Abstract
The recombinant vesicular stomatitis virus (rVSV) vector-based monovalent vaccine platform expressing a filovirus glycoprotein has been demonstrated to provide protection from lethal challenge with Ebola (EBOV) and Marburg (MARV) viruses both prophylactically and after exposure. This platform provides protection between heterologous strains within a species; however, protection from lethal challenge between species has been largely unsuccessful. To determine whether the rVSV-EBOV vaccines have the potential to provide protection against a newly emerging, phylogenetically related species, cynomolgus macaques were vaccinated with an rVSV vaccine expressing either the glycoprotein of Zaire ebolavirus (ZEBOV) or Côte d’Ivoire ebolavirus (CIEBOV) and then challenged with Bundibugyo ebolavirus (BEBOV), which was recently proposed as a new EBOV species following an outbreak in Uganda in 2007. A single vaccination with the ZEBOV–specific vaccine provided cross-protection (75% survival) against subsequent BEBOV challenge, whereas vaccination with the CIEBOV–specific vaccine resulted in an outcome similar to mock-immunized animals (33% and 25% survival, respectively). This demonstrates that monovalent rVSV-based vaccines may be useful against a newly emerging species; however, heterologous protection across species remains challenging and may depend on enhancing the immune responses either through booster immunizations or through the inclusion of multiple immunogens.
Ebola (EBOV) and Marburg (MARV) v iruses are enveloped, nonsegmented, negative-sense RNA viruses that belong to the family Filoviridae [1]. Infection frequently results in severe hemorrhagic fever in both humans and nonhuman primates, with case-fatality rates as high as 90% in humans [2, 3]. Outbreaks are geographically and temporally unpredictable but have primarily occurred in equatorial Africa [4]. Because there are no approved options for intervention, the development of prophylactic and therapeutic vaccines is highly desirable.
A number of vaccine platforms, including DNA, recombinant Adenovirus (rAd), combined DNA/rAd, virus-like particles (VLPs), human parainfluenza virus 3 (rHPIV3), and recombinant vesicular stomatitis virus (rVSV), have proven to be efficacious in nonhuman primates against EBOV and/or MARV challenge [5–11]. The viral glycoprotein (GP) seems to be the key viral immunogen for providing protection across divergent EBOV and MARV strains, as demonstrated in the DNA/rAd, rHPIV, VLP, and rVSV platforms [6–8, 10, 12–14]. Current data support the hypothesis that a vaccine that includes antigen from a single species will only be protective against that species. Based on the close phylogenetic relatedness of the MARV strains, it was suspected that a vaccine based on any of the MARV isolates might induce cross-protection against all strains, and this has been demonstrated [8]. However, current data suggest that there is no cross-protection between species and genera; for example, MARV and EBOV or the different EBOV species (which are much more distantly related than are the MARV strains) [7, 9]. Because there are multiple species of EBOV, including Zaire ebolavirus (ZEBOV), Sudan ebolavirus (SEBOV), Côte d’Ivoire ebolavirus (CIEBOV), Reston ebolavirus (REBOV) [1], and the proposed new species Bundibugyo ebolavirus (BEBOV) [15], this would suggest that multiple vaccines will be required. In some cases, different species have overlapping zones of endemicity [1, 4]; thus, there is a need for a single vaccine that can provide protection against multiple species. To date, there have only been 2 reports of cross-protection between EBOV species. The first report showed that a single-injection blended rVSV-vaccine containing ZEBOVgp, SEBOVgp, and MARVgp provided 100% protection from subsequent CIEBOV challenge [16], whereas the other report used multiple DNA vaccinations (with ZEBOVgp and SEBOVgp) followed by an rAd (ZEBOVgp) boost over the course of 1.5 years to provide protection from heterologous BEBOV challenge [17].
With the emergence of BEBOV in Uganda in 2007 [15] and the recent sequencing of a novel virus from bats in Spain that has been tentatively assigned to the proposed new filovirus genus Cuevavirus [18], the continued emergence of additional filoviruses seems inevitable. Furthermore, because certain fruit bat species appear to be the reservoir for both EBOV [19] and MARV [20], it is likely that sporadic outbreaks will continue, because vector control is not a viable option. A single-injection blended vaccine (containing equal amounts of rVSVΔG-ZEBOVgp, rVSVΔG-SEBOVgp, and rVSVΔG-MARVgp) can protect cynomolgus macaques against challenge with ZEBOV, SEBOV, CIEBOV, and MARV [16], illustrating that, in principle, it should be possible to generate a pan-filovirus rVSV-based, blended vaccine. This approach has also proven to be successful in the rAd and VLPs platforms [10, 13, 21]; however, in the event of a new emerging species, it is not expected that protection would necessarily be provided against this new species. Therefore, a vaccine that provides a broader cross-protection is still highly desired.
