Abstract
Infection with Ebola virus (EBOV) causes hemorrhagic fever in humans with high case-fatality rates. The EBOV-glycoprotein (EBOV-GP) facilitates viral entry and promotes viral release from human cells. African fruit bats are believed not to develop disease upon EBOV infection and have been proposed as a natural reservoir of EBOV. We compared EBOV-GP interactions with human cells and cells from African fruit bats. We found that susceptibility to EBOV-GP–dependent infection was not limited to bat cells from potential reservoir species, and we observed that GP displayed similar biological properties in human and bat cells. The only exception was GP localization, which was to a greater extent intracellular in bat cells as compared to human cells. Collectively, our results suggest that GP interactions with fruit bat and human cells are similar and do not limit EBOV tropism for certain bat species.
Ebola virus (EBOV), a negative-stranded RNA virus in the family Filoviridae, can cause hemorrhagic fever in humans. Ebola viruses are subdivided into 4 species, Zaire ebolavirus (ZEBOV), Sudan ebolavirus (SEBOV), Côte d’Ivoire ebolavirus (CIEBOV), and Reston ebolavirus (REBOV) [1, 2]. Bundibugyo ebolavirus (BEBOV) has been proposed as a fifth species [3]. Outbreaks of ZEBOV, SEBOV, and BEBOV hemorrhagic fever occur sporadically in Africa and are associated with case-fatality rates of up to 90% [2, 4]. REBOV is an Asian EBOV that might not be pathogenic for humans but induces hemorrhagic fever in experimentally infected macaques [2, 5].
The glycoprotein (GP) of EBOV mediates viral entry into host cells. EBOV exhibits a broad cell tropism [1, 6–8], but the cellular receptor(s) used by GP for viral entry is unknown. Cleavage of GP by cellular proteases upon viral uptake into target cells is thought to be essential for infectious viral entry [9], whereas the functional relevance of GP cleavage by subtilisin-like proteases in the secretory pathway of infected cells is unclear [10, 11]. Notably, GP has functions beyond mediating viral entry. Thus, EBOV-GP augments budding of viral particles [12, 13], which is driven by the viral matrix protein, VP40 [14, 15]. In addition, ZEBOV-GP has been shown to counteract the interferon-induced antiviral factor tetherin, which otherwise inhibits release of VP40-based virus-like particles [16]. Finally, GP interferes with the expression of cellular adhesion molecules and thereby induces detachment of cells from culture flasks [17]. It has been proposed that this activity could contribute to EBOV pathogenesis [17, 18], but this hypothesis has been refuted [19].
EBOV is transmitted from an animal reservoir, most likely bats, to humans or other end hosts, such as gorillas [20], either directly or via an intermediate host. EBOV sequences or EBOV-specific antibodies have been detected in African fruit bats, specifically in the species Hypsignathus monstrosus, Epomops franqueti, and Myonycteris torquata, and it has been suggested that these animals might constitute the natural reservoir of EBOV [21]. In addition, antibodies against EBOV were detected in Eidolon helvum and other fruit bat species [22, 23]. Experimental and natural infection did not seem to induce apparent signs of disease [22, 24], indicating that EBOV might be nonpathogenic in potential reservoir species, likely because of virus-host adaptation.
Here we examine and compare the in vitro interactions of EBOV-GP with human cells with the interactions with cells from African fruit bats. Specifically, we tested the hypotheses that susceptibility to EBOV infection of bat species might be determined at the stage of viral entry and that EBOV-GP interacts differentially with human and bat cells, thereby contributing to the differential pathogenicity of EBOV in these species. Such a scenario would not be unparalleled, considering that interactions of the severe acute respiratory syndrome (SARS)–coronavirus spike-protein with its receptor, ACE2, are thought to be a major determinant of the viral cell and species tropism and of viral pathogenicity [25]. We found that GP generally displayed similar biological properties in human and bat cells. In addition, we observed efficient ZEBOV-GP–mediated infectious entry into bat cells from potential reservoir and nonreservoir species, indicating that susceptibility of bat species to EBOV infection is not determined by GP-receptor interactions.
METHODS
Cell Culture
Cultivation and transfection of 293T, HeLa, BHK-21, and Vero E6 cells has been described elsewhere [26, 27]. Bats were caught in Ghana and Germany under valid licenses from the Ghanaian Forestry Commission and Veterinary Services Directorate, as well as the German Ministry of the Environment. Animals were typed morphologically and genetically as described by Vallo et al [28]. Cell lines used in this study stemmed from the African fruit bats Epomops buettikoferi (cell line EpoNi/22.1, adult kidney-derived), H. monstrosus (cell line HypNi/1.1, fetal kidney derived; cell line HypLu/45.1, fetal lung derived), and Rousettus aegyptiacus (cell line RoNi/7.1, adult kidney derived), as well as the European insectivorous bat Myotis daubentonii (cell line MyDauLu/47.1, adult lung-derived). For the generation of these cell lines, primary cells were cultured, immortalized, and cloned essentially as described [29].
