Abstract
Photoacoustic microscopy (PAM) was used to detect small animal brain activation in response to drug abuse. Cocaine hydrochloride in saline solution was injected into the blood stream of Sprague Dawley rats through tail veins. The rat brain functional change in response to the injection of drug was then monitored by the PAM technique. Images in the coronal view of the rat brain at the locations of 1.2 and 3.4 mm posterior to bregma were obtained. The resulted photoacoustic (PA) images showed the regional changes in the blood volume. Additionally, the regional changes in blood oxygenation were also presented. The results demonstrated that PA imaging is capable of monitoring regional hemodynamic changes induced by drug abuse.
Keywords: photoacoustic microscopy; imaging, brain; rat, drug abuse; cocaine
Introduction
The abuse of cocaine can cause insanity, strokes, seizures, and tremors.1, 2, 3 Imaging technologies have enabled the investigation of the pharmacological effects of chronic drug usage of cocaine on the brain noninvasively. Small animals have been used as experimental models to understand the effects of acute and chronic drug administration on regional brain function, neuroadaptations, and pharmacological interventions. Medical imaging methods, such as functional magnetic resonance imaging (MRI) and positron emission tomography (PET), have been used to globally map the effects of pharmacological actions on the central nervous system in small animals.4, 5 However, these techniques are associated with either high cost or using ionizing radiations.
Photoacoustic tomography (PAT) has been suggested as an imaging tool to detect the functional changes in the brain of small animal models for drug abuse/addiction.8 The administration of cocaine hydrochloride causes a dose-dependent neuronal activation with changes in brain metabolic rate. The changes in cerebral blood volume, which are affected by the level of dopamine and can be either increased or decreased,9, 10 can be detected by PA detection. Previously, we showed the overall changes in blood volume on the brain cortex surface due to drug abuse with a PAT system. In this paper, a photoacoustic microscopy (PAM) system is applied to detect the regional changes in the brain function induced by the injection of cocaine hydrochloride in small animals. With the PAM system, the brain images are shown at coronal views, and the regional changes in the total hemoglobin (HbT) concentration are presented. Additionally, the regional changes in blood oxygenation are also imaged.
Materials and Methods
For PAM imaging,11 a tunable OPO laser (Surelite OPO PLUS; Continuum, Clara, California), pumped by a Q-switched Nd:yttrium–aluminum–garnet laser (Surelite III; Continuum, Santa Clara, California) was used to generate laser light. During the conduction of PAM, we used 680 and 797 nm optical wavelength light. At 797 nm wavelength, the absorptions of oxy-hemoglobin (HbO2) and deoxy hemoglobin (Hb) are the same,12 and at 680 nm wavelength, the output power of the OPO laser research maximum. These two wavelengths were chosen for calculating the changes in blood oxygenation. The incident energy density of the laser that reached the surface of the rat head was less than 20 mJ/cm2, which complied with the safety limit for human skin exposure.13 During data collection, a laser beam was scanned across the surface, and subsequently generated PA signals were detected by a 5-MHz focused ultrasonic transducer (SU-108-013, Sonic Concepts), which has a focal length of 35 mm and diameter of 33 mm. The detected signals were amplified by a preamplifier (5072PR; Olympus-NDT, Waltham, Massachusetts), and collected through a multiple-channel data acquisition board (CS21G8-256MS; Gage, Lockport, Illinois) that used 8 bit resolution and 500 MS/s of sampling rate, and then downloaded to a personal computer for post-analysis. Photoacoustic images were reconstructed by using the envelope of each scan line from each scan position of the transducer. Essentially, the envelope of each signal at each scan position was projected in the depth direction. The distance along the depth direction was calculated by multiplying the time of flight by the speed of sound in soft tissue.