To determine whether monovalent rVSV vaccines against either ZEBOV or CIEBOV are protective against a phylogenetically closely related, proposed new EBOV species (BEBOV), cynomolgus macaques were vaccinated with rVSVΔG-ZEBOVgp or rVSVΔG-CIEBOVgp. These 2 candidate vaccines were chosen on the basis of the phylogenetic relationship of their GPs [15]. Although all animals developed signs of illness consistent with Ebola hemorrhagic fever (EHF), animals vaccinated with rVSVΔG-ZEBOVgp were partially protected (75% survival) from BEBOV challenge. Animals vaccinated with rVSVΔG-CIEBOVgp did not appear to be similarly protected from BEBOV challenge, because survival was not sufficiently enhanced over that of mock-immunized animals (33% and 25% survival, respectively).
MATERIALS AND METHODS
Vaccine Vectors and Challenge Virus
rVSV vectors containing the GP of either ZEBOV, strain Mayinga, or CIEBOV, strain Côte d’Ivoire, were generated as previously described [22, 23] using the infectious clone system lacking the VSV glycoprotein (g) gene (rVSVΔG) for VSV, serotype Indiana (kindly provided by Jack Rose, Yale University). The open reading frames for the EBOV GPs were generated by polymerase chain reaction (PCR) and cloned into the VSV genomic vectors. Insertion was confirmed by sequencing, and the recombinant viruses (rVSVΔG-ZEBOVgp and rVSVΔG-CIEBOVgp) were rescued as previously described [7, 22]. BEBOV, strain 200706291, was isolated from a patient during an outbreak in western Uganda in 2007 [15] and was subsequently obtained from the Centers for Disease Control and Prevention (Atlanta, GA).
Pilot Study
Prior to the vaccination study, 4 cynomolgus macaques (Macaca fascicularis) (female adults, 4–7 kg) were used to determine whether BEBOV resulted in a lethal infection. Two animals were infected intramuscularly (i.m.) with either 1000 or 10 000 50% tissue culture infective dose (TCID50) of BEBOV in 2 anatomical locations (left and right caudal thigh). Animals were checked twice daily for clinical signs of disease following challenge. The humane endpoint for euthanasia was determined using an approved clinical score sheet.
Vaccination and Challenge
Twelve cynomolgus macaques (M. fascicularis) (adult, 4–7 kg, male and female) were randomly assigned to one of three groups and received either 2 × 107 plaque-forming units (PFUs) of rVSVΔG-ZEBOVgp, 2 × 107 PFUs of rVSVΔG-CIEBOVgp or the identical volume of Dulbecco’s Modified Eagle medium (DMEM) (Sigma) by i.m. injection in the left and right caudal thigh. Following immunization, one of the animals in the rVSVΔG-CIEBOVgp group had to be euthanized due to an unrelated medical condition. Twenty-eight days after vaccination the animals were challenged with a total of 10 000 TCID50 of BEBOV i.m. in the left and right caudal thigh. The animals were checked twice daily for clinical signs of disease using an established clinical score sheet. Clinical examinations were performed on anaesthetized animals on days 0, 3, 7, 10, 14, 17, 21 and 28 after infection; at the same time points, animals were bled, and swab samples (rectal, nasal, and throat samples) were taken. The humane endpoint for euthanasia was determined using an approved clinical score sheet.
Virus Detection
RNA was isolated from nonhuman primate blood and swab samples with the RNA mini kit (Qiagen). BEBOV RNA was detected using the Lightcycler 480 RNA Masterhydrolysis kit (Roche) with primers (F 5′-TGAAGCCCCTGAGGGTGTAA-3′, R 5′-CCCTGTTCCAGAAACCTTGTG-3′)/MGB-probe (5′-AGGCTTCCCTCGCTGCCGTTATG-3′) targeting the GP gene. A TCID50 assay was performed as previously described [7]. Briefly, 10-fold serial dilutions of virus stock or clinical material were prepared and used to infect Vero E6 cells. Cells were monitored daily for cytopathic effect using light microscopy.
