Abstract
Type I interferon (IFN) signaling is mediated through several signaling pathways, including the Janus kinase and signal transducer and activator (JAK-STAT) and p38 mitogen-activated protein (MAP) kinase pathways. The VP24 protein of Ebolavirus is an IFN antagonist, blocking type I IFN signaling through the JAK-STAT pathway. Here, we show that, in 293 cells, VP24 also interferes with the p38 MAP kinase pathway by blocking IFN-β–stimulated phosphorylation of p38-α. Similar inhibition was not observed in HeLa cells, suggesting cell type–specific differences in signal transduction.
The interferon (IFN) response is the first line of defense against viral infections. Type I IFN (IFN-α and IFN-β) execute their antiviral activities via signal transduction pathways, resulting in the transcriptional upregulation of hundreds of genes whose products inhibit various stages of virus replication [1, 2]. Although the Janus kinase and signal transducer and activator (JAK-STAT) pathway is the best-characterized type I IFN–induced signal transduction pathway, the p38 mitogen-activated protein (MAP) kinase pathway is also critical for the type I IFN response [3]. Engagement of the type I IFN receptor by type I IFN activates a cascade of MAP kinases, eventually leading to the phosphorylation of the alpha isoform of p38 (p38-α) at threonine 180 and tyrosine 182 [4]. Phosphorylated p38 then triggers the phosphorylation of downstream transcription factors that participate in IFN responses [3, 4].
The ability of a virus to circumvent the IFN response influences its host range and pathogenicity [2]. To evade the antiviral effects of IFN, viruses have evolved multiple mechanisms that inhibit IFN production, IFN-activated signaling pathways, or IFN-induced antiviral proteins [1, 2, 5]. Zaire ebolavirus (ZEBOV), the highly pathogenic causative agent of Ebola hemorrhagic fever, causes mortality rates as high as 90% [6]. The ZEBOV VP35 protein, a viral polymerase cofactor, inhibits the production of type I IFN, but does not interfere with IFN signaling [7, 8]. The ZEBOV VP24 protein, a minor matrix protein, inhibits the cellular response to type I IFN by blocking the nuclear accumulation of phosphorylated STAT-1 [9, 10]. Here, we show that ZEBOV VP24 also inhibits the p38 MAP kinase pathway by preventing the phosphorylation of p38-α.
METHODS
Reagents
The p38 phosphorylation inhibitor, SB203580, was purchased from Promega; the c-Jun N-terminal kinase (JNK) phosphorylation inhibitor, SP600125, was purchased from Calbiochem. Human IFN-β was obtained from PBL Interferon Source. Antibodies against the phosphorylated form of human p38α (Thr180/Tyr182), total human p38-α, and human activation transcription factor-2 (ATF-2) (Thr71) were purchased from Cell Signaling Technologies, as was a p38 MAP kinase assay kit. Secondary antibodies were purchased from Invitrogen.
Cells and Plasmids
Human embryonic kidney 293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, L-glutamine, and penicillin-streptomycin solution. Cells were maintained at 37°C with 5% CO2.
Complementary DNA (cDNA) encoding the VP24 protein of ZEBOV (strain Mayinga) was cloned into a protein expression vector, pCAGGS/MCS as described previously [11].
