Abstract
The Ebola virus matrix protein VP40 plays an essential role in virus assembly and budding. In this study we reveal that transient VP40 expression results in the release into the culture medium of substantial amounts of soluble monomeric VP40 in addition to the release of virus-like particles containing an oligomeric form of this protein as previously described. We show that VP40 secretion is endoplasmic reticulum/Golgi–independent and is not associated with cell death. Soluble VP40 was observed during Ebola virus infection of cells and was also found in the serum of virus-infected animals albeit in lower amounts. Unconventional secretion of VP40 may therefore play a role in Ebola virus pathogenicity.
Ebola virus (EBOV) belongs to the Filoviridae family, which consists of 2 genera: Ebolavirus and Marburgvirus. Filoviruses, part of the order Mononegavirales, are a group of negative-strand RNA viruses responsible for severe hemorrhagic fevers in human and nonhuman primates. The matrix protein VP40 of EBOV is an important element of the virion structure and is essential for the assembly and budding of virus particles [1]. When synthesized in the absence of other viral proteins, VP40 promotes the formation of virus-like particles (VLPs) resembling filamentous virions [2]. Coexpression of VP40 and the surface glycoprotein GP further facilitates formation of VLPs, which have been used recently in vaccine development [3]. Structurally, VP40 consists of 2 functionally interrelated domains: an N-terminal oligomerization domain and a C-terminal membrane-binding domain [4]. Binding of VP40 with membranes is believed to induce a conformational change required for the protein’s oligomerization. Membrane-associated VP40 forms hexamers [5]. However, VP40 has also been demonstrated to bind RNA in a sequence-specific manner, inducing the formation of octameric ringlike structures [6]. Mutations in the region shown to be involved in VP40’s interaction with RNA severely affected the recovery of recombinant EBOV.
Two overlapping, so-called late-budding domains—PTAP and PPEY arranged as the sequence PTAPPEY—are present in the aminoterminal part of EBOV VP40 [7]. Similar to many other viral proteins that are involved in the budding process, late-budding domains of EBOV VP40 recruit the endosomal sorting complex required for transport machinery from its normal site of function (endosomes and multivesicular bodies) to sites of viral budding at the plasma membrane through interactions with TSG101 or Nedd4 [1, 8]. A recent study also revealed that VP40 interacts with Sec24C, a component of the COPII vesicular transport system [9]. This interaction was proposed to serve as an initial step in intracellular VP40 trafficking.
Taken together, it seems that there is a general acceptance of the concept that VP40 is primarily involved and responsible for virus budding through its ability to interact with membranes and membrane-associated cellular proteins. In this study, we report that EBOV VP40 is also secreted from cells in a soluble monomeric form, by an unconventional secretory pathway.
MATERIALS AND METHODS
Cells, Plasmids, Mutagenesis, Transfections, and Virus
HEK 293T and Vero E6 cells were cultured at 37°C in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal calf serum. The cells were grown for 24 hours to a confluence of ∼60%. Transfection of cells was performed using Fugene HD (Roche) according to the manufacturer’s instructions. The sequence encoding full-length wild-type VP40 of Zaire EBOV strain Mayinga was subcloned from plasmid pcDNA3.1(+) [10] into the vector phCMV using restriction endonuclease EcoRI. VP40 mutants were created using the QuikChange site-directed mutagenesis kit (Stratagene) according to the supplier’s instructions with phCMV-EBOV/VP40 serving as a template. Quantitative Western blotting was performed using secondary goat anti–mouse IgG conjugated with Cy3 (GE Healthcare) as a secondary antibody, and fluorescence measurement was performed using the Typhoon imaging system (GE Healthcare).
VLP Release and Ultracentrifugation
HEK 293T cells were transfected with phCMV-EBOV/VP40, and culture supernatants were collected 16 h after transfection. Culture supernatants were clarified by centrifugation at 1000 × g for 5 minutes at 4°C. The clarified supernatants were loaded on a 20% sucrose cushion and centrifuged at 250000 × g for 3 hours at 4°C in a Beckman Coulter LX100 ultracentrifuge. After ultracentrifugation, the upper phase containing soluble proteins and the pellet containing VLPs were harvested separately. The pellet was resuspended in phosphate-buffered saline (PBS) (1/15 of the original supernatant volume).
