 96, 835 (2007). 10.1002/jps.v96:4 [DOI] [PubMed] [Google Scholar]
- Lai B. E., Xie Y. Q., Lavine M., Szeri A. J., Owen D. H., and Katz D. F., “Dilution of microbicide gels with vaginal fluid and semen simulants: Effects on rheology and coating flow,” J. Pharm. Sci. 97, 1030 (2008). 10.1002/jps.v97:2 [DOI] [PubMed] [Google Scholar]
- Szeri A. J., Park S. C., Verguet S., Weiss A., and Katz D. F., “A model of transluminal flow of an anti-HIV microbicide vehicle: Combined elastic squeezing and gravitational sliding,” Phys. Fluids 20, 083101 (2008). 10.1063/1.2973188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby B. J., Mangnus M., de Groot W., Daniels R., and McLeish T. C. B., “Characterization of long chain branching: Dilution-rheology of industrial polyethylenes,” J. Rheology 46, 401 (2002). 10.1122/1.1451083 [DOI] [Google Scholar]
- Dealy J. M. and Larson R. G., Structure and Rheology of Molten Polymers (Hanser Publications, Cincinnati, OH, 2006). [Google Scholar]
- Owen D. H., Peters J. J., and Katz D. F., “Rheological properties of contraceptive gels,” Contraception 62, 321 (2000). 10.1016/S0010-7824(00)00184-0 [DOI] [PubMed] [Google Scholar]
- Roy S., Barrier Contraceptives: Current Status and Future Prospects (Wiley, New York, 1994). [Google Scholar]
- Coyle D. J., “Forward roll coating with deformable rolls: A simple one dimensional elastohydrodynamic model,” Chem. Eng. Sci. 43, 2673 (1988). 10.1016/0009-2509(88)80011-3 [DOI] [Google Scholar]
- Owen D. H. and Katz D. F., “A vaginal fluid stimulant,” Contraception 59, 91 (1999). 10.1016/S0010-7824(99)00010-4 [DOI] [PubMed] [Google Scholar]
- Crank J. A., “Theoretical investigation of the influence of molecular relaxation and internal stress on diffusion in polymers,” J. Polym. Sci. 11, 151 (1953). 10.1002/pol.1953.120110206 [DOI] [Google Scholar]
- Podual K., F.DoyleIII, and Peppas N. A., “Modeling of water transport in and release from glucose-sensitive swelling-controlled release systems based on Poly (Diethylaminoethyl Methacrylate-g-Ethylene Glycol),” Ind. Eng. Chem. Res. 43, 7500 (2004). 10.1021/ie0497238 [DOI] [Google Scholar]
- Verguet S., “Transport phenomena in microfluidics and microbicide drug delivery systems,” Ph.D. dissertation, University of California, Berkeley, 2008. [Google Scholar]
- Syrnikov Y. P., “Calculation of the self-diffusion coefficient of water,” J. Struct. Chem. 11, 698 (1970). 10.1007/BF00743446 [DOI] [Google Scholar]
- Acarturk F. and Robinson J. R., “Vaginal permeability and enzymatic activity studies in normal and ovariectomized rabbits,” Pharm. Res. 13, 5 (1996). 10.1023/A:1016016120392 [DOI] [PubMed] [Google Scholar]
- Kremer M., Squier C., Schlievert P., and Davis C., “Permeability of vaginal mucosa to toxic shock syndrome Toxin-1,” Abstr. Intersci. Conf. Antimicrob. Agents Chemother. 41, Abstract No. B-959 (2001).
- Mahalingam A., Smith E., Fabian J., Damian F. R., Peters J. J., Clark M. R., Friend D. R., Katz D. F., and Kiser P. F., “Design of a semisolid vaginal microbicide gel by relating composition to properties and performance,” Pharm. Res. 27, 2478 (2010). 10.1007/s11095-010-0244-1 [DOI] [PubMed] [Google Scholar]
- Schuenke M., Schulte E., Ross L. M., Schumacher U., Lamperti E. D., Atlas of Anatomy: Neck and Internal Organs (Thieme, New York, 2010), p. 250. [Google Scholar]