Western Blot Analysis
The 293 cells were transfected with pCAGGS VP24 or an empty protein expression vector, pCAGGS/MCS, by using TransIT-LT-1 (Mirus). Twenty-four hours after transfection, cells were treated with SB203580 (20 μM), SP600125 (20 μM), or an equal volume of dimethyl sulfoxide (DMSO) for 1 hour and then stimulated with IFN-β (1000 units/mL). At the indicated time points, cells were rapidly washed with ice-cold phosphate-buffered saline (PBS) and solubilized in cold lysis buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 0.5 mM phenylmethylsulfonyl fluoride, PhosSTOP [Roche], and complete protease inhibitors [Roche]). After incubation for 30 minutes on ice, lysates were centrifuged (12 000 rpm for 10 minutes) and supernatants were mixed with 4× SDS loading buffer (40% glycerol, 240 mM Tris, 8% SDS, 0.04% bromophenol blue, and 5% β-mercaptoethanol) and boiled for 3 minutes. Samples were loaded onto a 10% SDS-polyacrylamide gel (Biorad). After electrophoresis, proteins were transferred onto a nitrocellulose membrane (Invitrogen) and blocked for 4 hours at room temperature with 5% skim milk PBS solution containing 0.1% Tween 20 (PBS-T; Sigma). Membranes were incubated overnight at 4°C with primary antibody in PBS-T containing 5% bovine serum albumin (Sigma). Thereafter, membranes were washed 4 times with PBS-T for 10 minutes each, incubated with secondary antibody coupled to horseradish peroxidase in 5% skim milk PBS-T solution for 2 hours, and washed 4 times with PBS-T for 10 minutes each. Bound antibody was detected with a chemiluminescence reagent (Roche) and then exposed on Kodak Biomax film.
p38 MAP Kinase Assay
To measure p38 kinase activity, we performed a kinase assay according to the manufacturer’s protocol (Cell Signal Technology). Briefly, cell lysates were incubated with an immobilized monoclonal antibody to phosphorylated p38 MAP kinase (Thr180/Tyr182), then subjected to an in vitro kinase assay with purified ATF-2 as a substrate. ATF-2 phosphorylation was detected with Western blot analysis with an antibody to the phosphorylated form of ATF-2 (Thr71).
RESULTS
IFN-β Induces p38 and ATF-2 Phosphorylation in Human Embryonic Kidney Cells
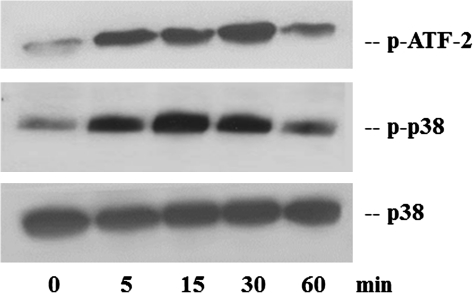
Before assessing the role of the ZEBOV VP24 protein in p38 MAP kinase signaling, we first confirmed that treatment of human embryonic kidney 293 cells with IFN-β induced p38 phosphorylation, and phosphorylation of the known p38 substrate, activating transcription factor 2 (ATF-2; 4). To accomplish this, we treated 293 cells with 1000 units/mL of IFN-β for different time periods. Cell lysates were then subjected to Western blot analysis with antibodies to the phosphorylated forms of p38α (p-p38), ATF-2 (p-ATF-2), or total p38 (Figure 1). In nontreated cells (0 min), basal levels of p-p38 and p-ATF-2 were detected. After IFN-β treatment, p-p38 peaked at 15 minutes posttreatment, whereas p-ATF-2 peaked at 30 minutes posttreatment (Figure 1). The total amount of p38 remained constant during IFN-β treatment. These results are consistent with previously published data [4].
Figure 1.
Phosphorylation of p38α and ATF-2 in interferon β (IFN-β)–stimulated human embryonic 293 cells. The 293 cells were stimulated with 1000 units/mL of IFN-β for the indicated times. Western blot analysis was performed by using antibodies against phosphorylated p38-α (Thr180/Tyr182), phosphorylated ATF-2 (Thr71), and total cellular p38. The data shown are representative of 2 independent experiments.