Clinical Chemistry, Hematology and Coagulation Analysis
Total white blood cell counts, red blood cell counts, platelet counts, hematocrit values, total hemoglobin concentration, mean cell volumes, mean corpuscular values and mean corpuscular hemoglobin concentrations were determined from blood samples that were collected in tubes containing ethylenediaminetetraacetic acid on the Vet ABC (Scil) according to the manufacturer’s instructions. The concentrations of albumin, amylase, alanine aminotransferase (ALT), alkaline phosphatase (ALP), glucose, total protein, total bilirubin, blood urea nitrogen (BUN), globulin, sodium, potassium, calcium, phosphorus and creatinine from serum samples were determined on the VetScan VS2 (Abaxis). Prothrombin (PT) and activated partial thromboplastin (aPTT) times were determined from citrated plasma on the Stago StArt4 coagulation analyzer with the Neoplastine CL Plus and PTT Automate kits (Diagnostica Stago) according to the manufacturer’s instructions.
Serological Testing
Serum samples were collected at the indicated points during examinations and stored at −80°C until analyzed as previously described [24]. Briefly, 96-well plates (Nunc) were coated with 1 μg/mL of recombinant purified ZEBOV, CIEBOV, or BEBOV GP1,2ΔTM (soluble GP1,2 lacking the transmembrane domain). Serum samples were assayed at 4-fold dilutions starting at 1/100 in 5% nonfat milk in phosphate-buffered saline. Wells were washed and incubated with anti-monkey immunoglobulin (Ig) G-horseradish peroxidase (KPL) at 1/1000 dilution. Wells were washed and incubated with the 2,2′-azine-di(3-ethylbenzthiazoline-6-sulfonate) peroxidase substrate system (KPL) and read at 405 nm on a plate reader.
Animal Work and Biosafety
The animal study protocol was approved by the Canadian Science Centre for Human and Animal Health Animal Care Committee. All procedures involving animals were performed by trained personnel according to the guidelines of the Canadian Council on Animal Care in certified facilities. All infectious in vitro and in vivo work was conducted in the Biosafety Level 4 facilities at the National Microbiology Laboratory of the Public Health Agency of Canada (Winnipeg, MB).
RESULTS
Pilot Study
At the time of the study, BEBOV had just recently emerged and was proposed as a new species in the genus Ebolavirus [15]; thus, there were no data on the outcome of infection in any animal model. Therefore, a pilot study was undertaken to determine whether BEBOV infection of cynomolgus macaques was lethal. Following infection, some animals developed clinical parameters consistent with EHF (elevated PT, aPTT, ALT, ALP, and BUN levels and decreased albumin levels and total protein). One animal in each group (1000 and 10 000 TCID50) developed peak viremia levels between 105 and 106 TCID50/mL and had to be euthanized with severe signs of EHF on day 11 after infection (data not shown). The second animal in the 1000 TCID50 group never developed any detectable viremia, developed only mild clinical symptoms (transient elevated temperature), and survived. The second animal in the 10 000 TCID50 group showed peak viremia of 104 TCID50/mL and developed a moderate EHF course. The animal then cleared viremia but simultaneously started to develop neurological symptoms, which finally lead to euthanasia. Overall, both challenge doses did not result in uniform lethality or typical signs of EHF; thus, the comparatively lower lethality in the cynomolgus macaque model might reflect the lower case fatality rate reported during the outbreak in Uganda [15].