Cross-linking of VP40/Sedimentation Assay
HEK 293T cells were transfected with phCMV-EBOV/VP40, and culture supernatants were harvested 16 hours after transfection. Supernatants were clarified by means of low-speed centrifugation and cross-linked using 2 mM DSP (Pierce) during 30 minutes with a subsequent quenching of the reaction by the addition of 100 mM Tris-HCL, pH 7.0. Cross-linked supernatants were loaded on a 20% sucrose cushion and ultracentrifuged as described above for VLP purification. Ultracentrifuged supernatants and the pellet containing VLPs were collected separately. The cell monolayer was cross-linked using a similar procedure. The cells were then lysed in coimmunoprecipitation (CO-IP) buffer (20 mM Tris-HCl, pH 7.0, 100 mM NaCl, 5 mM EDTA, 0.4% sodium deoxycholate, 1% Nonidet P40). Nuclei and cell debris were removed by means of low-speed centrifugation, and the resulting postnuclear supernatant was treated with 1% Triton X-100. The VLPs were resuspended in CO-IP buffer and then treated with Triton X-100 (1%). Ultracentrifugation supernatants, VLPs, and cell lysates were loaded onto 5%–25% sucrose linear gradients and ultracentrifuged during a period of 24 hours at 180 000 × g in a Beckman LX100. Fractions were collected from the bottom of the gradient using a fraction collector. Samples were analyzed by Western blot using anti-VP40 antibody.
Flotation Assay
HEK 293T cells were transfected with phCMV-EBOV/VP40. Culture supernatants and cells were collected separately 16 hours after transfection. Culture medium was clarified from cell debris by centrifugation at 1000 × g for 5 minutes and then adjusted to 40% sucrose wt/wt, loaded at the bottom of a tube, and layered with 30% sucrose and 10% sucrose solutions. The samples were centrifuged at 130000 × g for 20 hours in a Beckman LX100. Fractions were collected from the bottom to the top and analyzed by Western blot using anti-VP40 antibody.
Cytotoxicity Assay
Cytotoxicity was assayed using the LDH cytotoxicity detection kit (Clontech) following the manufacturer’s recommendations. Triton X-100 (1%) was used to determine 100% cell lysis.
The efficiency of transfection was approximately 70% and was verified by fluorescence-activated cell sorting (FACS) analysis using anti-VP40 antibodies. Propidium iodide/DiOC6 staining was performed following the manufacturer’s instructions. Briefly, HEK 293T cells were transfected with 1 μg of phCMV-EBOV/VP40 or phCMV-empty, and then cells were detached and incubated with DiOC6 (Invitrogen) at 40 nM for 20 minutes at 37°C, washed, and propidium iodide (Sigma-Aldrich) added just prior to analysis on a FACS Calibur (BD).
Stability of VP40 in the VLP Fraction
Culture supernatants were collected 14 hours after transfection with a plasmid expressing EBOV VP40 and low-speed clarified by centrifugation for 5 minutes at 1000 × g and then incubated for different intervals at 37°C. After incubation, the samples were loaded onto a 20% sucrose cushion and centrifuged at 250000 × g for 2 hours. The presence of VP40 in ultra-supernatants and pellet was analyzed by means of Western blot using anti-VP40 antibodies.
Ebola Virus Infections
Vero E6 or monocyte-derived dendritic cells were infected with EBOV at multiplicity of infection (MOI) 2 for 1 hour. After 2 days, supernatants were centrifuged at low speed (800 × g) and then loaded on 20% sucrose cushions and ultracentrifuged for 2 hours at 135000 × g and 4°C.
Guinea pigs were infected with 500 median tissue culture infective doses of recombinant guinea pig–adapted EBOV. This virus contains mutations in the gene encoding VP24 that provide a dramatic increase in EBOV pathogenicity for guinea pigs [11–13]. Animals were bled 6 days after infection and the serum samples subjected to ultracentrifugation as above. The pellet containing EBOV virions was resuspended in PBS (1/7 of original volume of the serum samples). Proteins from the pellet (virions) and ultra-supernatant were analyzed by Western blotting using anti-VP40, anti-sGP, and anti-NP antibodies. Research was conducted in compliance with European regulations, and protocols were approved by the Regional Committee on Ethics and Animal Experimentation (CREEA). Experiments using EBOV were performed in the Jean Merieux INSERM BSL-4 laboratory (Lyon, France).