ZEBOV VP24 Inhibits Phosphorylation of p38 in IFN-β–stimulated Human Embryonic Kidney Cells
ZEBOV VP24 blocks signaling through the JAK-STAT pathway [9, 10]. To investigate whether ZEBOV VP24 protein also interferes with p38 MAP kinase signaling, we examined the levels of phosphorylated p38 in the absence or presence of VP24. The 293 cells were transfected with a plasmid expressing ZEBOV VP24 (pCAGGS VP24) or an empty control plasmid. Twenty-four hours later, cells were treated with inhibitors to p38 (SB203580, 20 μM) or to c-Jun N-terminal kinase (JNK) (SP600125, 20 μM), or with an equal volume of DMSO for 1 hour and then stimulated with 1000 units/mL of IFN-β for 30 minutes; control cells remained unstimulated. In unstimulated cells, low basal levels of p-p38 were detected (Figure 2A). Stimulation with IFN-β resulted in increased levels of p38 phosphorylation, an effect that was inhibited in cells expressing ZEBOV VP24. The ZEBOV VP24-mediated inhibition of p38 phosphorylation was similar to that observed in cells treated with the p38 inhibitor SB203580; by contrast, p38 phosphorylation was not affected in cells treated with the JNK inhibitor SP600125 (Figure 2A). These findings indicate that ZEBOV VP24 prevents IFN-β–mediated phosphorylation of p38 MAP kinase.
Figure 2.
Zaire ebolavirus (ZEBOV) VP24 prevents p38 mitogen-activated protein (MAP) kinase and ATF-2 phosphorylation in interferon β (IFN-β)–stimulated human embryonic 293 cells. A, ZEBOV VP24 prevents p38 MAP kinase phosphorylation. Cells were transfected with a plasmid encoding ZEBOV VP24 or an empty control vector. Twenty-four hours after transfection, cells were treated with an inhibitor to p38 or JNK or with an equal volume of DMSO. One hour later, cells were stimulated with 1000 units/mL of IFN-β for 30 minutes or remained unstimulated. Western blot analysis was performed by using antibodies against phosphorylated p38α (Thr180/Tyr182) or total cellular p38. B, ZEBOV VP24 prevents ATF-2 phosphorylation. Cells were treated as indicated and described in A. Cell lysates were incubated with an immobilized monoclonal antibody to phosphorylated p38 MAP kinase (Thr180/Tyr182); an in vitro kinase assay was then performed with purified ATF-2 as a substrate. ATF-2 phosphorylation was detected by using Western blot analysis with an antibody to the phosphorylated form of ATF-2 (Thr71).
ZEBOV VP24 Inhibits Phosphorylation of ATF-2 in IFN-β–stimulated Human Embryonic Kidney Cells
ATF-2 is a known substrate of p38 [4]. Therefore, we also assessed the levels of ATF-2 phosphorylation upon IFN-β stimulation in the absence or presence of ZEBOV VP24. As expected, stimulation of 293 cells with IFN-β resulted in increased levels of p-ATF-2 (Figure 2B). This increase in p-ATF-2 levels did not occur in cells expressing ZEBOV VP24. Pretreatment of cells with SB203580, but not with SP600125 (Figure 2B), also resulted in the inhibition of ATF-2 phosphorylation, demonstrating that the observed effect was mediated through p38 MAP kinase and not JNK.
DISCUSSION
The interferon response is a potent first line of defense against viral infections. To evade the antiviral effects of IFN, viruses have evolved mechanisms to inhibit one or more of the 3 arms of the interferon response: IFN production, IFN-activated signaling, and IFN-induced antiviral proteins [1, 2, 5].
ZEBOV encodes several proteins that function as interferon antagonists. VP35, which is part of the viral replication complex and a component of the viral nucleocapsid [6], suppresses the transcriptional up-regulation of IFN-β messenger RNA by blocking the phosphorylation of interferon regulatory factor (IRF)-3 [7]. VP35 is also a pseudosubstrate for cellular kinases that phosphorylate IRF-3 and IRF-7 [8]. Moreover, VP35 inhibits the antiviral protein PKR [12]. Recently, the ZEBOV glycoprotein, GP, was shown to inhibit another antiviral protein, tetherin, which blocks the release of some viruses from infected cells [13].