Cross-protection Study
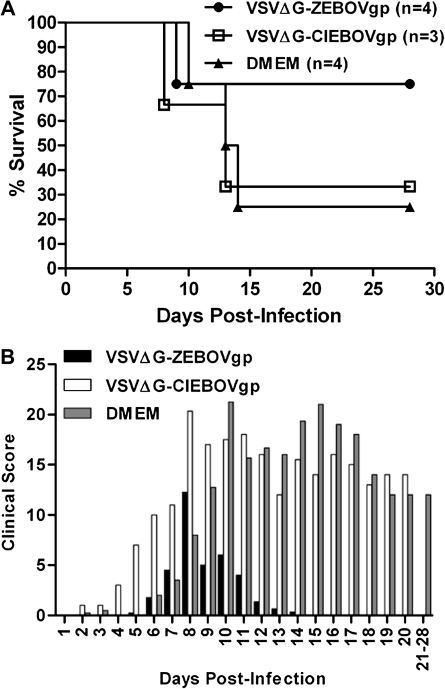
To evaluate the potential of the rVSV-EBOV platform to provide protection against a new emerging EBOV species, animals were vaccinated with either rVSVΔG-ZEBOVgp or rVSVΔG-CIEBOVgp and subsequently challenged with 10,000 TCID50 of BEBOV. All cynomolgus macaques vaccinated with either rVSVΔG-ZEBOVgp or rVSVΔG-CIEBOVgp did not show any signs of illness or distress following vaccination. This is consistent with previous studies in which no adverse effects were observed when nonhuman primates were vaccinated with rVSV-based vectors [9]. The cause of death for the 1 animal in the rVSVΔG-CIEBOVgp–vaccinated group was considered to be unrelated to vaccination and was probably due to a tumor that was discovered at the subsequent necropsy. Following BEBOV challenge, 3 of 4 rVSVΔG-ZEBOVgp–vaccinated animals survived (75% survival), whereas only 1 of 3 rVSVΔG-CIEBOVgp–vaccinated animals (33% survival) and 1 of 4 mock-immunized control animals survived (25% survival) (Figure 1A). This indicates partial protection against disease induced by the rVSVΔG-ZEBOVgp vector, with little or no protection following vaccination with the rVSVΔG-CIEBOVgp vector.
Figure 1.
Outcomes of cynomolgus macaques challenged with Bundibugyo ebolavirus (BEBOV). A, Kaplan-Meier survival curve for cynomolgus macaques vaccinated with sesicular stomatitis virus, lacking its glycoprotein gene (VSVΔG) containing the Zaire ebolavirus glycoprotein (VSVΔG-ZEBOVgp), VSVΔG containing the Côte d'Ivoire ebolavirus glycoprotein (VSVΔG-CIEBOVgp), or Dulbecco's modified Eagle medium (DMEM) and subsequently challenged with BEBOV on day 28 after vaccination. B, Mean clinical score following BEBOV challenge.
Following BEBOV infection, all animals developed clinical signs consistent with mild, moderate, or severe EHF. Although all animals had clinical scores above baseline following BEBOV infection, the rVSVΔG-ZEBOVgp–vaccinated animals had overall lower scores than those in the other 2 groups and returned to baseline the quickest (Figure 1B). The onset of clinical score occurred first in the rVSVΔG-CIEBOVgp–vaccinated animals, whereas the onset in the other 2 groups was comparable. Following the earlier onset of disease in the rVSVΔG-CIEBOVgp–vaccinated animals, there was no substantial difference in clinical scores between these animals and the mock-immunized control animals, again indicating a benefit of vaccination only for the rVSVΔG-ZEBOVgp–vaccinated group (Figure 1A and B).
Hematology and blood chemistry revealed a decrease in red blood cell and platelet counts as well as in hemoglobin and hematocrit values following BEBOV infection in all animals; however, in survivors, these values returned to prechallenge levels by the final sampling day (Figure 2A; data not shown). All animals showed decreased serum levels of albumin and total protein, whereas alkaline phosphatase and alanine aminotransferase levels were increased (Figure 2B and C; data not shown). Large increases in blood urea nitrogen and creatinine were observed in rVSVΔG-CIEBOVgp and control animals, whereas the increase in these parameters was attenuated in rVSVΔG-ZEBOVgp animals (Figure 2D; data not shown). Most animals showed alterations in coagulation, but these changes were less severe than those observed during ZEBOV infection [2, 3]. Three of 4 rVSVΔG-ZEBOVgp–vaccinated animals developed prolonged PT and aPTT values, and all 3 rVSVΔG-CIEBOVgp–vaccinated animals showed prolongation in PT and aPTT values, whereas 3 of 4 control animals developed prolonged PT and all control animals developed prolonged aPTT values (Figure 2E and F). In correlation with the blood chemistry data, the onset of coagulation abnormalities was slightly delayed in DMEM control animals.
Figure 2.
Clinical blood chemistry, blood counts, and coagulation analysis of cynomolgus macaques challenged with Bundibugyo ebolavirus (BEBOV). Whole blood, serum, and plasma samples were collected at the indicated time points and the mean levels of (A) platelets, (B) alanine aminotransferase (ALT), (C) albumin, (D) blood urea nitrogen (BUN), (E) prothrombin time (PT), and (F) activated partial thromboplastin time (aPTT) were determined.