Sprague Dawley rats (140 to 220 g body weight) were used for brain imaging. A total of eight animals were used in the experiments and the images presented here were from one of the animals. For the imaging procedure, the animals were initially anesthetized with a mixture of ketamine (87 mg/kg body weight) and xylazine (13 mg/kg body weight), and subsequent anesthesia was maintained with the inhalation of 1.0% to 2.0% isoflurane mixed with pure oxygen. After shaving the rat head with an electric shaver, the anesthetized rat was fixed on a custom-designed surgical stereotaxic frame, and the head surface was coated with ultrasonic gel, and placed under the acoustic coupling membrane in a water tank. The body temperature was maintained using a heating lamp and the heartbeat remained about 250±10/min. For PAM images, the rat was injected cocaine hydrochloride (Sigma-Aldrich, St. Louis, Missouri) in 0.9% sterile saline at the given dose of 5.0 mg/kg body weight. This dosage of cocaine hydrochloride was chosen because it can produce strong hemodynamic effects. About 2 h after administering the initial anesthesia, the cocaine hydrochloride in sterile saline solution was injected through the rat tail veins. During the experiment, the heart rate and blood oxygenation were monitored with a pulse-oximeter (Nonin medical, PulseSense VET, Plymouth, Minnesota). An initial PA image before the injection of cocaine hydrochloride solution was obtained to serve as the reference image. Then the drug was injected and PAM was employed to monitor the brain activation continuously. From the initial image, we could identify the coronal suture and confirm the position of bregma with ±0.2 mm error. All animal handling procedures are approved by the Institutional Animal Care and Use Committee of the University of Kansas.
After the injection of cocaine hydrochloride, the rat brain was scanned with 680 and 797 nm wavelengths. Reference images at 797 nm for a before-injection and an after-injection were acquired and compared with respective images at 680 nm for the same region. The respective images at 680 nm were obtained about 1 min after the reference images at 797 nm. We assumed the changes in brain activation were small during this 1 min because cocaine-induced brain activation is a relatively slow process. To sense the changes in blood oxygenation (sO2), functional changes (ΔIF) were calculated with the following equation:14
| (1) |
where I denotes the amplitude of PA signals, and IF denotes the ratio of PA signals at 680 and 797 nm. At 797 nm wavelength, which is an isospectral point for blood, the photoacoustic signal is proportional to the changes in HbT concentration.15 At 680 nm wavelength, the absorption of deoxy-hemoglobin is much higher than that of oxy-hemoglobin. As a result, IF is inversely proportional to changes in sO2 and independent of blood volume changes.14 Therefore, the functional change (ΔIF) reflects the level of sO2 change. Before calculating ΔIF, statistic analysis between images obtained at the same wavelength was performed. For the statistical significance, data (p-value of >0.05) were filtered to reduce noise. With the method, PA imaging data from 680 and 797 nm were used to map sO2 changes by pixel-wise normalization of images.14ΔIF is able to signify region of interest for sO2 changes at two positions, 1.2 mm posterior to bregma and 3.4 mm posterior to bregma.
Furthermore, PA imaging data were acquired to investigate the spatiotemporal distribution of relative HbT concentration changes due to the pharmadynamic effects of cocaine hydrochloride. At 797 nm, the reference signals and the subsequent signals 30 min after the cocaine injection were collected with noise-filtering and 0.8 mm spatial smoothing. Changes in HbT were calculated by 1+ΔHbT/HbT. On the B-scan image which showed the anatomical distribution of the HbT changes, positive HbT responses were used to indicate HbT increases between before and after cocaine injection.
Results and Discussion
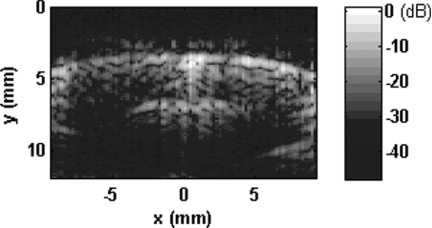
PA B-scan images on coronal view of rat brain at two positions of 1.2 and 3.4 mm posterior to bregma were monitored throughout the experiment for imaging cortical functional changes. Figure 1 shows an example of the coronal view of a rat brain when 680 nm light wavelength was used.
Figure 1.
PA B-scan image of a rat's brain at 3.2 mm posterior to bregma.
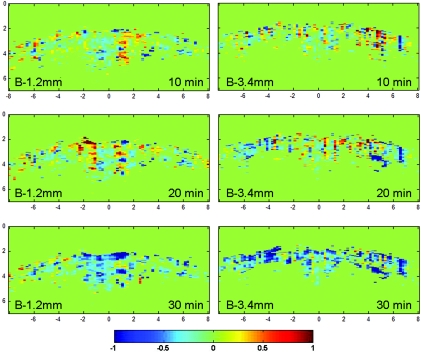
The functional images (ΔIF) show the changes of HbO2 and Hb on cerebral cortex (Fig. 2). The PA functional signals detected every 10 min after the injection of cocaine hydrochloride are shown. Because IF is the ratio of PA imaging signals at 680 nm to PA imaging signals at 797 nm, and at 680 nm, the optical absorption of Hb is dominant, a lower value of IF means a lower concentration of Hb. Furthermore, a negative value in ΔIF indicates the increase in sO2, and a positive value means the decrease in sO2. In Fig. 2, the negative area becomes broader as time increases, indicating an increase in HbO2 concentration. Meanwhile, Hb concentration decreased gradually until 30 min after injecting cocaine. This change in sO2 indicates the activation of the rat brain after the injection of cocaine hydrochloride. As a result, the increase of blood oxygenation level after cocaine injection indicates the change in the rat brain, which is consistent with the higher sO2 at 30 min than 10 min from previous studies.16, 17 Further, regions with the decreased sO2 were also observed at 10 and 20 min. The result may suggest that there are regions experiencing decreases in sO2 temporarily.