RESULTS AND DISCUSSION
Release of EBOV VP40 Into the Culture Medium
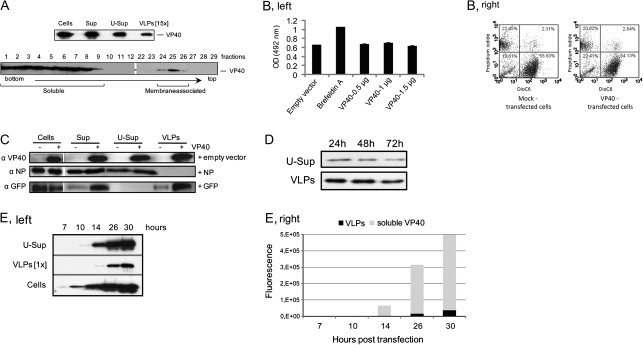
While analyzing transient expression of EBOV VP40 in HEK 293T cells, in particular, the subsequent release of VLPs containing VP40, we were puzzled by the observation that ultracentrifugation of culture medium yielded far less VP40 in the VLP form than the total amounts of this protein detected in the medium. It was of interest to clarify the nature of this phenomenon, because most publications related to VP40 VLP release experiments had been performed using a transient expression in HEK 293T cells but such an event had never been described. Surprisingly, we found that the “missing” VP40 was in the ultra-supernatant fraction, which presumably contains soluble proteins (Figure 1A, upper panel). Similar results were obtained when VP40 was transiently expressed in HuH7 cells (data not shown), suggesting that unconventional release of this protein may represent a particular VP40 property. To further investigate the characteristics of the protein present in the ultra-supernatant fraction, we performed a flotation assay (Figure 1A). Surprisingly, most of the VP40 was retained in the bottom fractions whereas relatively low amounts of the protein floated at the top of the gradient (fractions 24–26). Quantification of the VP40-containing fractions showed that only about 10% of the VP40 in the culture medium constituted the membrane-containing VLPs, whereas >90% represented non–membrane-associated, soluble VP40 (Figure 1A, bottom panel). Remarkably, both centrifugation assays showed approximately the same distribution between soluble and VLP-associated VP40 present in the culture medium. In our opinion, ascertainment of soluble VP40 presence in the culture medium of HEK 293T cells was underappreciated in the past, most likely because this form was always removed when harvesting VLPs in the sucrose cushion pelleting assay. To our knowledge, the article by Timmins et al [2] is the only publication that contains evidence of soluble VP40 in the culture medium of HEK 293T cells expressing VP40. However, quantification of soluble versus vesicular VP40 in the medium was not provided in this report, and the authors’ attention was mainly devoted to release of VP40 in vesicular form.
Figure 1.
Release of EBOV VP40 into the culture medium. A, Ultracentrifugation analysis (upper panel): 293T cells were transfected with phCMV-EBOV/VP40, and the culture medium was collected 14 h after transfection. After removal of cell debris by low-speed centrifugation, the culture medium was separated into ultra-supernatant and pellet. Samples of lysed cells (Cells), clarified culture medium (Sup), supernatant after ultracentrifugation (U-Sup), and pelleted materials (virus-like particles [VLPs]) were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) and analyzed by Western blot using anti-VP40 antibodies. The sample containing pelleted VLPs was resuspended in 1/15 of the original culture medium volume. Flotation analysis (bottom panel): gradient fractions were collected from the bottom to the top and analyzed by Western blotting using anti-VP40 antibodies. Fractions 1–10 represent soluble VP40 in 40% sucrose, fractions 11–25 represent 30% sucrose, and fractions 26–35 represent 10% sucrose. Membrane-associated VP40 was found at the interface of the 30% and 10% sucrose fractions. B, Cytotoxicity analysis. HEK 293T cells were transfected with increasing amounts of phCMV-EBOV/VP40 (VP40) or empty phCMV (Mock) and all with an equal amount of peGFP N1. Fourteen hours after transfection, cell-free culture supernatants were collected and analyzed using a LDH cytotoxicity detection kit (Clontech). For PI/DiOC6 analysis, cells were transfected in a similar manner but without adding peGFP N1. C, Corelease analysis. HEK 293T cells were transfected with phCMV-EBOV/VP40 and either empty plasmid, peGFP N1, or phCMV-EBOV/NP. VLPs, supernatant, ultra-supernatant, and cell lysates were prepared as described and loaded onto SDS-PAGE and analyzed by Western blot using anti-GFP, anti-NP, and anti-VP40 antibodies. D, Stability of VP40 in the VLP fraction. Culture supernatants from HEK 293T cells transfected with phCMV-EBOV/VP40 were clarified by low-speed centrifugation and then incubated for 24, 48, or 72 hours at 37°C. After incubation, all samples were separated for soluble (ultra-supernatant) and VLP fractions by ultracentrifugation. The presence of VP40 was analyzed by Western blot using anti-VP40 antibodies. E, Kinetics of VP40 release into the medium. HEK 293T cells were transfected with phCMV-EBOV/VP40 and samples of the cells and culture medium were harvested at different intervals after transfection. The samples of culture medium were subjected to ultracentrifugation and ultra-supernatant and pelleted fractions were analyzed by Western blotting using anti-VP40 antibodies (left panel). VLPs in the pellet were resuspended in phosphate-buffered saline (1/1 to original volume of culture medium). Quantitation of the VP40 bands on Western blot (right panel). VP40 in the VLP fraction and in the ultrasupernatant fraction (soluble VP40) are presented in comparison.