The results of quantitative real-time reverse-transcription PCR (RT-PCR) (Figure 3A) performed on blood specimens indicated that all animals developed BEBOV viremia and that sterile protection was not achieved through immunization (Figure 3). All groups showed near-equivalent levels of genome copies in blood specimens on the initial day for which specimens were analyzed (day 3). Overall, mock-immunized animals had the highest levels and longest durations of viral RNA in their blood (Figure 3A). rVSVΔG-ZEBOVgp–vaccinated animals cleared viremia the fastest, whereas the single surviving animal in the rVSVΔG-CIEBOVgp–vaccinated group cleared viremia at a time similar to that at which the single surviving control animal cleared viremia. rVSVΔG-ZEBOVgp–vaccinated animals showed lower levels of BEBOV viremia, as did the other surviving animals. Overall, viremia levels in mock-immunized controls and nonprotected vaccinated animals were lower than those reported from ZEBOV infections [2, 3]; however, viremia above 104 genome copies/mL at peak times usually correlated with severe disease progression.
Figure 3.
Genome copies of Bundibugyo ebolavirus (BEBOV) in samples from BEBOV-challenged cynomolgus macaques. Blood (A), rectal (B), and throat (C) swab samples were obtained at the time point indicated, total RNA was extracted, quantitative real-time reverse-transcription polymerase chain reaction was performed, and genome copies were calculated. Survivors are indicated by a closed box, whereas animals that were euthanized due to clinical score are indicated by an open box. Data points along the x-axis represent surviving animals that did not have detectable viremia. (VSVΔG-ZEBOVgp), vesicular stomatitis virus lacking its glycoprotein gene, containing the Zaire ebolavirus glycoprotein; (VSVΔG-CIEBOVgp), vesicular stomatitis virus lacking its glycoprotein gene, containing the Côte d'Ivoire ebolavirus glycoprotein; DMEM, Dulbecco's modified Eagle medium.
Vaccinated animals showed a shorter duration and lower levels of viral RNA in rectal, nasal, and throat swab samples (Figure 3B and C; data on nasal swab samples not shown). As observed in the blood, rVSV-ZEBOVgp–vaccinated animals were negative for viral genomes in the swab samples earlier than were the other 2 groups. Overall, control animals showed the highest levels of viral RNA in all samples and at all time points. For most time points, the rVSVΔG-ZEBOVgp–vaccinated animals, compared with control animals, had viral genome copy levels that were 1 to 2 logs lower in clinical specimens, supporting the cross-protective potential of this rVSV vector and indicating less potential for virus shedding and transmission.
All VSV-vaccinated animals developed an IgG titer against the EBOV GP that was included in the vaccine construct (Figure 4A). The mean reciprocal titer of VSVΔG-ZEBOVgp–vaccinated animals at 3 days after challenge was 25 600, whereas VSVΔG-CIEBOVgp–vaccinated animals had markedly lower titers, with a mean reciprocal titer of 1200 (Figure 4A). There was no correlation between survival and antibody titer in vaccinated animals. Two of 4 VSVΔG-ZEBOVgp–vaccinated animals and all 3 VSVΔG-CIEBOVgp–vaccinated animals developed antibodies that cross-reacted with the BEBOV GP antigen, with mean reciprocal titers of 800 and 3200, respectively (Figure 4B). Following BEBOV challenge (at the time of euthanasia), all vaccinated animals had developed increased antibody titers to the BEBOV GP antigen, but titers in the VSVΔG-CIEBOVgp–vaccinated animals were significantly higher (mean reciprocal titer of 25 600) than were titers in the VSVΔG-ZEBOVgp–vaccinated animals (mean reciprocal titer of 1600) (Figure 4B). The DMEM control animals did not develop any antibody response to the BEBOV GP antigen, with the exception of the 1 surviving animal, which showed a reciprocal titer of 1600 at the time of euthanasia.
Figure 4.
Antibody response in cynomolgus macaques following vesicular stomatitis virus (VSV)–based vaccination and Bundibugyo ebolavirus (BEBOV) infection. The reciprocal endpoint immunoglobulin (Ig) G titer from serum samples was determined against (A) the Ebola virus glycoprotein (GP) delivered by the vaccine (Zaire ebolavirus [ZEBOV] GP for animals vaccinated with vesicular stomatitis virus lacking its glycoprotein [VSVΔG] containing ZEBOVgp [VSVΔG-ZEBOVgp] or VSVΔG containing Côte d'Ivoire ebolavirus (CIEBOV) GP [VSVΔG-CIEBOVgp] and Dulbecco's modified Eagle medium [DMEM]-treated animals) at 3 days after BEBOV infection and (B) BEBOV GP at 3 days after BEBOV infection and on the day of euthanasia (Euth). Survivors are indicated by a closed box, whereas animals that were euthanized due to clinical score are indicated by an open box. No titer was detected in DMEM-vaccinated animals against ZEBOV GP (data not shown).
