Abstract
To gain further insight into the interdependent pathogenic processes in Ebola hemorrhagic fever (EHF), we have examined the dynamics of host responses in individual rhesus macaques infected with Zaire ebolavirus over the entire disease course. Examination of coagulation parameters revealed that decreased coagulation inhibitor activity triggered severe coagulopathy as indicated by prolonged coagulation times and decreased fibrinogen levels. This has been proposed as one of the significant mechanisms underlying disseminated intravascular coagulation in EHF patients. Furthermore, monitoring of expression levels for cytokines/chemokines suggested a mixed anti-inflammatory response syndrome (MARS), which indicates that a catastrophic uncontrolled immunological status contributes to the development of fatal hemorrhagic fever. These results highlight the pathological analogies between EHF and severe sepsis and not only contribute to our understanding of the pathogenic process, but will also help to establish novel postexposure treatment modalities.
Zaire ebolavirus (ZEBOV) infections result in case-fatality rates of up to 90%, making this one of the most severe viral hemorrhagic fevers (VHFs) worldwide [1, 2]. Gaining further insight into the details of interdependent pathogenic processes, including both the host immune and pathophysiological responses, involved in triggering this severe hemorrhagic syndrome is essential for the development of effective countermeasures for Ebola hemorrhagic fever (EHF) [2, 3].
Currently, the nonhuman primate (NHP) is the gold standard animal model for studying pathogenesis and developing countermeasures [4]. NHPs are both highly susceptible to ZEBOV infection (nearly 100% lethality) and show the hallmarks of human EHF [5–7]. To date, both cynomolgus (Macaca fascicularis) and rhesus (Macaca mulatta) macaques have been used for the development of countermeasures against EHF, while characterization of the pathogenic processes in NHPs infected with ZEBOV has mainly been carried out in cynomolgus macaques [6–8]. In contrast to cynomolgus macaques, detailed studies on pathogenesis with rhesus macaques, which are often used for postexposure treatment studies [9–12], have not been carried out, with a few exceptions from the early literature [13–16]. Several serial sacrifice studies have been conducted using cynomolgus macaque models to elucidate the pathogenic processes associated with ZEBOV infection. These studies have provided us with significant insights into the role of primary target cells in pathogenesis and the mechanisms underlying coagulation abnormalities [6–8]. On the other hand, continuous monitoring of immunological and pathophysiological host responses in individually infected animals is important to fully understand EHF pathogenesis [7, 14–16]. This cannot be achieved with serial sacrifice pathology studies, because of the variable host responses in outbred animals such as NHPs. To date, only a limited number of studies have been carried out for the comprehensive monitoring of host responses in rhesus macaques infected with ZEBOV [15, 16]. We, therefore, studied the host response dynamics through daily monitoring of blood biochemistry and coagulation parameters, as well as cytokine/chemokine profiles in individual animals over time. The new data obtained here advance our understanding of the pathogenesis of ZEBOV infection in macaques and will facilitate development of novel postexposure treatment strategies to combat EHF.
MATERIALS AND METHODS
Cells and Viruses
Vero E6 (African green monkey kidney) cells were grown and maintained at 37°C in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, L-glutamine, penicillin, and streptomycin. The Mayinga strain of ZEBOV was grown in Vero E6 cells. Virus infectivity titers, expressed as focus-forming units (FFU), were obtained by counting the number of infected cell foci detected using an indirect immunofluorescent antibody assay with a rabbit polyclonal anti-ebolavirus (EBOV) VP40 as previously described [17].
Nonhuman Primate Experiments
Three healthy, filovirus-seronegative, male rhesus macaques designated as subjects 14, 15, and 16, weighing 10.0, 9.4, and 12.0 kg, respectively, were inoculated in the caudal thigh with 1 mL of virus stock containing 1000 FFU of ZEBOV. The animals were monitored daily through clinical scoring (nonanesthetized) and clinical examination (anesthetized). Clinical examinations included body temperature, blood pressure, heart rate, respiration rate, pulse oximetry, venous bleeding, and collection of swabs from nasal, oral, and rectal mucosa. The preclinical examinations were done 2 days before and simultaneously with infection (day 0). All animals were euthanized when clinical signs indicated terminal disease according to an approved endpoint-scoring sheet. Upon necropsy, various tissues were collected for virological assays. Infectivity titers from all blood, swab, and tissue samples were determined by median tissue culture infective dose (TCID50) assay on Vero E6 cells.