ZEBOV VP24, the minor matrix protein [6], inhibits JAK-STAT signaling by blocking the nuclear accumulation of phosphorylated STAT-1, thereby preventing the transcriptional up-regulation of IFN-stimulated genes [9, 10]. Here, we present evidence that, in 293 cells, the ZEBOV VP24 protein also targets the p38 MAP kinase pathway, which is also activated in response to IFN-α and IFN-β [3, 4]. The p38 MAP kinase pathway induces an antiviral state against hepatitis C virus [14], but viral proteins that counteract type I IFN–induced antiviral activities by targeting the p38 MAP kinase pathway have not been described. Our preliminary results show that inhibition of IFN-β–stimulated phosphorylation of p38-α by VP24 does not occur in HeLa cells (data not shown), suggesting that this phenomenon may be cell type specific. Nonetheless, our findings indicate that VP24 participates in a novel mechanism for eluding host antiviral defenses, in which it may prevent the phosphorylation of p38 MAP kinase.
In mouse-adapted ZEBOV, a mutation in VP24 is critical for the virulent phenotype observed in mice [15]. However, this mutation does not affect VP24-mediated inhibition of the JAK-STAT pathway in mouse NIH-3T3 cells [10]. It remains to be seen whether this mutation affects the VP24-mediated inhibition of the p38 MAP kinase pathway.
In summary, our findings suggest ZEBOV resistance to the antiviral effects of IFN is embodied by at least a 2-pronged strategy: VP35-mediated inhibition of IFN synthesis [7, 10] and VP24-mediated inhibition of IFN-induced signaling cascades, including the JAK-STAT [8, 15] and p38 MAP kinase pathways. Understanding how the p38 MAP kinase pathway regulates the antiviral response and elucidating the mechanism by which VP24 inhibits the p38 MAP kinase pathway could help to clarify the unusual virulence of ZEBOV and may lend impetus to the development of effective antiviral drugs.
Funding
This work was supported by Public Health Service research grants from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) and by the NIH/NIAID Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (RCE) Program and the Region X “Pacific Northwest” RCE (NIH award 1-U54-AI-081680).
Acknowledgments
We thank Sue Watson for editing the manuscript.
References
- 1.Sen G. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–81. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Randall R, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 3.Platanias L. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Immunol. 2005;5:375–86. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 4.Uddin S, Majchrzak B, Woodson J, et al. Activation of the p38 mitogen-activated protein kinase by type interferon. J Biol Chem. 1999;274:30127–31. doi: 10.1074/jbc.274.42.30127. [DOI] [PubMed] [Google Scholar]
- 5.Versteeg G, Garcia-Sastre A. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol. 2010;13:508–16. doi: 10.1016/j.mib.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez A, Geisbert TW, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, Griffin DE, Martin MA, Lamb RA, Roizman B, et al., editors. Fields virology. 5 ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007. pp. 1409–48. [Google Scholar]
- 7.Basler C, Wang X, Muhlberger E, et al. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci U S A. 2000;97:12289–94. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prins K, Cardenas W, Basler C. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol. 2009;83:3069–77. doi: 10.1128/JVI.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid SP, Leung L, Hartman A, et al. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol. 2006;80:5156–67. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid SP, Valmas C, Martinez O, Sanchez F, Basler C. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily. J Virol. 2007;81:13469–77. doi: 10.1128/JVI.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe S, Watanabe T, Noda T, et al. Production of novel Ebola virus-like particles from cDNAs; an alternative to Ebola virus generation by reverse genetics. J Virol. 2004;78:999–1005. doi: 10.1128/JVI.78.2.999-1005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Z, Cerveny M, Yan Z, He B. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J Virol. 2007;81:182–92. doi: 10.1128/JVI.01006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaletsky R, Francica J, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus buding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A. 2009;106:2886–91. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida H, Ohkawa K, Hosui A, et al. Involvement of p38 signaling pathway in interferon-alpha-mediated anitiviral activity toward hepatitis C virus. Biochem Biophys Res Commun. 2004;321:722–7. doi: 10.1016/j.bbrc.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Ebihara H, Takada A, Kobasa D, et al. Molecular determinants of Ebola virus virulence in mice. Plos Pathog. 2006;2:0705–11. doi: 10.1371/journal.ppat.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]