To clarify whether the release of soluble VP40 could be due to damage to cells caused by the protein’s expression, HEK 293T cells were transfected with either empty phCMV vector or increasing amounts of phCMV-EBOV/VP40. Fourteen hours after transfection, the cytotoxicity was assayed using the LDH-cytotoxicity-detection kit (Clontech) by measuring the release of the cytosolic enzyme LDH. Because the majority of the transfected cells expressed VP40 (>68%; data not shown) and because LDH release from the mock- and phCMV-EBOV/VP40-transfected cells was at comparable levels (Figure 1B, left panel), we conclude that VP40 expression does not lead to a loss of cell integrity during at least 14–20 hours after transfection. In addition, analysis of cell integrity by DiOC6/propidium iodide flow cytometry (Figure 1B, right panel) did not reveal any difference in apoptosis or the necrosis state between VP40- and mock-transfected cells. Previously, it had been shown that coexpression of EBOV NP and VP40 resulted in incorporation of both proteins into the VLPs [14], suggesting that the 2 proteins may interact during intracellular trafficking and/or VLP assembly. It was therefore of interest to investigate whether NP in a soluble form could be released when coexpressed with VP40. The 2 proteins were coexpressed or expressed separately in HEK 293T cells, and the culture medium and cells were analyzed as described above. As expected, NP appeared in the medium in the form of the VLPs after coexpression with VP40. However, we did not observe the release of NP in soluble form, confirming our conclusion on the absence of damage to cell integrity by VP40 expression (Figure 1C). Interestingly, under the same experimental conditions, GFP expressed with or without VP40 was also released in a soluble form, confirming earlier observations by Tanudji et al [15] that GFP can be released from cells by unconventional secretion.
Next, we investigated the possibility that soluble VP40 may originate from destabilization of VLPs after their release into the medium. The data obtained indicated that both VLPs and soluble VP40 are relatively stable during incubation at 37°C (Figure 1). In an attempt to clarify the mechanism of VP40 release in soluble form, we monitored protein expression over time. Samples of the culture medium were collected at 7, 10, 14, 26, and 30 hours after transfection, and soluble and membrane-associated VP40 were separated as described above. In order to quantify VP40 in different fractions, the VLPs were resuspended in the same volume as the initial amount of culture medium. Western blot analysis showed that soluble VP40 was detected as early as 10 hours after transfection whereas VLPs were detected later, most likely reflecting the difference in the amounts of VP40 released in the 2 different forms (Figure 1). Quantitation of VP40 showed that the proportions of soluble and vesicular protein remained approximately the same during the whole experiment (∼92% for soluble vs ∼8% for vesicular), suggesting the existence of a specific mechanism for the protein’s secretion.