Hematology, Blood Biochemistry, and Coagulation Parameter Assays
Hematological and serum biochemical analysis was carried out following standard protocols [7]. Hematological values and parameters were determined from EDTA blood with the HemaVet 950FS+ laser-based hematology analyzer (Drew Scientific). Blood chemistry values were analyzed from heparinized whole blood using the blood chemistry analyzer iSTAT1 (Abbott Point of Care) with the EC8+ and Crea cartridges. Blood samples for the coagulation parameter assays were collected into 1.8-mL citrate vacutainers. Plasma was separated by centrifugation and analyzed for activated partial thromboplastin time (aPTT), prothrombin time (PT), thrombin time (TT), fibrinogen concentration, and protein S and protein C activity with the STart4 instrument using the PTT Automate, STA Neoplastine CI plus, STA Thrombin, Fibri-Prest automate, and STA Staclot protein S and protein C kits, respectively (all from Diagnostica Stago).
Plasma Cytokine and Chemokine Analysis
Plasma samples for cytokine assay were inactivated by γ-irradiation (5 mrad) prior to removal from Biosafety Level 4 (BSL4) for further analysis. Concentrations of granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, interferon (IFN)–γ, interleukin (IL)–1β, IL-1 receptor antagonist, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23 (p40), IL-13, IL-15, IL-17, MCP-1 and macrophage inflammatory protein (MIP)–1α, MIP-1β, soluble CD40-ligand (sCD40L), transforming growth factor-α, tumor necrosis factor (TNF)–α, vascular endothelial growth factor (VEGF), and IL-18 were measured on a Bio-Plex 200 instrument (Bio-Rad) using the Non-Human Primate Cytokine MILLIPLEX map kit (Millipore) according to the manufacturer’s instructions.
Biosafety and Animal Ethics
All in vitro and in vivo work with infectious ZEBOV was performed in the BSL4 laboratories of the Integrated Research Facility of the Rocky Mountain Laboratories, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. All animal work was approved by the local Institutional Animal Care and Use Committee (IACUC) and performed following the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC) by certified staff in an AALAC-approved facility.
RESULTS
Clinical Signs, Disease Progression, and Viremia in Infected Rhesus Macaques
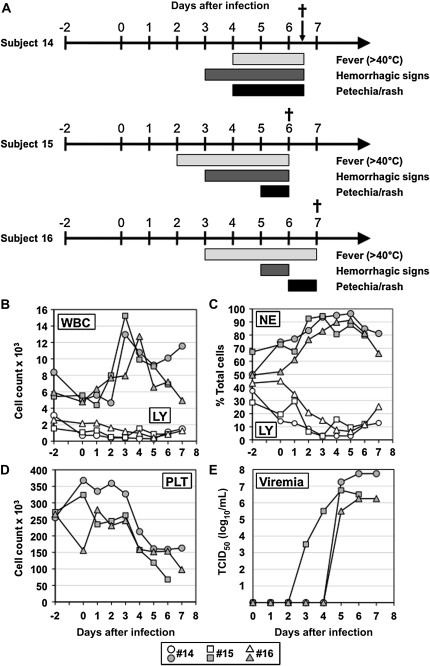
The clinical, hematological, and virological findings are summarized in Figure 1A. Signs of illness were visible starting from days 2–3 after infection. All 3 animals became febrile (>40°C) between days 2 and 4 and remained febrile until euthanized. The first hemorrhagic manifestations observed were bleeding signs, such as blood on nasal or rectal swabs, which were observed on day 3 for subjects 14 and 15, and on day 5 for subject 16 (Figure 1A). Following the appearance of early bleeding signs, all animals developed a macular cutaneous rash/petechia on several body parts (eg, arms, chest, groin, thorax, and face) starting between days 4 and 7 . In consultation with the facility veterinarian, animals were euthanized according to a predetermined clinical humane endpoint-scoring sheet when clinical disease progression was considered irreversible (subject 14 on day 6.5, 15 on day 6, and 16 on day 7) (Figure 1A). Necropsy findings and gross pathological changes were consistent with the observations reported previously [7, 13].
