Abstract
In vitro DNA synthesis on a phi X174 template primed with a restriction fragment and catalyzed by the Escherichia coli DNA polymerase I large (Klenow) fragment (pol I) terminates at the nucleotide preceding a site that has been altered by ultraviolet irradiation or treatment with N-acetylaminofluorene. Termination on ultraviolet-irradiated templates is similar when synthesis is catalyzed by E. coli DNA polymerase III holoenzyme (pol III), phage T4 DNA polymerase, a polymerase alpha from human lymphoma cells, or avian myeloblastosis virus reverse transcriptase. 3' leads to 5' exonuclease activity cannot be detected in the reverse transcriptase and DNA polymerase alpha preparations. On N-acetylaminofluorene templates, pol I, pol III, and T4 polymerase reactions terminate immediately preceding the lesion, whereas reverse transcriptase-catalyzed reactions and, at some positions in the sequence, polymerase alpha-catalyzed reactions terminate at the site of the lesion. Substitution of Mn2+ for Mg2+ changes the pattern of pol I-catalyzed termination sites. The data suggest that termination is a complicated process that does not depend exclusively on the 3' leads to 5' exonuclease activity associated with many polymerases.
Full text
PDF
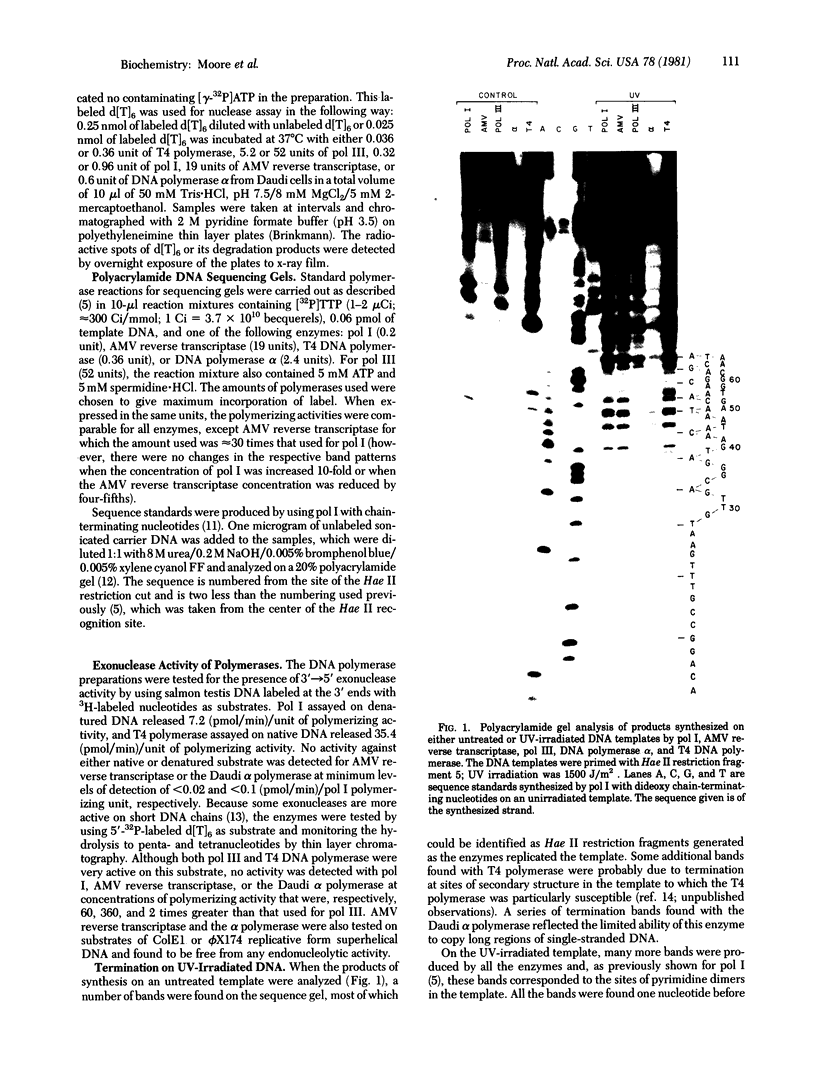


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. A role for single-strand breaks in bacteriophage phi-X174 genetic recombination. J Mol Biol. 1974 Sep 25;88(3):629–651. doi: 10.1016/0022-2836(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Bose K., Karran P., Strauss B. Repair of depurinated DNA in vitro by enzymes purified from human lymphoblasts. Proc Natl Acad Sci U S A. 1978 Feb;75(2):794–798. doi: 10.1073/pnas.75.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges B. DNA polymerase and mutation. Nature. 1978 Oct 19;275(5681):591–592. doi: 10.1038/275591b0. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M., Radman M. Molecular mechanisms of induced mutagenesis. Replication in vivo of bacteriophage phiX174 single-stranded, ultraviolet light-irradiated DNA in intact and irradiated host cells. J Mol Biol. 1977 Nov 25;117(1):95–110. doi: 10.1016/0022-2836(77)90025-0. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. The effect of template secondary structure on vaccinia DNA polymerase. J Biol Chem. 1979 Aug 25;254(16):7820–7826. [PubMed] [Google Scholar]