Figure 2.
Functional images (ΔIF) at 1.2 mm posterior to bregma, and 3.4 mm posterior to bregma. sO2 changes are observed at 10, 20, and 30 min. The negative values in ΔIF indicated the increases in sO2 levels, and vice versa.
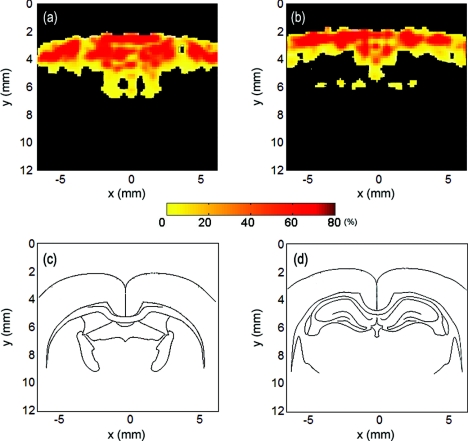
Figure 3 illustrates the percentage change in HbT concentration, and stereotaxic atlas reference lines are shown below the images for reference. We can confirm the increase in regional HbT concentration and the activated region in the rat brain cortex due to the effect of cocaine. The increase in HbT concentration is not uniform, with a maximum increase of 80% in some regions. From these results, we are able to identify the regional response of the brain to the injection of cocaine hydrochloride by identifying the anatomical region from stereotaxic atlas reference lines. A change in HbT implies a change in relative cerebral blood volume (rCBV). The conspicuous changes of rCBV on cortical layers have been shown by a previous study by Marota et al. with MRI (Ref. 18) and the increase in rCBV was shown to be as high as 49% with a 1 mg/kg concentration of cocaine.
Figure 3.
The difference in HbT between 0 min and 30 min after 5 mg/kg of cocaine administration at (a) 1.2 mm posterior to bregma and (b) 3.4 mm posterior to bregma. (c) and (d) are the corresponding rat brain atlas referent lines. They correspond to (a) and (b), respectively.
When PA imaging is used to detect brain activation, PA imaging does not directly measure changes in neuronal and glial signaling. Instead, PA imaging detects changes in hemodynamic activities, and normal neurovascular coupling is assumed. Although it is believed that blood flow and cell activities are coupled under normal conditions, recent reports, however, described that neurovascular coupling may be compromised during progression of certain diseases or in response to certain drugs.19, 20 These results may limit the direct application of PA imaging on neuroimaging. A better solution may be to directly detect the metabolic rate by combining PA imaging and PA Doppler detection in the future.
Conclusions
We used the PAM technique to monitor the pharmacological effect with photoacoustic functional detection in small animal brain in vivo. The regional change in HbT, which can be detected by PA imaging system effectively, was observed from the result of injecting cocaine hydrochloride. PA imaging on coronal view also shows the changes in the distribution of oxygenated hemoglobin in the rat brain. The results demonstrate that PA imaging can be used to monitor regional hemodynamic changes induced by drug abuse.
Acknowledgments
This work was supported by NIH Grant No. 1R03DA026987.