Plasmids
The pcDNA6 plasmids expressing SEBOV-GP and ZEBOV-GP have been described elsewhere [17]. The pcDNA3.1 plasmids encoding CIEBOV-GP and REBOV-GP were generated using the restriction sites HindII and XbaI. Note that pcDNA6 and pcDNA3.1 share the same promoter. The pCAGGS plasmids [30] encoding EBOV-GPs with a C-terminal V5-tag were generated using the following restriction sites: SEBOV-GP and ZEBOV-GP (KpnI, NheI), CIEBOV-GP (EcoRI, NheI), REBOV-GP (EcoRI, XhoI). The plasmids encoding EYFP fused to the C-terminus of ZEBOV- and REBOV-GP were generated using pEYFP-N1 [31] and the NheI, AgeI and XhoI, SacII restriction sites, respectively. The p96ZM651gag-opt plasmid encoding human immunodeficiency virus type 1 Gag (p55) has been described elsewhere [32]. The pCR3.1 plasmids encoding VP40-c-myc, VP40-EGFP, and human tetherin have been described elsewhere [33, 34]. The plasmid encoding for replication-incompetent recombinant vesicular stomatitis virus (VSV*ΔG-Luc) was generated by replacing the gene encoding the glycoprotein (VSV-G) with the gene encoding enhanced green fluorescent protein (EGFP) followed by the gene encoding firefly luciferase.
Antibodies
An anti-p24 hybridoma supernatant (183-H12-5C) was used for p55-Gag detection [35]. For detection of V5-tagged EBOV-GP, a mouse anti-V5 antibody (Invitrogen) was used. A mouse anti-c-myc antibody (Biomol) was used to detect VP40-c-myc. Secondary antibodies were purchased from Dianova. For loading controls, anti–β-actin antibodies (Sigma) were used.
Analysis of the Release of Virus-like Particles
To analyze the effect of EBOV-GPs on VP40-driven release of virus-like particles, 293T and EpoNi/22.1 cells were transfected with plasmids expressing VP40-c-myc and EBOV-GPs at a ratio of 1:1 (3 μg each). Then, 48 hours after transfection, supernatants were cleared from debris by centrifugation and subsequently concentrated through a 20% sucrose cushion by centrifugation at 21 000 × g for 2 hours. Concentrated supernatants and pellets from virus-like particle–producer cells were harvested in sodium dodecyl sulfate–loading buffer and analyzed by Western blotting.
Flow Cytometry
The number of cells in the supernatant of 293T or EpoNi/22.1 cells transfected with the different EBOV-GPs was counted using a Cytomics FC500 flow cytometer (Beckman Coulter). For this, the supernatant of 1 well of a 12-well plate (1 mL) was harvested and concentrated to a volume of 200 μL. Cells were then washed, and each sample was measured for the same time period (110 sec) in the “high flow” setting.
Fluorescence Microscopy
HeLa, EpoNi/22.1, and MyDauLu/47.1 cells were transfected with plasmids expressing ZEBOV- and REBOV-GP-EYFP or VP40-EGFP fusion protein. Then, 48 hours after transfection, cells were fixed with 4% paraformaldehyde and washed with 1 × phosphate-buffered solution, and nuclei were counterstained with 4',6-diamidino-2-phenylindole. Images were acquired using the Zeiss Observer Z1 microscope.
Production of Pseudotypes
For preparation of VSV pseudotypes, BHK-21 cells were transfected with EBOV-GP or VSV-G expression plasmids or empty vector, washed, and infected with a previously described replication-deficient VSV, complemented with VSV-G [27] at a multiplicity of infection (MOI) of 3. After 1 hour the inoculum was removed, the cells were washed, and residual helper virus was neutralized with a polyclonal rabbit anti-VSV serum. Subsequently, the cells were washed again and fresh medium was added. At 24 hour after infection, the supernatant, containing VSV pseudotypes, was collected and clarified by centrifugation at 600 × g. To normalize the different VSV pseudotypes to comparable titers, Vero E6 cells were infected with 10-fold serial dilutions of the clarified supernatants. Infection efficiency was monitored by assessing luciferase and GFP expression, and the different pseudotypes were subsequently diluted in minimum essential medium to reach the luciferase activity of the least infectious construct.
