Abstract
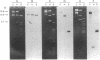
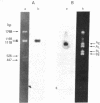
We report the molecular cloning of African green monkey genomic DNA segments that include regions of homology to the origin of replication of simian virus 40 (SV40). Three clearly different cloned segments 14 to 17 kilobase pairs (kb) long were isolated from a genomic library in lambda phage. We estimate that each of the three is repeated fewer than four times in the monkey genome. The SV40-like regions represent a small portion of the cloned segments, and these regions cross hybridize only weakly with one another. One of the three segments is described here in detail. Although the entire segment occurs only once or twice in the monkey genome, it contains DNA sequences (other than the SV40-like sequences) that are repeated elsewhere in the genome including in the other two cloned segments. The homology to SV40 is contained within about 300 base pairs of monkey DNA and is limited to the region around the viral replication origin. The nucleotide sequence of the SV40-like region was determined. It contains a large number of short stretches homologous to three specific noncoding domains around the SV40 origin of replication: the 27-base-pair region of dyad symmetry, the first set of (short) repeats that occur just on the late side of the origin, and, further in the late direction, the two 72-base-pair-long repeats. Although these components are grouped in the monkey DNA, as they are in SV40 DNA, their relative juxtaposition is scrambled.
Full text
PDF
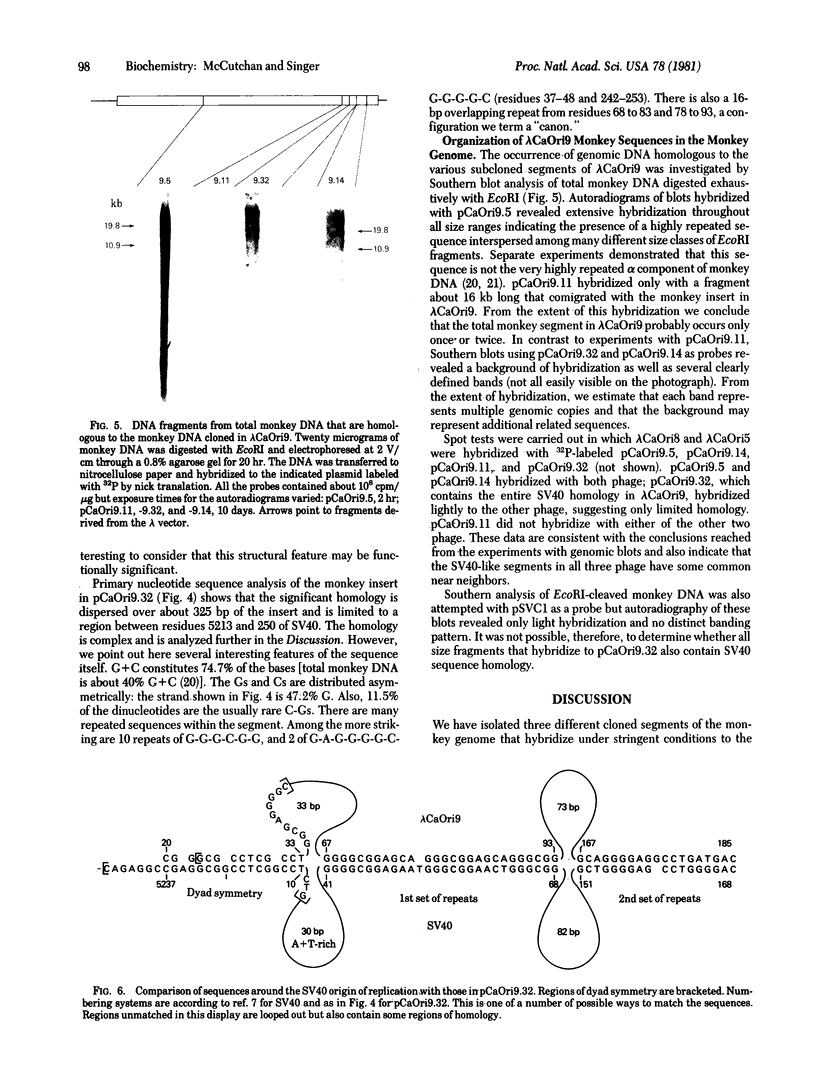

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. Deletions covering the putative promoter region of early mRNAs of simian virus 40 do not abolish T-antigen expression. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3865–3869. doi: 10.1073/pnas.77.7.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Characterization of early simian virus 40 transcriptional complexes: late transcription in the absence of detectable DNA replication. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5443–5447. doi: 10.1073/pnas.74.12.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Sambrook J. F., Frisque R. J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio J. J. DNA strand reassociation and polyribonucleotide binding in the African green monkey, Cercopithecus aethiops. J Mol Biol. 1971 Mar 28;56(3):579–595. doi: 10.1016/0022-2836(71)90403-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Singer M., Rosenberg M. Highly reiterated sequences of SIMIANSIMIANSIMIANSIMIANSIMIAN. Science. 1978 Apr 28;200(4340):394–402. doi: 10.1126/science.205944. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Kleinberger T., Livingston D. M. Mapping of SV40 DNA replication origin region binding sites for the SV40 T antigen by protection against exonuclease III digestion. Cell. 1980 Jun;20(2):411–422. doi: 10.1016/0092-8674(80)90627-3. [DOI] [PubMed] [Google Scholar]