Figure 1.
Disease progression in rhesus macaques following Zaire ebolavirus (ZEBOV) infection. Three rhesus macaques (subjects 14, 15, and 16) were infected via intramuscular injection with 1000 focus-forming units of ZEBOV (Mayinga strain). Clinical examination and sample collections were performed daily until animals were euthanized. (A) Disease progression and clinical presentations. Dagger: euthanasia, when clinical signs indicated terminal disease according to an endpoint-scoring sheet. (B) Total white blood cell (WBC) and lymphocyte (LY) counts. (C) Differential neutrophil (NE) and LY counts. (D) Platelet (PLT) counts. (E) Viremia. Viral infectivity titration was performed on Vero E6 cells by use of a median tissue culture infective dose (TCID50) assay. Infectivity titers are presented as log10 TCID50/mL.
Total white blood cell (WBC) counts peaked between days 3 and 4 and indicated leukocytosis (Figure 1B). Differential WBC counts confirmed leukocytosis mainly due to neutrophilia (Figure 1C). Concomitantly, lymphocytopenia was observed as differential lymphocyte counts dropped from day 2 to day 5 (Figure 1C). Thrombocytopenia developed between days 3 and 4 with cell counts dropping below 150000 or even 100000 (Figure 1D). These findings were consistent with the observations in cynomolgus and rhesus macaques reported previously [7, 15].
Plasma viremia was first detected on day 3 in subject 15. This animal was also the first to succumb to the infection. In subjects 14 and 16, viremia was delayed, starting on day 5 (Figure 1E). Viremia levels constantly increased and peaked at the time of euthanasia, ranging from 106 to 108 TCID50/mL. At necropsy, various tissues (40 tissues per animal) were collected and infectious virus was detected in all tissues with titers ranging from approximately 104 to 108 TCID50/g (data not shown).
Coagulation Abnormalities in Infected Rhesus Macaques
We further examined coagulation parameters, including PT, aPTT, TT, fibrinogen level, and protein C and S coagulation inhibitor activity in plasma of infected animals. Changes over baseline were observed in all parameters tested starting between days 3 and 4 (Figure 2, A–C). The clotting time parameters PT, aPTT, and TT were prolonged in all animals starting at day 3 for PT and aPTT and day 5 for TT (Figure 2). Fibrinogen levels were increased and peaked on days 4–5, then strikingly declined to below the detection limit in the terminal stage of illness (Figure 2D). A rapid decline in protein C activity was observed in all animals on day 3, whereas decline in protein S activity was moderate and not distinct (Figure 2, E and F). Subject 15 showed low levels of protein C and S activity until the terminal stage of infection, whereas the levels in subjects 14 and 16 appeared to slightly rebound between days 4 and 6 and then rapidly decline during the terminal stage of illness (Figure 2, E and F). These parameters indicate that ZEBOV-infected animals displayed severe dysfunctions in coagulation resembling disseminated intravascular coagulation (DIC), although confirmation of DIC ideally includes detection of fibrin split products and D-dimers.
Figure 2.
Development of coagulation abnormalities in rhesus macaques during Zaire ebolavirus (Mayinga strain) infection. (A) Prothrombin time (PT). (B) Activated partial thromboplastin time (aPTT). (C) Thrombin time (TT). (D) Fibrinogen concentration in plasma. (E) Plasma protein C coagulation inhibitor activity. The y-axis describes values as percentage of normal human coagulation inhibitor activity. (F) Plasma protein S coagulation inhibitor activity. The y-axis describes values as percentage of normal human coagulation inhibitor activity.
Cytokine and Chemokine Profiles in Infected Rhesus Macaques
To examine the correlation between clinical disease progression, coagulation, and host immune responses, we monitored the expression of cytokines, chemokines, and other soluble mediators in the plasma of infected animals. Twelve of the 23 soluble mediators examined showed significant changes from baseline values (day –2 and day 0 at challenge). First, plasma levels of representative proinflammatory cytokines and chemokines (IL-1β, IL-6, MIP-1α, and TNF-α), which are upregulated during EHF in humans [18–20], were examined. IL-1β, IL-6, and MIP-1α were elevated from day 4 onward in all infected animals (Figure 3, A–D), whereas TNF-α was only detected during the terminal stage of infection (days 5 and 6) and only in 2 of the 3 animals (Figure 3C).