ZEBOV Infection Experiments
All cell lines were seeded in 6-well plates and infected in duplicate with ZEBOV (Kikwit strain) at an MOI of 0.1 for 1 hour. Subsequently, cells were washed twice with medium, 3 mL of fresh medium was added, and the cells were incubated at 37°C. Samples (250 μL) were taken at various time points after inoculation, and the removed medium was replaced by an equal amount of fresh medium. ZEBOV titers were determined by means of endpoint titration in Vero E6 cells, essentially as described [36]. All work with infectious ZEBOV was performed in the high-containment facility (BSL4) at the Integrated Research Facility, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, in Hamilton, Montana.
RESULTS
The Ebola Virus Glycoprotein Is Efficiently Cleaved in Human and Bat Cells
The EBOV-GP is synthesized as a precursor protein, GP0, which is cleaved by subtilisin-like pro-protein convertases in the secretory pathway of infected cells [37]. We compared cleavage of ZEBOV-, SEBOV-, CIEBOV-, and REBOV-GP in the human embryonic kidney cell line 293T and in EpoNi/22.1 kidney cells derived from E. buettikoferi, a member of the same bat genus as the potential EBOV reservoir species E. franqueti (the EpoNi/22.1 cell line was chosen because of reasonable transfection efficiency, compared with other bat cell lines tested). For this, we transfected the cell lines with expression plasmids encoding EBOV-GPs equipped with a C-terminal V5-tag and assessed GP cleavage by Western blot. If GP is efficiently cleaved, mainly the transmembrane unit GP2 should be detected, whereas if cleavage is inefficient, mainly the precursor protein GP0 should be detected. Expression of all GPs was detected in transfected 293T and EpoNi/22.1 cells (Figure 1), although expression in the latter cells was less efficient, as a result of reduced transfection efficiency (70%–80% for 293T cells vs 20%–30% for EpoNi/22.1 cells). Expression efficiency among GPs varied, with ZEBOV-GP and SEBOV-GP being more robustly expressed than CIEBOV-GP and REBOV-GP (Figure 1). All GPs were cleaved in 293T and EpoNi/22.1 cells, and no difference in cleavage efficiency was apparent between these cell lines. However, marked differences in cleavage efficiency were observed between EBOV-GPs. Thus, ZEBOV- and SEBOV-GP were almost fully cleaved, whereas a large proportion of CIEBOV- and particularly REBOV-GP remained uncleaved (Figure 1). In sum, GP cleavage does not seem to differ between the tested bat and human cells and only the GPs derived from ZEBOV and SEBOV are cleaved efficiently.
Figure 1.
Cleavage of Ebola virus glycoproteins (EBOV-GPs) by subtilisin-like proteases in human and bat cells. We transfected 293T cells and EpoNi/22.1 cells with pCAGGS plasmids encoding the indicated V5-tagged EBOV-GPs or empty pCAGGS plasmid (Mock). Cells were harvested at 48 h after transfection. EBOV-GP expression in cell lysates was determined using anti-V5 antibody. Detection of β-actin served as loading control. Similar results were obtained in 3 independent experiments and with Hela, HypNi/1.1, and MyDauLu/47.1 cells. CIEBOV, Côte d’Ivoire ebolavirus; REBOV, Reston ebolavirus; SEBOV, Sudan ebolavirus; ZEBOV, Zaire ebolavirus.
The EBOV Glycoprotein Augments Particle Release in Human and in Bat Cells
The matrix protein VP40 of EBOV drives assembly and egress of progeny particles from infected cells [14, 15]. Expression of VP40 alone is sufficient to induce release of virus-like particles, and release can be augmented by coexpression of GP [12–15]. We investigated whether GP enhanced the release of VP40 from human 293T cells and bat EpoNi/22.1 cells. Expression and release of VP40 was detectable in both 293T and EpoNi/22.1 cells. The generation of 2 forms of VP40 was likely due to use of an internal AUG codon in the VP40 open reading frame [15]. Coexpression of VP40 with EBOV-GPs augmented the release of VP40 relative to that in cells transfected with empty plasmid instead of a GP-encoding plasmid (Mock), despite the presence of comparable amounts of GP in cell lysates (Figure 2). Thus, GP might augment particle release in infected humans and bats, and the ability to augment release seems to be conserved between the GPs of all EBOV species.
Figure 2.
Ebola virus glycoproteins (EBOV-GPs) enhance budding of VP40-based virus-like particles (VLPs) in human and bat cells. We cotransfected 293T and EpoNi/22.1 cells with a VP40-c-myc expression plasmid and the indicated EBOV-GP-V5 expression plasmids or empty vector (Mock). Expression of VP40 in cell lysates and supernatants was detected using an anti-c-myc antibody. Detection of β-actin served as loading control. Similar results were obtained in 3 independent experiments. CIEBOV, Côte d’Ivoire ebolavirus; REBOV, Reston ebolavirus; SEBOV, Sudan ebolavirus; ZEBOV, Zaire ebolavirus.