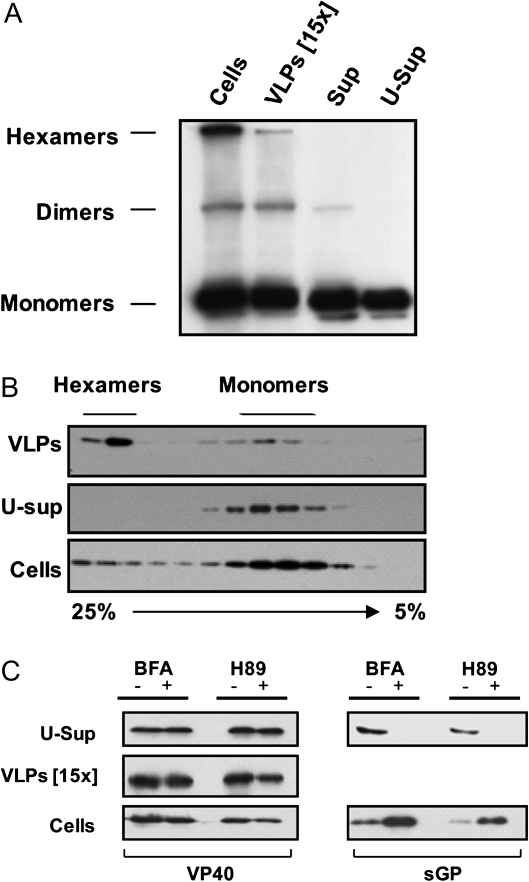
Vesicular release of VP40 (VLPs) is known to be associated with VP40’s interaction with membranes and its further oligomerization [4]. In order to identify the oligomeric state of soluble VP40, we used a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)/Western blot approach. As previously reported [5], oligomeric VP40 is partly resistant to SDS treatment under nonreducing conditions. Dimeric and hexameric forms of VP40 along with the monomeric protein were detected in samples from the cells, VLPs, and culture supernatants (Figure 2A). It seemed that ultracentrifugation of culture supernatants pelleted the oligomeric VP40, whereas in the ultra-supernatants, the predominantly monomeric VP40 was retained. Indeed, this observation correlates well with the non–membrane-associated nature of soluble VP40. To further confirm the monomeric structure of soluble VP40, we used the membrane-permeable reversible cross-linker DSP that preserved the oligomers from possible dissociation. HEK 293T cells were transfected with phCMV-EBOV/VP40, and the culture medium and cells were harvested 16 hours after transfection. Culture medium clarified by low-speed centrifugation and cells were treated with 2 mM of DSP following the manufacturer’s instructions. To separate VLPs from soluble VP40, the medium was subjected to ultracentrifugation as described earlier. Soluble VP40, VLPs, and cells were then solubilized in CO-IP buffer containing 1% Triton X-100 and subjected to sedimentation analysis using a linear 5%–25% sucrose gradient. Gradient fractions were collected from the bottom to the top and treated with 5% β-mercaptoethanol (MET) and 2% SDS to remove cross-linking. The Western blot analysis in Figure 2B confirms that VP40 in the VLP fraction is mostly present in the oligomeric form, whereas small amounts of monomeric VP40 in this fraction can be explained by contamination or partial instability of the VLPs caused by ultracentrifugation followed by pellet resuspension. Remarkably, the ultra-supernatant fraction contained only monomeric VP40, whereas both oligomeric forms of VP40 were found in the cell lysates. These results clearly confirm the existence of 2 distinct forms of VP40 in the medium.
Figure 2.
Characterization of soluble VP40. A, VP40 oligomeric forms. Culture supernatants (Sup) were collected 14 hours after transfection of cells with phCMV-EBOV/VP40. The supernatants were subjected to ultracentrifugation as previously described (Figure 1A), and then the samples containing either soluble proteins or pelleted virus-like particles (VLPs) were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) (8% polyacrylamide gel) under nonreducing conditions followed by Western blotting using anti-VP40 antibodies. Oligomeric forms of VP40 were identified according to molecular weight markers. B, Soluble VP40 is a monomer. Culture supernatants of 293T cells expressing EBOV VP40 were treated with DSP. Fractions representing soluble and membrane-associated VP40 (VLPs) were obtained by ultracentrifugation through a 20% sucrose cushion as above. VP40 fractions from the supernatant and cells pretreated with cross-linker were lysed in lysis buffer and then loaded onto a 5%–25% sucrose gradient. After ultracentrifugation, gradient fractions collected from the bottom to the top were analyzed by Western blotting using anti-VP40 antibodies. To remove cross-linking, the samples were treated with SDS-PAGE loading buffer (2% SDS, 5% β-MET). Fractions containing monomeric and oligomeric VP40 are indicated. C, VP40 is released by an unconventional secretion pathway. 293T cells were transfected with either phCMV-EBOV/VP40 or phCMV-EBOV/sGP. Fourteen hours after transfection, the cells were either left untreated or treated with protein transport inhibitors: 3.6 μM Brefeldin A (BFA) (BD, Golgi plug) or 50 μM H89. Cells and culture supernatants were collected after 7 hours of treatment. Culture supernatants were separated into soluble (ultra-supernatant [U-Sup]) and VLP fractions by ultracentrifugation as described above. Cells and culture supernatant fractions were lysed and the proteins separated by SDS-PAGE. Viral proteins present in the culture medium and the cells were analyzed by Western blotting using anti-VP40 and anti-sGP antibodies.