DISCUSSION
Infection of cynomolgus macaques with BEBOV leads to the development of a viral hemorrhagic fever with clinical signs comparable to those associated with infection due to other EBOV species, with the exception of 1 animal in the pilot study that developed neurological signs following what appeared to be mild EHF. However, BEBOV infection is not uniformly lethal; there was a 50% survival rate in the pilot study. Therefore, we used a challenge dose that was an order of magnitude higher (10 000 TCID50) than that used in previous studies with other filovirus species. When all studies using this BEBOV isolate are combined (our pilot study, this vaccine study [which had 25% survival], and another study [which had 25% survival [17]]), BEBOV infection alone has resulted in the survival of 4 of 12 cynomolgus macaques (33% survival). As with any drug or vaccine efficacy studies that use low numbers of animals per treatment group, this low lethality in the cynomolgus macaque model calls for caution when drawing conclusions. Certainly this model needs further investigation, including evaluation of the potential for atypical clinical symptoms, as were observed in 1 of the animals in the pilot study.
Although protection induced by rVSVΔG-ZEBOVgp against BEBOV was incomplete (75% survival), it was considerably higher than that in the control and rVSVΔG-CIEBOVgp–vaccinated animals. This suggests that the rVSVΔG-ZEBOVgp vaccine indeed induced cross-protection against BEBOV; however, they were not protected from disease. In previous studies, successfully vaccinated animals typically did not show changes in blood chemistry or hematological findings [9], whereas, in this study, all animals had blood chemistry results that showed pathophysiologic parameters (eg, elevated liver enzymes) (Figure 2). This indicates that, regardless of survival, pathological processes occur following infection. In contrast to homologous vaccination and challenge studies, all vaccinated animals in our study had detectable viremia (Figure 3A). This is in contract to previous studies where rVSV-vaccinated macaques that were protected from challenge showed no evidence of EBOV or MARV viremia by virus isolation and/or RT-PCR [7, 8, 16, 25]. In the only other successful demonstration of cross-protection using the rVSV platform, a blended vaccine containing equal amounts of rVSVΔG-ZEBOVgp, rVSVΔG-SEBOVgp, and rVSVΔG-MARVgp resulted in complete protection from CIEBOV challenge [16]. Although CIEBOV infection alone resulted in 40% survival, animals vaccinated with the blended vaccine did not show any clinical signs of disease nor changes in blood chemistry or hematological test results [16], which suggests that this approach also resulted in protection from disease. In this study, it is possible that the higher challenge dose, combined with a heterologous vaccination, results in lower efficacy of the vaccine than would be seen with a lower challenge dose.
The only other EBOV vaccine platform to also demonstrate cross-protection involved a multiple DNA prime, rAd5 boost vaccination strategy that included both ZEBOV and SEBOV GP over the course of 1.5 years. Although 100% survival was observed following BEBOV challenge, viremia and clinical disease were noted in some of the animals [17]. This suggests that, despite evidence of cross-protection (eg, survival), there is definitely an absence of sterilizing immunity following cross-species heterologous challenge. Furthermore, this study used a lower challenge dose, which may result in enhanced survival of the vaccinated group, despite having the identical survival in the control group. To date, nearly all vaccination and challenge studies with the rVSV platform have involved a single vaccination [7, 8, 16, 25–27]; however, recent data suggest that the addition of an rVSV boost following the initial vaccination enhances cross-protection in the ZEBOV guinea pig model [23]. As an emergency intervention, a long-duration vaccination and boost schedule is of little use, whereas a rapid-acting (ie, acting following a single vaccination) vaccine that, in addition, would show enhanced efficacy or cross-protection following a booster dose would be highly desirable. Future studies should address whether a booster vaccination with the rVSV platform would enhance cross-protection.