Ebola Virus Glycoproteins Induce Detachment of Human and Bat Cells
Expression of EBOV-GPs was shown to induce rounding and detachment of human cells from culture dishes [17, 18]. The ability to induce cell rounding and detachment correlated with the viral pathogenicity in humans, with ZEBOV- and SEBOV-GP but not REBOV-GP being able to efficiently trigger rounding of GP-expressing cells [18]. We assessed whether GP expression induced detachment of 293T and EpoNi/22.1 cells. Quantification of cells present in the supernatant of transfected cultures showed that ZEBOV-GP, SEBOV-GP, and REBOV-GP were able to induce detachment of 293T cells, whereas CIEBOV-GP was inactive (Figure 3A). In EpoNi/22.1 cells, detachment was observed upon expression of all GPs tested, with ZEBOV-GP being most active (Figure 3B). Because the results obtained for 293T cells did not match those previously published [17], we asked whether differences in GP expression levels might be the cause. To investigate this, we cloned all EBOV-GP sequences in commonly used pcDNA plasmids and compared GP expression by pcDNA plasmids (used in the previous study) with expression by pCAGGS plasmids [30], which have been used throughout the present study. Expression of REBOV-GP and CIEBOV-GP upon transfection of pcDNA plasmids was barely detectable, whereas expression of these GPs was readily detected in cells transfected with pCAGGS plasmids (Figure 3C). Notably, when cell detachment was analyzed with the pcDNA plasmids containing GP, ZEBOV-GP and SEBOV-GP were active whereas REBOV-GP was not (Figure 3D), in agreement with published data [17, 18]. These results show that GP-induced cellular detachment does not differ markedly between human and bat cells. In addition, our findings suggest that previously noted differences between the ability of ZEBOV- and REBOV-GP to induce cell rounding might have been due to differences in expression levels.
Figure 3.
Expression of Ebola virus glycoproteins (EBOV-GPs) induces detachment of human and bat cells. A, 293T cells were transfected with pCAGGS plasmids encoding EBOV-GP-V5 or empty pCAGGS plasmid (Mock), and the number of cells in the culture supernatant was counted at 48 hours after transfection via fluorescence-activated cell sorting. The mean of 12 independent experiments is shown. Error bars indicate standard error of the mean (SEM). B, EpoNi/22.1 cells were transfected with pCAGGS plasmids encoding EBOV-GP-V5, and the number of cells in the supernatant was counted as described in A. The mean of 11 independent experiments is shown. Error bars indicate SEM. C, 293T cells were transfected with pCAGGS or pcDNA plasmids encoding EBOV-GPs with C-terminal V5 tag and treated as in Figure 1. D, 293T cells were transfected with pcDNA plasmids encoding EBOV-GP-V5, and the number of cells in the supernatant was counted as in A. The mean of 11 independent experiments is shown. Error bars indicate SEM. Asterisks indicate statistically significant differences (P < .05) relative to mock as determined by means of 2-tailed Student t test. CIEBOV, Côte d’Ivoire ebolavirus; REBOV, Reston ebolavirus; SEBOV, Sudan ebolavirus; ZEBOV, Zaire ebolavirus.
Plasma Membrane Localization of Ebola Virus Glycoprotein Is More Prominent in Human Compared With Bat Cells
Production of progeny particles requires that EBOV-GP and VP40 be transported to the site of viral budding, the plasma membrane. We compared the localization of these proteins in transfected HeLa cells, a human cervix carcinoma cell line particularly suitable for immunofluorescence studies, and in EpoNi/22.1 cells. In HeLa cells, ZEBOV-GP and REBOV-GP were mainly located at the cell surface and to a lesser extent within intracellular compartments (Figure 4A). GP was also detected at the cell surface of EpoNi/22.1 cells, but intracellular localization was more prominent, compared with that in HeLa cells (Figure 4). In addition, accumulation of GP in intracellular compartments was frequently detected in EpoNi/22.1 cells (Figure 4). However, this phenotype was not unique to EpoNi/22.1 cells but was also observed for potential reservoir cells, generated from H. monstrosus, and for nonreservoir cells, generated from M. daubentonii (Figure 4B). Finally, VP40 was found at the plasma membrane and distributed in the cellular cytoplasm in all cell lines tested (Figures 4A and 4B). Collectively, there were no substantial differences in VP40 localization in human and reservoir cells, whereas plasma membrane localization of GP was more prominent in human than in reservoir cells.