Figure 3.
Kinetics of plasma proinflammatory cytokine and chemokine levels in rhesus macaques following Zaire ebolavirus (Mayinga strain) infection. (A) IL-1β, (B) IL-6, (C) TNF-α, (D) MIP-1α. IL, interleukin; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor.
Among the proinflammatory cytokines associated with T cell activation, IFN-γ and IL-15 were found to be elevated between days 2 and 4 in all animals (Figure 4, A and C). Subject 16 showed massive expression of IFN-γ in the terminal disease stage. In subjects 14 and 16, the level of IL-15 peaked between days 4 and 5, then declined before the animal entered the terminal disease stage (Figure 4C). Interestingly, decreased levels of IL-12/23 p40 were observed in all infected animals from day 1 and those levels continuously decreased until animals succumbed to the infection (Figure 4B). All animals showed elevated IL-18 levels beginning between days 3 and 4, but the levels varied over time and among animals (Figure 4D). In subject 14, IL-18 levels peaked on day 6 and then rapidly declined immediately before the animal succumbed to infection. Subject 15 showed the smallest increase in levels of IL-18. Interestingly, the elevation of IL-18 levels in subject 16 was biphasic (Figure 4D).
Figure 4.
Kinetics of plasma proinflammatory cytokine and chemokine levels activating T cells in rhesus macaques following Zaire ebolavirus (Mayinga strain) infection. (A) IFN-γ, (B) IL-12/23 p40, (C) IL-15, (D) IL-18. IFN, interferon; IL, interleukin.
Anti-inflammatory responses were also upregulated in all infected animals. While IL-13 and IL-1 receptor antagonist (IL-1ra) levels were increased from day 2 in all animals (Figure 5, B and C), the expression kinetics of IL-10 was totally different among the 3 animals (Figure 5A). Subject 15, which showed the shortest survival time, had increased IL-10 expression beginning at day 1, whereas subject 14 showed decreased levels of IL-10 at early stages of infection and then rebounded at the terminal stage of infection. Subject 16 had elevated IL-10 expression only in the terminal disease stage (Figure 5A).
Figure 5.
Kinetics of plasma anti-inflammatory mediators, and soluble CD40 ligand (sCD40L) levels in rhesus macaques following Zaire ebolavirus (Mayinga strain) infection. (A) IL-10, (B) IL-13, (C) IL-1 receptor antagonist (IL-1ra), (D) sCD40L. IL, interleukin.
Finally, we also monitored soluble CD40-ligand as a marker for platelet activation [21]. Overall, sCD40L levels were increased in the early stage of infection (Figure 5D), and then decreased in the late to terminal stages of disease, which were also associated with thrombocytopenia in 2 of 3 animals, both showing a prolonged period with hemorrhagic signs (Figure 1A, subjects 14 and 15; Figure 1D). Subject 14 showed high levels of sCD40L expression prior to infection. Levels then rapidly declined until day 3 when they rebounded and declined again toward the terminal stage of disease. In subject 15, sCD40L levels peaked on day 2 and then rapidly declined toward the terminal stage of disease. Subject 16 only showed a transient increase in sCD40L levels and maintained a low level of sCD40L throughout the infection (Figure 5D).
DISCUSSION
In the present study, we investigated the dynamics of pathogenesis-related host responses occurring during ZEBOV infection through daily monitoring of multiple parameters in individual rhesus macaques throughout the entire disease course. It is noteworthy that the time to death (average 6.5 days) of rhesus macaques infected with the ZEBOV Mayinga strain in the present study was shorter than that of recent rhesus macaque studies with the ZEBOV-95 Kikwit strain (average 8.4 days) [9–12]. A shorter time to death in rhesus macaques infected with another ZEBOV-76 strain was also observed in previous studies [13–16]. These data suggest that ZEBOV-76 strains might be more virulent than ZEBOV-95 in rhesus macaques.