Classical secretion of cellular proteins from mammalian cells requires a signal peptide sequence and occurs via the endoplasmic reticulum (ER)–Golgi network. Although examination of the EBOV VP40 primary sequence reveals the absence of a classical signal-peptide sequence, that still does not exclude an involvement of these compartments in VP40 secretion. To investigate ER-Golgi dependence of VP40 release, we used 2 inhibitors: Brefeldin A (BFA) and H89. BFA targets ER-to-Golgi transport [16], whereas H89 is known to inhibit both trans-Golgi-network–to–cell-surface transport and ER-to-Golgi transport [17, 18]. HEK 293T cells were transfected with either phCMV-EBOV/VP40 or phCMV-EBOV/sGP, a plasmid encoding the soluble glycoprotein (sGP) of EBOV [19]. Fourteen hours after transfection, the cells were rinsed with fresh culture medium and incubated in the presence or absence of 3.6 μM BFA (BD, Golgi plug) or 50 μM H89 (Sigma-Aldrich) for 7 h. The phenomenon of sGP secretion occurs through the trans-Golgi network [20, 21], and the presence of both BFA and H89 completely abrogated sGP secretion and led to intracellular accumulation of the protein (Figure 2C, right panels). However, soluble VP40 release was insensitive to BFA and H89 (Figure 2A, left panels), reflecting the independence of VP40 secretion from ER-Golgi–related transport.
Interestingly, in silico analysis using Secretome P software (Technical University Denmark), a program designed to identify signal peptide–less secretory proteins [22], revealed that VP40 is predicted with fairly high probability to enter the unconventional secretory pathway. The molecular details of the mechanisms involved in unconventional or nonclassical secretion remain elusive, but there are a growing number of secreted proteins found to be released by this pathway. These include interleukin-1α, fibroblast growth factor (FGF) 1 and 2, Annexin 1 and 2, GFP, and a number of viral proteins [23–25]. Translocation across the plasma membrane of FGF-2 [26], for example, is believed to be linked to the existence of a plasma membrane transporter or an as yet unknown capacity to destabilize the plasma membrane [27]. Secretion of GFP in a nonfluorescent, presumably unfolded form represents another example of nonclassical secretion that has been explained to represent the capacity of cells to remove excess levels of certain proteins via an as yet unknown mechanism [15]. The release of VP40 could in some degree resemble the release of GFP, especially if it is considered that both proteins are of foreign origin and unlikely to have an importance for cell function. Future studies will need to address the mechanism of VP40 release.
Finally, it was of particular interest to investigate whether soluble VP40 is released during EBOV infection. Vero E6 and monocyte-derived dendritic cells were infected with EBOV at a MOI of 1 and the culture supernatants assayed for release of soluble VP40 using ultracentrifugation as previously described. In comparison with transient expression, it seemed that less VP40 was released in soluble form and substantially more VP40 was found in the pellet (Figure 3A). This result was somewhat expected, because in the viral context VP40 is heavily involved in assembly and the subsequent budding of virus particles. However, as a result of increases in the amounts of viral proteins in cells during infection, certain VP40 molecules may be surplus to their main function and thus could be released via the same mechanism as in the case of transient VP40 expression. We also investigated whether soluble VP40 is present in the serum of virus-infected animals. Guinea pigs were infected intraperitoneally with 500 plaque-forming units of guinea pig–adapted recombinant EBOV [28], and 6 days after infection, serum samples were subjected to ultracentrifugation through a 20% sucrose cushion. The pellet and ultra-supernatant were analyzed by Western blot for the presence of VP40, sGP, and NP. Both VP40 and soluble sGP were detected in the ultra-supernatant fraction of the serum samples, whereas NP was present only in the pellet containing viral particles (Figure 3). The work of Steele et al [29] provides support for our observations: Immunohistochemical analysis of tissues from EBOV-infected nonhuman primates and guinea pigs performed by these researchers showed that the epithelial cells of renal proximal convoluted tubules contained VP40 antigen but no other viral antigens or virus-specific RNA. The authors suggested that tubular epithelial cells might concentrate VP40 present in the blood. That VP40 is released in a soluble form from cells spreading throughout the body of infected animals seems very likely. It should be mentioned that, to this end, we cannot exclude the possibility that in addition to a secretion mechanism soluble VP40 could be released as a result of a cytopathic effect induced by virus infection [30]. However, the low presence of other structural proteins in soluble form in culture supernatants of EBOV-infected cells or in the serum of infected animals points to a particular property of VP40 and the existence of a specific mechanism for the release of this protein.
Figure 3.