Figure 4.
Plasma membrane localization of Ebola virus glycoprotein (EBOV-GP) is more prominent in human than in bat cells. A, HeLa and EpoNi/22.1 cells were transfected with the indicated EBOV-GP-EYFP or VP40–enhanced green fluorescent protein expression plasmids. Cells were fixed at 48 hours after transfection, and cellular distribution of GP and VP40 was determined by fluorescence microscopy. Similar results were obtained in 3 independent experiments. White scale bar represents 20 μm. B, EpoNi/22.1, MyDauLu/47.1, and HypNi1.1 cells were transfected and analyzed as in A. Similar results were obtained in 2 independent experiments. White scale bar represents 20 μm. REBOV, Reston ebolavirus; ZEBOV, Zaire ebolavirus.
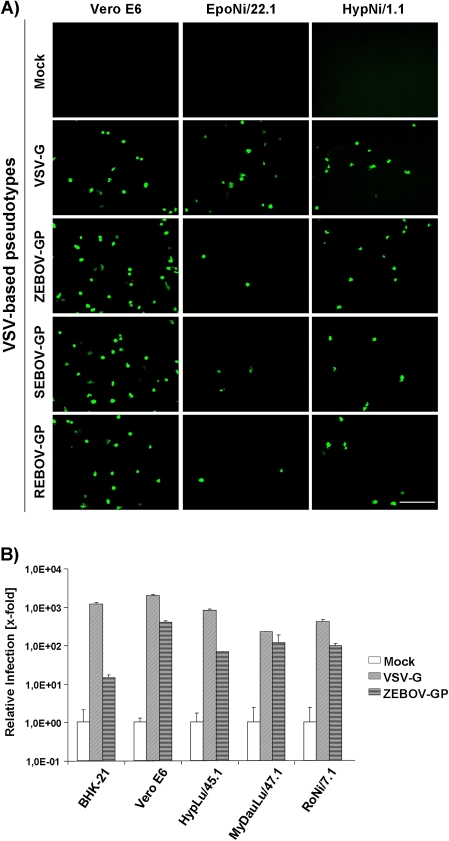
Zaire ebolavirus Glycoprotein Mediates Entry Into Cells From Potential Reservoir and Nonreservoir Bat Species
EBOV nucleic acid was found in 3 African bat species but not in several others, suggesting that only certain bats are susceptible to filovirus infection. Using VSV-based pseudotypes bearing EBOV-GPs, we assessed whether susceptibility of bats to filovirus infection may be restricted at the stage of viral entry. For this, infectivity-normalized VSV pseudotypes bearing VSV-G or EBOV-GPs were used for infection of bat cell lines. Pseudotypes bearing neither VSV-G nor EBOV-GP served as negative controls. Infectious entry of VSV-G–bearing control pseudotypes into all cell lines tested was readily measurable. ZEBOV-, SEBOV-, and REBOV-GP–driven infection of EpoNi/22.1 and HypNi/1.1 cells was also detected (Figure 5A). The latter were obtained from the potential reservoir species H. monstrosus. In general, the infectivity of pseudotypes was relatively low, and we suspected that the C-terminal V5 tag might be incompatible with efficient GP incorporation into VSV particles. We therefore used VSV pseudotypes bearing untagged ZEBOV-GP to clarify whether susceptibility to ZEBOV-GP–dependent entry is a characteristic of cells from reservoir species. These pseudotypes were indeed more infectious for the control cell lines Vero E6 and BHK-21 than were their counterparts with a V5-tag (data not shown), and infection of cells derived from the potential reservoir species H. monstrosus and R. aegyptiacus [23] and nonreservoir bat species M. daubentonii was readily detectable (Figure 5B), indicating that receptor-GP interactions might not limit the susceptibility of bat species to EBOV infection.
Figure 5.
The glycoproteins (GPs) of the Ebola virus (EBOV) species Zaire, Sudan, and Reston mediate entry into potential reservoir and nonreservoir cells. A, The indicated cell lines were infected with vesicular stomatitis virus (VSV)–based pseudotypes bearing either VSV-G or EBOV-GPs as envelope proteins. The different pseudotypes were normalized to comparable titers on Vero E6 cells prior to infection of bat cells. Pseudotypes bearing no GPs were used as negative control (mock) and not normalized. Infectivity was determined by microscopy, detecting green fluorescent protein signals of infected cells. One representative experiment of 3 independent experiments is shown. White scale bar represents 200 μm. B, The indicated cell lines were infected as in A. Infectivity was measured by luciferase readout. The signals obtained for samples infected with pseudotypes bearing no GP (mock) were set as 1. Infection was performed in duplicate. One representative experiment of 3 independent experiments is shown. Error bars indicate standard deviation (SD). REBOV, Reston ebolavirus; SEBOV, Sudan ebolavirus; ZEBOV, Zaire ebolavirus.