Among the pathological processes examined in this study, the coagulation disorders are believed to be key pathogenic events responsible for inducing lethal disease in humans and NHPs infected with ZEBOV [3, 6, 8, 22]. We show here that thrombocytopenia was temporally associated with the appearances of abnormalities in coagulation parameters, including prolonged PT, aPTT, and TT (Figure 1D and Figure 2, A–C). Notably, decline in protein C coagulation inhibitor activity due to its consumption occurred 1 to 2 days prior to prolongation in coagulation times (Figure 2, A–E). This suggests that enhancement of the procoagulant state, an event that has been proposed as an important mechanism for DIC development, might trigger severe coagulation impairment in the infected animals [3, 6, 8, 22]. Therefore, ZEBOV infection in NHPs (8; this study) behaves similarly to severe sepsis, where protein C is also consumed and/or production of protein C is decreased during systemic coagulopathy [23–25]. In addition to being a regulator of coagulation, protein C has also been reported to have anti-inflammatory, antiapoptotic, and cytoprotective activities [26, 27]. Particularly for sepsis, the anti-inflammatory function of protein C is thought to play an important role in controlling severe inflammatory responses that lead to hypercoagulation [24, 28]. Here and in other studies [7, 18, 20] it has been reported that aberrant inflammatory/anti-inflammatory responses are a hallmark of ebolavirus infection, suggesting that reduced protein C activity may contribute to severe disease progression through defective control of coagulation and severe inflammatory responses. Moreover, protein C and fibrinogen (see below) are synthesized in the liver [24], where the hepatic synthesis of both protein C and fibrinogen have been reported to be reduced in cases of septic liver dysfunction [24]. The liver is a main target organ for filovirus infection, with pathological changes that include severe hepatocellular degeneration and necrosis with inflammation, most likely resulting in decreased production of protein C and fibrinogen (see below) [7, 24, 29]. Supporting these concepts, postexposure treatment of ZEBOV-infected rhesus macaques with recombinant human activated protein C was found to protect 18% of animals and resulted in a significantly prolonged survival time with decreased plasma fibrin degradation products (D-dimer levels) in animals that succumbed to infection [30]. Similarly, activated protein C reduced the relative risk for lethal outcome among patients with sepsis and DIC by 38% [25]. Altogether, impaired protein C and S activities and the effect on coagulation and inflammatory responses are common pathogenic processes of severe sepsis/septic shock and filoviral hemorrhagic fever.
Another striking finding in ZEBOV-infected rhesus macaques was the rapid reduction of plasma fibrinogen levels in the terminal stage of disease following an initial abnormal increase in fibrinogen levels (Figure 2D). Plasma fibrinogen concentrations decline due to the subsequent generation of large amounts of fibrin by the conversion of fibrinogen. This observation clearly suggests the induction of uncontrolled coagulation in ZEBOV-infected animals. Widespread intravascular fibrin deposition is a hallmark of DIC during severe sepsis [24, 31] and, similarly, both fibrin deposition in tissues and rapid increases in D-dimer plasma levels were observed in ZEBOV-infected cynomolgus macaques [8]. Thus, consumption of plasma fibrinogen indirectly reflects progression to DIC. Hypofibrinogenemia, however, can only be detected in a limited number of severe DIC cases, because levels of fibrinogen, an acute-phase reactant, are generally elevated during systemic inflammatory response syndrome (SIRS), such as in sepsis and VHFs [23, 25]. Therefore, in certain circumstances, apparently normal fibrinogen plasma levels may actually conceal fibrinogen consumption. In fact, fibrinogen concentrations were increased before the ZEBOV-infected animals entered the terminal stage of illness (Figure 2D), which might be explained by the strong systemic inflammatory response during infection (Figures 3 and 4). Nevertheless, infected animals showed a dramatic drop in fibrinogen during the terminal disease stage, suggesting uncontrolled coagulopathy as a result of ZEBOV infection.