Release of VP40 during Ebola virus (EBOV) infection. (A) Release of VP40 from virus-infected cells: 3 × 107 monocyte-derived dendritic or Vero E6 cells were infected with recombinant EBOV at a multiplicity of infection of 2, and the culture supernatants were collected 2 days after infection and subsequently subjected to ultracentrifugation through a 20% sucrose cushion. Cells, original culture supernatants (Sup) and ultra-supernatants (U-Sup), and the virus pellet (Virions) were resuspended in lysis buffer and analyzed by Western blotting using anti-VP40 and anti-NP antibodies. The samples representing the virus pellet are 10-fold concentrated in comparison with the original culture supernatants. (B) Release of VP40 during EBOV infection of guinea pigs. Guinea pigs were infected with 500 median tissue culture infective doses of recombinant guinea pig–adapted EBOV. Animals were bled 6 days after infection and the serum samples subjected to ultracentrifugation as above. The pellet containing EBOV virions was resuspended in phosphate-buffered saline (1/7 of original volume of the serum). Proteins from the pellet (Virions) and ultra-supernatant were analyzed by Western blotting using anti-VP40, anti-sGP, and anti-NP antibodies.
The importance of VP40 release in soluble form is currently difficult to predict. The early appearance of anti-VP40 antibodies in EBOV patients could be explained by the release of soluble VP40 [31, 32]. Future studies must provide further insight into what the consequences of the release on EBOV replication and pathogenesis are. Active virus replication in different cells and tissues may not be the only explanation for the immune and vascular dysregulation shadowing EBOV infection. There is a growing volume of evidence that severe EBOV pathogenesis can also be explained by massive and uncontrolled release of cellular mediators. In this regard, we hypothesize that release of soluble VP40 may contribute to an overall dysregulation of the host defense system, resulting in inadequate host responses causing the severe pathogenesis of EBOV infection.
Funding
This work was supported by INSERM, France, and grants from ANR-07-MIME-006-01, Deutsche Forschungsgemeinschaft (SFB 593), FRM DMI20091117323 and the National Institutes of Health AI059536 to C. F. B. and V. E. V.
Acknowledgments
We are grateful to Robin Buckland for helpful comments on the manuscript and Winfried Weissenhorn for providing mouse anti-VP40 monoclonal antibodies (9B2). All experiments involving live Ebola virus were carried out in the INSERM BSL-4 laboratory Jean Merieux in Lyon, France. We thank the biosafety team members for their assistance in conducting experiments with infected animals.
References
- 1.Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci U S A. 2000;97:13871–6. doi: 10.1073/pnas.250277297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmins J, Scianimanico S, Schoehn G, Weissenhorn W. Vesicular release of Ebola virus matrix protein VP40. Virology. 2001;283:1–6. doi: 10.1006/viro.2001.0860. [DOI] [PubMed] [Google Scholar]
- 3.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(suppl 2):S430–7. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 4.Ruigrok RW, Schoehn G, Dessen A, et al. Structural characterization and membrane binding properties of the matrix protein VP40 of Ebola virus. J Mol Biol. 2000;300:103–12. doi: 10.1006/jmbi.2000.3822. [DOI] [PubMed] [Google Scholar]
- 5.Scianimanico S, Schoehn G, Timmins J, Ruigrok RH, Klenk HD, Weissenhorn W. Membrane association induces a conformational change in the Ebola virus matrix protein. Embo J. 2000;19:6732–41. doi: 10.1093/emboj/19.24.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoenen T, Volchkov V, Kolesnikova L, et al. VP40 octamers are essential for Ebola virus replication. J Virol. 2005;79:1898–905. doi: 10.1128/JVI.79.3.1898-1905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licata JM, Simpson-Holley M, Wright NT, Han Z, Paragas J, Harty RN. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J Virol. 2003;77:1812–9. doi: 10.1128/JVI.77.3.1812-1819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmins J, Schoehn G, Ricard-Blum S, et al. Ebola virus matrix protein VP40 interaction with human cellular factors Tsg101 and Nedd4. J Mol Biol. 2003;326:493–502. doi: 10.1016/s0022-2836(02)01406-7. [DOI] [PubMed] [Google Scholar]