Zaire ebolavirus Replicates in Bat Cells With High Efficiency
Finally, we investigated whether the EpoNi/22.1 and HypNi/1.1 cells, which were susceptible to infection with EBOV-GP–bearing pseudotypes (Figure 6), allowed replication of infectious ZEBOV. The African green monkey–derived cell line Vero E6, which is routinely used to replicate filoviruses, was included as positive control. For this, cells were inoculated with ZEBOV and washed, and the median tissue culture infective dose of supernatants was determined at different points after infection. The virus replicated with high efficiency and similar kinetics in EpoNi/22.1 and HypNi/1.1 cells, and replication in these cell lines was faster, compared with that in Vero E6 cells. Thus, EpoNi/22.1 and HypNi/1.1 are highly permissive to EBOV infection and can be used as models for the analysis of EBOV interactions with cells derived from reservoir species.
Figure 6.
Efficient replication of Ebola virus in EpoNi/22.1 and HypNi/1.1 cells. The indicated cell lines were seeded in 6-well plates; infected in duplicate with Zaire Ebola virus (ZEBOV), Kikwit strain (multiplicity of infection of 0.1); washed; and cultured. Samples were taken at the indicated time points after inoculation, and their titers were determined by endpoint titration in Vero E6 cells, using the development of cytopathic effects as readout (TCID50, tissue culture infectious dose that leads to 50% cytopathic effects). Infectious titers were calculated from 3 replicates by the method of Spearman-Karber. The results represent the geometric mean titers obtained from 2 independent experiments. Error bars indicate standard deviation.
DISCUSSION
We assessed whether biological processes associated with EBOV-GP expression in human cells also proceed in cells from African fruit bats, the potential reservoir of EBOV. We found that most EBOV-GP–induced processes occurred in human and potential reservoir cells with comparable efficiency. In addition, we found that reservoir and nonreservoir bat cells were susceptible to EBOV-GP–driven infectious cellular entry, indicating that the susceptibility of bat species to EBOV infection is not determined at the stage of viral entry. Collectively, these findings indicate that GP interactions with human cells and cells from potential reservoir species do not differ fundamentally.
Proteolytic activation of viral GPs by host cell proteases is often essential for infectious viral entry. The EBOV-GP has been shown to be activated by endosomal proteases, cathepsins B and L, during host cell entry [9]. In addition, EBOV-GP was found to be cleaved in the secretory pathway of transfected or infected cells by furin [37], a subtilisin-like endoprotease. In contrast to GP cleavage by cathepsins, GP processing by furin was found to be dispensable for viral spread in cell culture [10] and in animals [11], and the biological relevance of GP cleavage by furin is at present unclear. The EBOV-GPs tested here were cleaved in human and bat cells with similar efficiency, indicating that a bat homolog of furin processes EBOV-GP with efficiency comparable to that of its human counterpart. However, processing of the GPs of ZEBOV and SEBOV was clearly more efficient than cleavage of the GPs of CIEBOV and REBOV. The decreased cleavability of REBOV-GP was expected, because 2 arginines present in the furin consensus site of ZEBOV-, SEBOV-, and CIEBOV-GP are replaced by lysines in REBOV-GP [37]. However, similar substitutions are not present in CIEBOV-GP, and the reasons for reduced cleavage are at present unclear.
Similar to the findings for GP cleavage, no profound differences in GP and VP40 localization in bat and human cells were observed. However, localization of GP at the plasma membrane, the site of viral budding [38], was less pronounced in bat cells than in human cells, and accumulation of GP in intracellular compartments was more frequently observed in bat cells than in human cells. However, intracellular accumulation was observed in potential reservoir and nonreservoir bat cells and may thus not affect the viral host range.
The ability of EBOV-GP to augment budding driven by VP40 and to induce detachment of GP-expressing cells was also conserved between human and bat cells. Notably, the latter process was dependent on GP expression levels. Thus, when conditions of high GP expression were chosen (GP sequences contained in pCAGGS; Figure 1), all EBOV-GPs induced cellular detachment with similar efficiency in human and reservoir cells. In contrast, when GP was inserted in the commonly used plasmids pcDNA3.1 and pcDNA6, which was used in a previous analysis of GP-induced cellular detachment [17], expression of CIEBOV- and REBOV-GP was barely detectable and both proteins were unable to induce cellular detachment. These findings highlight that cytopathogenic effects of GP leading to cell detachment occur only upon high expression, in agreement with results reported by a previous study [39]. In addition, the comparable GP-dependent detachment of human and bat cells suggests that this process might not play a major role in EBOV pathogenesis.