Aberrant cytokine/chemokine responses are thought to be another significant factor for disease progression in EHF cases and experimentally infected nonhuman primates [5, 18–20, 32, 33]. The most consistent observation is uncontrolled systemic inflammatory responses (hypersecretion/cytokine storm) resembling SIRS, which may represent a critical factor in the induction of vascular leakage, coagulation abnormalities (DIC), and multiorgan failure [1, 3, 5, 7, 18–20, 22, 32–34]. In our present study, upregulated proinflammatory cytokines/chemokines included IL-1β, IL-6, MIP-1α, IL-15, and IL-18 (Figures 3 and 4), which have been previously reported to be upregulated in EHF patients [18, 19, 32, 33] and experimentally infected cynomolgus macaques [7]. Interestingly, TNF-α, which has been recognized as a critical cytokine for VHF pathogenesis, was only detected in 2 of 3 animals at the terminal disease stage (Figure 3C). The role of TNF-α in EHF remains controversial [18–20, 32–34]. Data from outbreaks in Gabon (1996) and the Democratic Republic of the Congo (1995) associated moderate to high plasma levels of TNF-α with fatal outcomes [19, 20]. In contrast, investigations of later outbreaks in Gabon and the Republic of Congo [18] as well as the Sudan ebolavirus outbreak in Uganda, did not find the same association [32, 33]. In addition, early moderate levels of TNF-α have been described as key immune responses in survivors of Zaire EHF and asymptomatic EBOV infections [19, 35], suggesting that a TNF-α response in the late stages of infection might be deleterious and contribute to lethal disease progression. TNF-α is also produced by EBOV and Marburg virus (MARV)–infected monocytes and was shown to increase endothelial cell permeability in a tissue culture system [36, 37]. This may suggest that TNF-α triggers abnormal vascular leakage in EHF patients and infected macaques [1, 5, 6]. In contrast to other proinflammatory mediators, plasma levels of the p40 subunit of IL-12 and IL-23 were decreased in all infected animals (Figure 4B). Both of these cytokines are released from dendritic cells and regulate innate and adaptive immunity [38]. Therefore, depression of both cytokines in the plasma of ZEBOV-infected macaques may reflect impaired dendritic cell function, a premise that is supported by in vitro studies showing that ZEBOV infection or expression of ZEBOV VP35 in dendritic cells impaired their maturation and suppressed IL-12 expression [39–42].
Synchronous proinflammatory and anti-inflammatory responses have been previously reported as an abnormality in the immunological status of EHF patients [18–20]. Our study supports the finding that the induction of a SIRS-like syndrome during EBOV infection might induce a hypoinflammatory status referred to as compensatory anti-inflammatory response syndrome [43], which itself is known to trigger an abnormal immunological status known as mixed anti-inflammatory response syndrome (MARS) [43, 44]. MARS has also been described in severe sepsis cases and may be a marker for early mortality [42–44]. In the present study, IL-13 and IL-1ra levels were highly elevated in infected macaques (Figure 5, B and C). This suggests that MARS may contribute to pathogenesis in EHF. Notably, increased levels of anti-inflammatory cytokines including IL-10 and IL-13 were associated with fatal EBOV cases, and were related to severity and fatality in sepsis patients [19, 20, 32, 45], although levels of IL-10 were variable among the 3 animals in this study. These findings emphasize the potential for preventing the progression to MARS by controlling the development of SIRS as a treatment option for EHF.
Similar to anti-inflammatory mediators, higher plasma levels of sCD40L, a known marker of platelet activation [21], were found in 2 animals (subjects 14 and 15), although levels of sCD40L were variable and inconclusive among the 3 animals (Figure 5D). This implies that platelet activation might play a role in the pathogenesis of EHF. Increased sCD40L levels have also been observed in patients with acute coronary syndrome, SIRS, and septic shock [21, 46]. In acute coronary syndrome, circulating sCD40L levels were strongly associated with higher neutrophil counts (neutrophilia) [46]. This was also observed in ZEBOV-infected macaques (Figure 1C) [7, 15], further suggesting that activation of platelets and/or sCD40L release might lead to neutrophil activation and inflammation in infected animals [47].
One of the guiding concepts in filoviral hemorrhagic fever pathogenesis focuses on the pathophysiological and immunological similarities between EHF and severe sepsis [8, 22, 28, 48]. Our findings here further support these concepts and strongly emphasize the pathophysiological analogies between filoviral hemorrhagic fever, particularly EHF, and severe sepsis/septic shock syndrome. Therefore, we propose that successful treatment strategies for sepsis cases be evaluated in EHF animal models and, if effective, be considered for treatment of EBOV and MARV infections.