- 9.Yamayoshi S, Noda T, Ebihara H, et al. Ebola virus matrix protein VP40 uses the COPII transport system for its intracellular transport. Cell Host Microbe. 2008;3:168–77. doi: 10.1016/j.chom.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basler CF, Wang X, Muhlberger E, et al. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci U S A. 2000;97:12289–94. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prins KC, Delpeut S, Leung DW, et al. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J Virol. 2010;84:3004–15. doi: 10.1128/JVI.02459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateo M, Carbonnelle C, Reynard O, et al. VP24 is a molecular determinant of virulence in guinea-pigs. J Infect Dis. 2011;204(Suppl 3):S1019–29. doi: 10.1093/infdis/jir338. [DOI] [PubMed] [Google Scholar]
- 13.Reynard O, Mokhonov V, Mokhonova E, et al. Kunjin virus replicon-based vaccines expressing Ebola virus glycoprotein GP protect the guinea pig against lethal Ebola virus infection. J Infect Dis. 2011;204(Suppl 3):S1068–73. doi: 10.1093/infdis/jir347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noda T, Watanabe S, Sagara H, Kawaoka Y. Mapping of the VP40-binding regions of the nucleoprotein of Ebola virus. J Virol. 2007;81:3554–62. doi: 10.1128/JVI.02183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanudji M, Hevi S, Chuck SL. Improperly folded green fluorescent protein is secreted via a non-classical pathway. J Cell Sci. 2002;115:3849–57. doi: 10.1242/jcs.00047. [DOI] [PubMed] [Google Scholar]
- 16.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–80. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muniz M, Alonso M, Hidalgo J, Velasco A. A regulatory role for cAMP-dependent protein kinase in protein traffic along the exocytic route. J Biol Chem. 1996;271:30935–41. doi: 10.1074/jbc.271.48.30935. [DOI] [PubMed] [Google Scholar]
- 18.Muniz M, Martin ME, Hidalgo J, Velasco A. Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc Natl Acad Sci U S A. 1997;94:14461–6. doi: 10.1073/pnas.94.26.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volchkov VE, Volchkova VA, Slenczka W, Klenk HD, Feldmann H. Release of viral glycoproteins during Ebola virus infection. Virology. 1998;245:110–9. doi: 10.1006/viro.1998.9143. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez A, Yang ZY, Xu L, Nabel GJ, Crews T, Peters CJ. Biochemical analysis of the secreted and virion glycoproteins of Ebola virus. J Virol. 1998;72:6442–7. doi: 10.1128/jvi.72.8.6442-6447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volchkova VA, Klenk HD, Volchkov VE. Delta-peptide is the carboxy-terminal cleavage fragment of the nonstructural small glycoprotein sGP of Ebola virus. Virology. 1999;265:164–71. doi: 10.1006/viro.1999.0034. [DOI] [PubMed] [Google Scholar]
- 22.Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng Des Sel. 2004;17:349–56. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- 23.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–33. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein G. HIV-1 Tat protein as a potential AIDS vaccine. Nat Med. 1996;2:960–4. doi: 10.1038/nm0996-960. [DOI] [PubMed] [Google Scholar]
- 25.Lecellier CH, Vermeulen W, Bachelerie F, Giron ML, Saib A. Intra- and intercellular trafficking of the foamy virus auxiliary Bet protein. J Virol. 2002;76:3388–94. doi: 10.1128/JVI.76.7.3388-3394.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickel W. Unconventional secretion: an extracellular trap for export of fibroblast growth factor 2. J Cell Sci. 2007;120:2295–9. doi: 10.1242/jcs.011080. [DOI] [PubMed] [Google Scholar]
- 27.Temmerman K, Ebert AD, Muller HM, Sinning I, Tews I, Nickel W. A direct role for phosphatidylinositol-4,5-bisphosphate in unconventional secretion of fibroblast growth factor 2. Traffic. 2008;9:1204–17. doi: 10.1111/j.1600-0854.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- 28.Volchkov VE, Chepurnov AA, Volchkova VA, Ternovoj VA, Klenk HD. Molecular characterization of guinea pig-adapted variants of Ebola virus. Virology. 2000;277:147–55. doi: 10.1006/viro.2000.0572. [DOI] [PubMed] [Google Scholar]
- 29.Steele K, Crise B, Kuehne A, Kell W. Ebola virus glycoprotein demonstrates differential cellular localization in infected cell types of nonhuman primates and guinea pigs. Arch Pathol Lab Med. 2001;125:625–30. doi: 10.5858/2001-125-0625-EVGDDC. [DOI] [PubMed] [Google Scholar]
- 30.Ryabchikova EI, Kolesnikova LV, Netesov SV. Animal pathology of filoviral infections. Curr Top Microbiol Immunol. 1999;235:145–73. doi: 10.1007/978-3-642-59949-1_9. [DOI] [PubMed] [Google Scholar]
- 31.Leroy EM, Baize S, Debre P, Lansoud-Soukate J, Mavoungou E. Early immune responses accompanying human asymptomatic Ebola infections. Clin Exp Immunol. 2001;124:453–60. doi: 10.1046/j.1365-2249.2001.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baize S, Leroy EM, Georges-Courbot MC, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–6. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]