The antiviral host cell factor tetherin inhibits the release of VP40-based virus-like particles [40] and is counteracted by ZEBOV-GP [16]. It can be speculated that tetherin could inhibit EBOV spread in the host and, in agreement with this hypothesis, we found that tetherin counteraction was conserved among all EBOV-GPs tested (Supplementary Figure 1). Antagonism of human tetherin by EBOV-GPs was much more pronounced in human, compared with bat, cells (Supplementary Figure 1), suggesting that the latter might lack a cofactor required for tetherin counteraction by EBOV-GP. Whether tetherin homologs are expressed in bat cells and exhibit antiviral function remains to be examined.
Sequences of EBOV have so far been detected in only 3 fruit bat species, H. monstrosus, E. franqueti, and M. torquata. Several parameters might determine permissiveness of bats to EBOV infection, one being GP interactions with receptors on the surface of host cells. Our results suggest that ZEBOV-, SEBOV-, and REBOV-GP can mediate entry into cells from potential reservoir species, which is remarkable for REBOV, because the virus should be adapted to Asian reservoir hosts, potentially also bats. In addition, our findings show that susceptibility to EBOV-GP–driven infection is not a feature unique to cells from potential reservoir species, in agreement with a previous study [41], indicating that GP-receptor interactions do not limit the susceptibility of bat species for EBOV infection. Notably, the bat cell lines tested here were highly permissive to EBOV replication and can be used to study interactions of EBOV with cells derived from potential reservoir species. However, it remains to be examined whether they adequately model EBOV infection of primary cells or cells from other organs of potential reservoir species. In sum, EBOV-GP–mediated host cell entry does not limit EBOV infection to certain bat species, and cell lines from potential bat reservoir species can allow efficient EBOV replication. The latter finding is in agreement with a recent report revealing that Marburg virus readily replicates in a cell line established from the fruit bat R. aegyptiacus [36], a suspected reservoir host [42].
Supplementary Data
Supplementary data are available at The Journal of Infectious Diseases online.
Funding
This work was supported by the German Federal Ministry of Education and Research (BMBF) via the project Ecology and Pathogenesis of SARS, an Archetypical Zoonosis (project codes 01KIO701, 01KIO703); International Research Training Group 1273 and Hannover Biomedical Research School (S.P. and G.B.); the European Community's Seventh Framework Programme FP7 EMPERIE, (project code 223498); and in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Supplementary Material
Acknowledgments
We thank Prof T. F. Schulz for support, Prof P. D. Bieniasz for the human tetherin expression plasmid, and Prof G. Simmons for EBOV-GP-V5 expression plasmids.
References
- 1.Geisbert TW, Hensley LE. Ebola virus: new insights into disease aetiopathology and possible therapeutic interventions. Expert Rev Mol Med. 2004;6:1–24. doi: 10.1017/S1462399404008300. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn JH. Filoviruses: a compendium of 40 years of epidemiological, clinical, and laboratory studies. Arch Virol Suppl. 2008;20:13–360. [PubMed] [Google Scholar]
- 3.Towner JS, Sealy TK, Khristova ML, et al. Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4:e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoenen T, Groseth A, Falzarano D, Feldmann H. Ebola virus: unravelling pathogenesis to combat a deadly disease. Trends Mol Med. 2006;12:206–15. doi: 10.1016/j.molmed.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Barrette RW, Metwally SA, Rowland JM, et al. Discovery of swine as a host for the Reston ebolavirus. Science. 2009;325:204–6. doi: 10.1126/science.1172705. [DOI] [PubMed] [Google Scholar]
- 6.Geisbert TW, Hensley LE, Larsen T, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163:2347–70. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wool-Lewis RJ, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J Virol. 1998;72:3155–60. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Delgado R, Xu L, et al. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–7. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 9.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science. 2005;308:1643–5. doi: 10.1126/science.1110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann G, Feldmann H, Watanabe S, Lukashevich I, Kawaoka Y. Reverse genetics demonstrates that proteolytic processing of the Ebola virus glycoprotein is not essential for replication in cell culture. J Virol. 2002;76:406–10. doi: 10.1128/JVI.76.1.406-410.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann G, Geisbert TW, Ebihara H, et al. Proteolytic processing of the Ebola virus glycoprotein is not critical for Ebola virus replication in nonhuman primates. J Virol. 2007;81:2995–8. doi: 10.1128/JVI.02486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Licata JM, Johnson RF, Han Z, Harty RN. Contribution of Ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J Virol. 2004;78:7344–51. doi: 10.1128/JVI.78.14.7344-7351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noda T, Sagara H, Suzuki E, Takada A, Kida H, Kawaoka Y. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J Virol. 