It has been previously reported that survival of CIEBOV infection was protective against subsequent ZEBOV or SEBOV challenge [16]. One hypothesis is that CIEBOV may provide cross-protection against other EBOV species. In contrast, rVSVΔG-CIEBOVgp–vaccinated animals in this study had an outcome that was similar to that of mock-vaccinated animals, which suggests that there was no or limited cross-protection between CIEBOV and BEBOV. This was somewhat surprising, specifically in regards to BEBOV challenge, because BEBOV and CIEBOV GPs have the greatest phylogenetic similarity. Because the correlates of immunity are still not well defined, it is difficult to postulate why a more distantly related species would provide cross-protection when a more closely related species does not. It is possible that there are shared antigenic epitopes (B and/or T cell) between ZEBOV and BEBOV or that rVSVΔG-ZEBOVgp is a better immunogen (ie, has higher expression of the GP) than rVSVΔG-CIEBOVgp. The difference in IgG titers against the vaccine antigen (Figure 4A) suggests that this may be a possibility. It is currently unknown why one vaccine construct would be more immunogenic than the other; however, this would explain why the ZEBOV-based vaccine provided greater protection. Moreover, it has not been determined whether rVSVΔG-CIEBOVgp alone provides protection against homologous challenge. At time of euthanasia, all vaccinated animals had an increase in titer, which suggests that replication of a heterologous virus (in this case, BEBOV) boosts the immune response that is primed by the vaccine. However, the IgG titers are not substantially increased in rVSVΔG-ZEBOVgp–vaccinated animals (Figure 4B), which indicates that these animals are better able to control infection, which correlates with vaccine efficacy (Figure 1A).
An alternative hypothesis for survival of heterologous challenge following CIEBOV infection is that, in the absence of vaccination, survival of infection results in an immune response against multiple viral antigens. Subsequent challenge with a heterologous virus (eg, ZEBOV) results in cross-protection because of the more diverse immune response. This is also in accordance with previous results in which vaccinated animals that survived homologous challenge did not survive heterologous back-challenge [7], because vaccination results in sterilizing immunity, and an immune response against additional viral antigens is likely not generated. This is further supported by data from Marzi et al [23], who reported that guinea pigs that survived wild-type virus infection with different EBOV species were protected from lethal heterologous back-challenge with guinea pig–adapted ZEBOV.
Interestingly, 2 animals that were vaccinated (1 with VSVΔG-CIEBOVgp and 1 with VSVΔG-ZEBOVgp) but did not survive succumbed to disease earlier than did unvaccinated control animals (Figure 1A). Antibody-dependent enhancement has been suggested as a mechanism of pathogenesis during EHF [28, 29], and it is possible that development of antibodies that cross-react with BEBOV may actually enhance infection. Cross-reactive antibody titers against BEBOV GP were higher in rVSVΔG-CIEBOVgp–vaccinated animals following vaccination, which suggests that this is a possibility. Future vaccination campaigns may have to keep this in mind in areas where infection with >1 species of EBOV is possible.
Although the possibility of cross-protection between EBOV species is encouraging for future vaccine development (ie, there is the possibility of providing protection against a new emerging species), this protection is far from complete and may have to be taken in the context of the pathogenicity of the challenge viruses. For example, compared with ZEBOV and SEBOV, BEBOV appears to be less virulent. This is indicated by the comparably low (25%) case-fatality rate in humans [15]; the inability of BEBOV to cause 100% lethality in the cynomolgus macaque model (as demonstrated in our study and [17]); and its lower level of replication, its reduced ability to stimulate proinflammatory cytokines, and the delayed death of macrophages in human peripheral blood mononuclear cells in vitro [30]. Together, this indicates that the ability of the rVSV platform, as well as of other vaccine platforms, to provide cross-protection may be somewhat limited, because such cross-protection has only been demonstrated in the case of BEBOV and CIEBOV challenge. However, because the rVSV platform has been demonstrated to have the potential to be reused [16], upon the emergence of a new species of EBOV, this platform could be rapidly adapted to include the GP from the new species and be used in subsequent vaccination campaigns, thus providing enhanced protection against an emerging virus.
Funding
This work was supported by the National Microbiology Laboratory, Public Health Agency of Canada; the Manitoba Health Research Council (to D. F.); and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Acknowledgments
We thank John Rose (Yale University, New Haven, CT), for kindly providing us with the VSV reverse-genetics system, and the Special Pathogens Branch, Division of Viral and Rickettsial Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention (Atlanta, GA), in particular Thomas Ksiazek (now University of Texas Medical Branch, Galveston TX), Pierre Rollin, and Stuart Nichol, for providing the BEBOV virus isolate.