- Clayton L. K., Goodman M. F., Branscomb E. W., Galas D. J. Error induction and correction by mutant and wild type T4 DNA polymerases. Kinetic error discrimination mechanisms. J Biol Chem. 1979 Mar 25;254(6):1902–1912. [PubMed] [Google Scholar]
- Fersht A. R. Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: spontaneous mutation by misincorporation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4946–4950. doi: 10.1073/pnas.76.10.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberger D., Blobstein S. H., Weinstein I. B. Modification of ribonucleic acid by chemical carcinogens. VI. Effect of N-2-acetylaminofluorene modification of guanosine on the codon function of adjacent nucleosides in oligonucleotides. J Mol Biol. 1974 Feb 5;82(4):459–468. doi: 10.1016/0022-2836(74)90241-1. [DOI] [PubMed] [Google Scholar]
- Hibner U., Alberts B. M. Fidelity of DNA replication catalysed in vitro on a natural DNA template by the T4 bacteriophage multi-enzyme complex. Nature. 1980 May 29;285(5763):300–305. doi: 10.1038/285300a0. [DOI] [PubMed] [Google Scholar]
- Kriek E. Carcinogenesis by aromatic amines. Biochim Biophys Acta. 1974 Sep 9;355(2):177–203. doi: 10.1016/0304-419x(74)90003-1. [DOI] [PubMed] [Google Scholar]
- Low R. L., Rashbaum S. A., Cozzarelli N. R. Purification and characterization of DNA polymerase III from Bacillus subtilis. J Biol Chem. 1976 Mar 10;251(5):1311–1325. [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Strauss B. S. Termination of vitro DNA synthesis at AAF adducts in the DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4473–4484. doi: 10.1093/nar/8.19.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Strauss B. S. Sites of inhibition of in vitro DNA synthesis in carcinogen- and UV-treated phi X174 DNA. Nature. 1979 Apr 12;278(5705):664–666. doi: 10.1038/278664a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M. A., Dube D. K., Loeb L. A. On the fidelity of DNA replication. Metal activation of Escherichia coli DNA polymerase I. J Biol Chem. 1979 Jan 10;254(1):107–111. [PubMed] [Google Scholar]
- Villani G., Boiteux S., Radman M. Mechanism of ultraviolet-induced mutagenesis: extent and fidelity of in vitro DNA synthesis on irradiated templates. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3037–3041. doi: 10.1073/pnas.75.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymouth L. A., Loeb L. A. Mutagenesis during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1924–1928. doi: 10.1073/pnas.75.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]