2002;76:4855–65. doi: 10.1128/JVI.76.10.4855-4865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci U S A. 2000;97:13871–6. doi: 10.1073/pnas.250277297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasenosky LD, Neumann G, Lukashevich I, Kawaoka Y. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J Virol. 2001;75:5205–14. doi: 10.1128/JVI.75.11.5205-5214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A. 2009;106:2886–91. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons G, Wool-Lewis RJ, Baribaud F, Netter RC, Bates P. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J Virol. 2002;76:2518–28. doi: 10.1128/jvi.76.5.2518-2528.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med. 2000;6:886–9. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 19.Geisbert TW, Young HA, Jahrling PB, et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163:2371–82. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroy EM, Rouquet P, Formenty P, et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303:387–90. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- 21.Leroy EM, Kumulungui B, Pourrut X, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–6. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 22.Hayman DT, Emmerich P, Yu M, et al. Long-term survival of an urban fruit bat seropositive for Ebola and Lagos bat viruses. PLoS One. 2010;5:e11978. doi: 10.1371/journal.pone.0011978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pourrut X, Souris M, Towner JS, et al. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect Dis. 2009;9:159. doi: 10.1186/1471-2334-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanepoel R, Leman PA, Burt FJ, et al. Experimental inoculation of plants and animals with Ebola virus. Emerg Infect Dis. 1996;2:321–5. doi: 10.3201/eid0204.960407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Wong SK, Li F, et al. Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-protein interactions. J Virol. 2006;80:4211–9. doi: 10.1128/JVI.80.9.4211-4219.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glowacka I, Bertram S, Muller MA, et al. Evidence that TMPRSS2 activates the SARS-coronavirus spike-protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–34. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanika A, Larisch B, Steinmann E, Schwegmann-Wessels C, Herrler G, Zimmer G. Use of influenza C virus glycoprotein HEF for generation of vesicular stomatitis virus pseudotypes. J Gen Virol. 2005;86:1455–65. doi: 10.1099/vir.0.80788-0. [DOI] [PubMed] [Google Scholar]
- 28.Vallo P, Guillén-Servent A, Benda P, Pires DB, Koubek P. Variation of mitochondrial DNA reveals high cryptic diversity in Hipposideros caffer complex. Acta Chiropt. 2008;10:193–206. [Google Scholar]
- 29.Hofmann A, Kessler B, Ewerling S, et al. Epigenetic regulation of lentiviral transgene vectors in a large animal model. Mol Ther. 2006;13:59–66. doi: 10.1016/j.ymthe.2005.07.685. [DOI] [PubMed] [Google Scholar]
- 30.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–9. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 31.Banning C, Votteler J, Hoffmann D, et al. A flow cytometry-based FRET assay to identify and analyse protein-protein interactions in living cells. PLoS One. 2010;5:e9344. doi: 10.1371/journal.pone.0009344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao F, Li Y, Decker JM, et al. Codon usage optimization of HIV type 1 subtype C gag, pol, env, and nef genes: in vitro expression and immune responses in DNA-vaccinated mice. AIDS Res Hum Retroviruses. 2003;19:817–23. doi: 10.1089/088922203769232610. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7:1313–9. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- 34.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–30. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 35.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–54. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krahling V, Dolnik O, Kolesnikova L, et al. Establishment of fruit bat cells (Rousettus aegyptiacus) as a model system for the investigation of filoviral infection. PLoS Negl Trop Dis. 2010;4:e802. doi: 10.1371/journal.pntd.0000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volchkov VE, Feldmann H, Volchkova VA, Klenk HD. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc Natl Acad Sci U S A. 1998;95:5762–7. doi: 10.1073/pnas.95.10.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bavari S, Bosio CM, Wiegand E, et al. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alazard-Dany N, Volchkova V, Reynard O, et al. Ebola virus glycoprotein GP is not cytotoxic when expressed constitutively at a moderate level. J Gen Virol. 2006;87:1247–57. doi: 10.1099/vir.0.81361-0. [DOI] [PubMed] [Google Scholar]
- 40.Jouvenet N, Neil SJ, Zhadina M, et al. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83:1837–44. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strong JE, Wong G, Jones SE, et al. Stimulation of Ebola virus production from persistent infection through activation of the Ras/MAPK pathway. Proc Natl Acad Sci U S A. 2008;105:17982–7. doi: 10.1073/pnas.0809698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Towner JS, Pourrut X, Albarino CG, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2:e764. doi: 10.1371/journal.pone.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.