References
- 1.Feldmann H, Geisbert T, Jahrling P, et al. Filoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy: VIIIth report of the International Committee on the Taxonomy of Viruses. San Diego, CA: Elsevier; 2005. pp. 645–53. [Google Scholar]
- 2.Bray M, Geisbert TW. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol. 2005;37:1560–6. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2010;377:849–62. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez JP, Pourrut X, Leroy E. Ebolavirus and other filoviruses. Curr Top Microbiol Immunol. 2007;315:363–87. doi: 10.1007/978-3-540-70962-6_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–4. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukreyev A, Rollin PE, Tate MK, et al. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol. 2007;81:6379–88. doi: 10.1128/JVI.00105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones SM, Feldmann H, Stroher U, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 8.Daddario-DiCaprio KM, Geisbert TW, Geisbert JB, et al. Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J Virol. 2006;80:9659–6. doi: 10.1128/JVI.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisbert TW, Bausch DG, Feldmann H. Prospects for immunisation against Marburg and Ebola viruses. Rev Med Virol. 2010;20:344–57. doi: 10.1002/rmv.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swenson DL, Wang D, Luo M, et al. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol. 2008;15:460–7. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riemenschneider J, Garrison A, Geisbert J, et al. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine. 2003;21:4071–80. doi: 10.1016/s0264-410x(03)00362-1. [DOI] [PubMed] [Google Scholar]
- 12.Geisbert TW, Feldmann H. Vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis. 2011 doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratt WD, Wang D, Nichols DK, et al. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol. 2010;17:572–81. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan NJ, Geisbert TW, Geisbert JB, et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006;3:e177. doi: 10.1371/journal.pmed.0030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towner JS, Sealy TK, Khristova ML, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4:e1000212. doi: 10.1371/journal.ppat.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geisbert TW, Geisbert JB, Leung A, et al. Single-injection vaccine protects nonhuman primates against infection with Marburg virus and three species of Ebola virus. J Virol. 2009;83:7296–304. doi: 10.1128/JVI.00561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hensley LE, Mulangu S, Asiedu C, et al. Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus species. PLoS Pathog. 2010;6:e1000904. doi: 10.1371/journal.ppat.1000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn JH, Becker S, Ebihara H, et al. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol. 2010;155:2083–103. doi: 10.1007/s00705-010-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroy EM, Kumulungui B, Pourrut X, et al. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–6. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 20.Towner JS, Pourrut X, Albarino CG, et al. Marburg virus infection detected in a common African bat. PLoS One. 2007;2:e764. doi: 10.1371/journal.pone.0000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swenson DL, Warfield KL, Negley DL, Schmaljohn A, Aman MJ, Bavari S. Virus-like particles exhibit potential as a pan-filovirus vaccine for both Ebola and Marburg viral infections. Vaccine. 2005;23:3033–42. doi: 10.1016/j.vaccine.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 22.Garbutt M, Liebscher R, Wahl-Jensen V, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78:5458–65. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzi A, Ebihara H, Callison J, et al. Vesicular stomatitis virus based Ebola vaccines with improved cross-protective efficacy. J Infect Dis. 2011 doi: 10.1093/infdis/jir348. in press, Filovirus supplement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakayama E, Yokoyama A, Miyamoto H, et al. Enzyme-linked immunosorbent assay for the detection of filovirus species-specific antibodies. Clin Vaccine Immunol. 2010;17:1723–28. doi: 10.1128/CVI.00170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisbert TW, Daddario-Dicaprio KM, Lewis MG, et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 2008;4:e1000225. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu X, Fernando L, Alimonti JB, et al. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One. 2009;4:e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takada A, Feldmann H, Ksiazek TG, Kawaoka Y. Antibody-dependent enhancement of Ebola virus infection. J Virol. 2003;77:7539–44. doi: 10.1128/JVI.77.13.7539-7544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada A, Watanabe S, Okazaki K, Kida H, Kawaoka Y. Infectivity-enhancing antibodies to Ebola virus glycoprotein. J Virol. 2001;75:2324–30. doi: 10.1128/JVI.75.5.2324-2330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta M, Goldsmith CS, Metcalfe MG, Spiropoulou CF, Rollin PE. Reduced virus replication, proinflammatory cytokine production, and delayed macrophage cell death in human PBMCs infected with the newly discovered Bundibugyo ebolavirus relative to Zaire ebolavirus. Virology. 2010;402:203–8. doi: 10.1016/j.virol.2010.03.024. [DOI] [PubMed] [Google Scholar]