- Singer D. S. Arrangement of a highly repeated DNA sequence in the genome and chromatin of the African green monkey. J Biol Chem. 1979 Jun 25;254(12):5506–5514. [PubMed] [Google Scholar]
- Soeda E., Maruyama T., Arrand J. R., Griffin B. E. Host-dependent evolution of three papova viruses. Nature. 1980 May 15;285(5761):165–167. doi: 10.1038/285165a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Smith K., Padgett T., McCombe P., Roulland-Dussoix D., Moscovici C., Varmus H. E., Bishop J. M. Uninfected avian cells contain RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):371–379. doi: 10.1016/0092-8674(78)90205-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Radonovich M. F., Salzman N. P. Characterization of the 5'-terminal structure of simian virus 40 early mRNA's. J Virol. 1979 Aug;31(2):437–446. doi: 10.1128/jvi.31.2.437-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Tolun A., Aleström P., Pettersson U. Sequence of inverted terminal repetitions from different adenoviruses: demonstration of conserved sequences and homology between SA7 termini and SV40 DNA. Cell. 1979 Jul;17(3):705–713. doi: 10.1016/0092-8674(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn H., Fiers W. Nucleotide sequence of the Hind-C fragment of simian virus 40 DNA. Comparison of the 5'-untranslated region of wild-type virus and of some deletion Mutants. Eur J Biochem. 1979 Oct;100(1):51–60. doi: 10.1111/j.1432-1033.1979.tb02032.x. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Sundin O., Bohn M. A stretch of "late" SV40 viral DNA about 400 bp long which includes the origin of replication is specifically exposed in SV40 minichromosomes. Cell. 1979 Feb;16(2):453–466. doi: 10.1016/0092-8674(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Wigmore D. J., Eaton R. W., Scott W. A. Endonuclease-sensitive regions in SV40 chromatin from cells infected with duplicated mutants. Virology. 1980 Jul 30;104(2):462–473. doi: 10.1016/0042-6822(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Williams B. G., Blattner F. R. Construction and characterization of the hybrid bacteriophage lambda Charon vectors for DNA cloning. J Virol. 1979 Feb;29(2):555–575. doi: 10.1128/jvi.29.2.555-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. C., Wu R. Comparative study of papovavirus DNA: BKV(MM), BKV(WT) and SV40. Nucleic Acids Res. 1979 Oct 10;7(3):651–668. doi: 10.1093/nar/7.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]