Funding
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
Acknowledgments
The authors are grateful to Lisa Kercher, Kathleen Meuchel, Rocky Rivera, Ed Schreckendgust, Sandra Skorupa, and Mike Parnell of the Rocky Mountain Veterinary Branch for help with animal care and veterinary service. We also thank Allison Groseth, Institute for Virology at the Philipps-University Marburg, Germany; James E. Strong, National Microbiology Laboratory, Public Health Agency of Canada; and Darryl Falzarano, Laboratory of Virology, Division of Intramural Research, NIAID, NIH, for helpful discussions and editing of the manuscript. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the NIH.
References
- 1.Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004;10:S110–21. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez A, Geisbert T, Feldmann H. Filoviridae: Marburg and Ebola viruses. In: Knipe D, Howley P, editors. Fields virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1409–48. [Google Scholar]
- 3.Hoenen T, Groseth A, Falzarano D, Feldmann H. Ebola virus: unravelling pathogenesis to combat a deadly disease. Trends Mol Med. 2006;12:206–15. doi: 10.1016/j.molmed.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis Model Mech. 2009;2:12–7. doi: 10.1242/dmm.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensley LE, Geisbert TW. The contribution of the endothelium to the development of coagulation disorders that characterize Ebola hemorrhagic fever in primates. Thromb Haemost. 2005;94:254–61. doi: 10.1160/TH05-03-0153. [DOI] [PubMed] [Google Scholar]
- 6.Geisbert TW, Young HA, Jahrling PB, et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163:2371–82. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geisbert TW, Hensley LE, Larsen T, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163:2347–70. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188:1618–29. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 9.Geisbert TW, Hensley LE, Jahrling PB, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362:1953–8. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann H, Jones SM, Daddario-DiCaprio KM, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 2007;3:e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geisbert TW, Lee AC, Robbins M, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisbert TW, Daddario-DiCaprio KM, Williams KJ, et al. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol. 2008;82:5664–8. doi: 10.1128/JVI.00456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baskerville A, Bowen ET, Platt GS, McArdell LB, Simpson DI. The pathology of experimental Ebola virus infection in monkeys. J Pathol. 1978;125:131–8. doi: 10.1002/path.1711250303. [DOI] [PubMed] [Google Scholar]
- 14.Bowen ET, Platt GS, Simpson DI, McArdell LB, Raymond RT. Ebola haemorrhagic fever: experimental infection of monkeys. Trans R Soc Trop Med Hyg. 1978;72:188–91. doi: 10.1016/0035-9203(78)90058-5. [DOI] [PubMed] [Google Scholar]
- 15.Fisher-Hoch SP, Platt GS, Neild GH, et al. Pathophysiology of shock and hemorrhage in a fulminating viral infection (Ebola) J Infect Dis. 1985;152:887–94. doi: 10.1093/infdis/152.5.887. [DOI] [PubMed] [Google Scholar]
- 16.Fisher-Hoch SP, Platt GS, Lloyd G, Simpson DI, Neild GH, Barrett AJ. Haematological and biochemical monitoring of Ebola infection in rhesus monkeys: implications for patient management. Lancet. 1983;2:1055–8. doi: 10.1016/s0140-6736(83)91041-3. [DOI] [PubMed] [Google Scholar]
- 17.Ebihara H, Takada A, Kobasa D, et al. Molecular determinants of Ebola virus virulence in mice. PLoS Pathog. 2006;2:e73. doi: 10.1371/journal.ppat.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal Zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000837. pii: e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baize S, Leroy EM, Georges AJ, et al. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–8. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villinger F, Rollin PE, Brar SS, et al. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(Suppl 1):S188–91. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 21.Chew M, Rahman M, Ihrman L, Erson A, Zhang S, Thorlacius H. Soluble CD40L (CD154) is increased in patients with shock. Inflamm Res. 2010;59:979–82. doi: 10.1007/s00011-010-0213-5. [DOI] [PubMed] [Google Scholar]
- 22.Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4:487–98. doi: 10.1016/S1473-3099(04)01103-X. [DOI] [PubMed] [Google Scholar]