References
- Anthony J. C. and Petronis K. R., “Early-onset drug-use and risk of later drug problems,” Drug Alcohol Depend. 40(1), 9–15 (1995). 10.1016/0376-8716(95)01194-3 [DOI] [PubMed] [Google Scholar]
- Schwartz B. G., Rezkalla S., and Kloner R. A., “Cardiovascular effects of cocaine,” Circulation 122(24), 2558–2569 (2010). 10.1161/CIRCULATIONAHA.110.940569 [DOI] [PubMed] [Google Scholar]
- Cregler L. L. and Mark H., “Medical complications of cocaine abuse,” New England J. Med. 315(23), 1495–1500 (1986). 10.1056/NEJM198612043152327 [DOI] [PubMed] [Google Scholar]
- Lu H., Xi Z. X., Gitajn L., Rea W., Yang Y., and Stein E. A., “Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI),” Proc. Natl. Acad. Sci. U.S.A. 104(7), 2489–2494 (2007). 10.1073/pnas.0606983104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Mullani N., Gould K. L., Adler S., and Krajewski K., “Cerebral blood flow in chronic cocaine users: a study with positron emission tomography,” Br. J. Psychiatry 152, 641–648 (1988). 10.1192/bjp.152.5.641 [DOI] [PubMed] [Google Scholar]
- Jo J. and Yang X., “Detection of cocaine induced rat brain activation by photoacoustic tomography,” J. Neurosci. Methods 195(2), 232–235 (2011). 10.1016/j.jneumeth.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar M. J., Ritz M. C., and Boja J. W., “The Dopamine Hypothesis of the Reinforcing Properties of Cocaine,” Trends Neurosci. 14(7), 299–302 (1991). 10.1016/0166-2236(91)90141-G [DOI] [PubMed] [Google Scholar]
- Luo F., Schmidt K. F., Fox G. B., and Ferris C. F., “Differential responses in CBF and CBV to cocaine as measured by fMRI: Implications for pharmacological MRI signals derived oxygen metabolism assessment,” J. Psychiatr. Res. 43(12), 1018–1024 (2009). 10.1016/j.jpsychires.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Cui H., Staley J., and Yang X., “The integration of photoacoustic imaging and high intensity focused ultrasound,” J. Biomed. Opt. 15, 021312 (2010). 10.1117/1.3365948 [DOI] [PubMed] [Google Scholar]
- Kim C., Favazza C., and Wang L. H. V., “In vivo photoacoustic tomography of chemicals: high-resolution functional and molecular optical imaging at new depths,” Chem. Rev. 110(5), 2756–2782 (2010). 10.1021/cr900266s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laser Institute of America, “American National Standard for Safe Use of Lasers ANSI Z136.1-2000,” I. American National Standards Institure, Ed. (2000).
- Liao L. D., Li M. L., Lai H. Y., Shih Y. Y. I., Lo Y. C., Tsang S. N., Chao P. C. P., Lin C. T., Jaw F. S., and Chen Y. Y., “Imaging brain hemodynamic changes during rat forepaw electrical stimulation using functional photoacoustic microscopy,” Neuroimage 52(2), 562–570 (2010). 10.1016/j.neuroimage.2010.03.065 [DOI] [PubMed] [Google Scholar]
- Wang X. D., Xie X. Y., Ku G. N., and Wang L. H. V., “Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography,” J. Biomed. Opt. 11(2), 024015 (2006). 10.1117/1.2192804 [DOI] [PubMed] [Google Scholar]
- Du C. W., Tully M., Volkow N. D., Schiffer W. K., Yu M., Luo Z. C., Koretsky A. P., and Benveniste H., “Differential effects of anesthetics on cocaine's pharmacokinetic and pharmacodynamic effects in brain,” Eur. J. Neurosci. 30(8), 1565–1575 (2009). 10.1111/j.1460-9568.2009.06931.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K. F., Febo M., Shen Q., Luo F., Sicard K. M., Ferris C. F., Stein E. A., and Duong T. Q., “Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI,” Psychopharmacology 185(4), 479–486 (2006). 10.1007/s00213-006-0319-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marota J. J. A., Mandeville J. B., Weisskoff R. M., Moskowitz M. A., Rosen B. R., and Kosofsky B. E., “Cocaine activation discriminates dopaminergic projections by temporal response: An fMRI study in rat,” Neuroimage 11(1), 13–23 (2000). 10.1006/nimg.1999.0520 [DOI] [PubMed] [Google Scholar]
- Yuan Z. J., Luo Z. C., Volkow N. D., Pan Y. T., and Du C. W., “Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo,” Neuroimage 54(2), 1130–1139 (2011). 10.1016/j.neuroimage.2010.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M., Deouell L. Y., and Gazzaley A., “Alterations in the bold FMRI signal with ageing and disease: A challenge for neuroimaging,” Nat. Rev. Neurosci. 4(11), 863–872 (2003). 10.1038/nrn1246 [DOI] [PubMed] [Google Scholar]