- 23.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2010;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knoebl P. Blood coagulation disorders in septic patients. Wien Med Wochenschr. 2010;160:129–38. doi: 10.1007/s10354-009-0738-9. [DOI] [PubMed] [Google Scholar]
- 25.Levi M. Current understanding of disseminated intravascular coagulation. Br J Haematol. 2004;124:567–76. doi: 10.1046/j.1365-2141.2003.04790.x. [DOI] [PubMed] [Google Scholar]
- 26.Esmon CT. The protein C anticoagulant pathway. Arterioscler Thromb. 1992;12:135–45. doi: 10.1161/01.atv.12.2.135. [DOI] [PubMed] [Google Scholar]
- 27.Castellino FJ, Ploplis VA. The protein C pathway and pathologic processes. J Thromb Haemost. 2009;7(Suppl 1):140–5. doi: 10.1111/j.1538-7836.2009.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi M, Keller TT, van Gorp E, ten Cate H. Infection and inflammation and the coagulation system. Cardiovasc Res. 2003;60:26–39. doi: 10.1016/s0008-6363(02)00857-x. [DOI] [PubMed] [Google Scholar]
- 29.Geisbert TW, Daddario-DiCaprio KM, Geisbert JB, et al. Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein c2. J Infect Dis. 2007;196(Suppl 2):S372–81. doi: 10.1086/520608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hensley LE, Stevens EL, Yan SB, et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J Infect Dis. 2007;196(Suppl. 2):S390–9. doi: 10.1086/520598. [DOI] [PubMed] [Google Scholar]
- 31.Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128:2864–75. doi: 10.1378/chest.128.4.2864. [DOI] [PubMed] [Google Scholar]
- 32.Hutchinson KL, Rollin PE. Cytokine and chemokine expression in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196(Suppl 2):S357–63. doi: 10.1086/520611. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez A, Lukwiya M, Bausch D, et al. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78:10370–7. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baize S, Leroy EM, Georges-Courbot MC, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–6. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 35.Leroy EM, Baize S, Volchkov VE, et al. Human asymptomatic Ebola infection and strong inflammatory response. Lancet. 2000;355:2210–5. doi: 10.1016/s0140-6736(00)02405-3. [DOI] [PubMed] [Google Scholar]
- 36.Ströher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H. Infection and activation of monocytes by Marburg and Ebola viruses. J Virol. 2001;75:11025–33. doi: 10.1128/JVI.75.22.11025-11033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldmann H, Bugany H, Mahner F, Klenk HD, Drenckhahn D, Schnittler HJ. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol. 1996;70:2208–14. doi: 10.1128/jvi.70.4.2208-2214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 39.Jin H, Yan Z, Prabhakar BS, et al. The VP35 protein of Ebola virus impairs dendritic cell maturation induced by virus and lipopolysaccharide. J Gen Virol. 2010;91:352–61. doi: 10.1099/vir.0.017343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol. 2003;170:2797–801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- 41.Bosio CM, Aman MJ, Grogan C, et al. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis. 2003;188:1630–8. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- 42.Bray M, Geisbert TW. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol. 2005;37:1560–6. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 44.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–74. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 45.Collighan N, Giannoudis PV, Kourgeraki O, Perry SL, Guillou PJ, Bellamy MC. Interleukin 13 and inflammatory markers in human sepsis. Br J Surg. 2004;91:762–8. doi: 10.1002/bjs.4521. [DOI] [PubMed] [Google Scholar]
- 46.Setianto BY, Hartopo AB, Gharini PP, Anggrahini DW, Irawan B. Circulating soluble CD40 ligand mediates the interaction between neutrophils and platelets in acute coronary syndrome. Heart Vessels. 2010;25:282–7. doi: 10.1007/s00380-009-1199-1. [DOI] [PubMed] [Google Scholar]
- 47.Vanichakarn P, Blair P, Wu C, Freedman JE, Chakrabarti S. Neutrophil CD40 enhances platelet-mediated inflammation. Thromb Res. 2008;122:346–58. doi: 10.1016/j.thromres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Bray M, Mahanty S. Ebola hemorrhagic fever and septic shock. J Infect Dis. 2003;188:1613–7. doi: 10.1086/379727. [DOI] [PubMed] [Google Scholar]