Abstract
The transmission of vector-borne pathogens is greatly influenced by the ecology of their vector, which is in turn shaped by genetic ancestry, the environment, and the hosts it feeds on. One group of vectors, the mosquitoes in the Culex pipiens complex, play key roles in the transmission of a range of pathogens including several viruses such as West Nile and St. Louis encephalitis viruses, avian malaria (Plasmodium spp.), and filarial worms. The Cx. pipiens complex includes Cx. pipiens pipiens with two forms, pipiens and molestus, Cx. pipiens pallens, Cx. quinquefasciatus, Cx. australicus, and Cx. globocoxitus. While several members of the complex have limited geographic distributions, Cx. pipiens pipiens and Cx. quinquefasciatus are found in all known urban and sub-urban temperate and tropical regions, respectively, across the world, where they are often principal disease vectors. In addition, hybrids are common in areas of overlap. Although gaps in our knowledge still remain, the advent of genetic tools has greatly enhanced our understanding of the history of speciation, domestication, dispersal, and hybridization. We review the taxonomy, genetics, evolution, behavior, and ecology, of members of the Cx. pipiens complex and their role in the transmission of medically important pathogens. The adaptation of Cx. pipiens complex mosquitoes to human-altered environments led to their global distribution through dispersal via humans and, combined with their mixed feeding patterns on birds and mammals (including humans), increased the transmission of several avian pathogens to humans. We highlight several unanswered questions that will increase our ability to control diseases transmitted by these mosquitoes.
Keywords: Vector borne disease, invasive, West Nile virus, arbovirus, bridge vector, vector competence, Plasmodium
1. Introduction
Vector-borne diseases such as malaria, plague, yellow fever, lymphatic filariasis have shaped our genetic make-up (Aubry, 2008; Tarantola et al., 2009), driven the rise and fall of civilizations (Vazeille et al., 2008), and the outcome of wars (Delatte et al., 2008). These and other vector-borne diseases such as dengue, Lyme disease and West Nile encephalitis, affect our ability to enjoy the outdoors (Coffinet et al., 2007; Kiehn et al., 2008) and, by separating humans from nature, potentially affect how we value biodiversity. Vector-borne pathogens include a wide range of organisms that are transmitted by a diverse set of species, including arthropods such as fleas, sandflies, ticks and mosquitoes (Anosike et al., 2007). It follows that the specific life-history demands, abilities, and limitations of the vectors must have an enormous impact on transmission and thus the severity of disease outbreaks.
Determining the principal vectors for pathogens and what influences their transmission rates is a critical step in understanding patterns of transmission in space and time and in developing effective control interventions. Frequently an initial strategy for prevention of human diseases is to target the vectors most likely to bite humans. For pathogens where humans are an infectious host, a vector that bites humans exclusively with no or few “lost” bites to incompetent hosts such as pets, livestock, or wildlife, would generate the highest transmission rates (Kilpatrick et al., 2007; Townson and Nathan, 2008). This is the case for the dengue viruses , the filarial worms that cause lymphatic filariasis (Wuchereria bancrofti), and Plasmodium falciparum, the protozoon that causes human malaria. For these pathogens, humans are infectious hosts and the pathogens are primarily (but not exclusively) transmitted by mosquitoes that feed extensively on humans (Chandler et al., 1975; Siriyasatien et al., 2010).
Many human vector-borne diseases, however, are zoonoses that have amplification cycles involving species other than humans. These include Lyme disease, rickettsia, plague, and arboviral diseases such as yellow fever, West Nile, St. Louis, and Eastern Equine encephalitis, which have primates, small mammals, or birds as reservoirs. For many avian arboviruses, humans are dead-end hosts, because viremia (the concentration of virus in the blood) in humans for these viruses is too low to result in infection in biting vectors. This sometimes creates an apparent paradox because the principal vector of a human disease may be one that feeds primarily on non-human hosts and only a small fraction of its bloodmeals are derived from humans. This paradox is particularly well illustrated by Cx. pipiens complex mosquitoes and the transmission of WNV in North America, as discussed in detail below.
In this review we examine in detail the taxonomy, phylogeny, ecology, population genetics, behavior, and vector competence of the Cx. pipiens complex, a group of morphologically and evolutionarily closely related mosquitoes with a long history of association with humans (Vinogradova, 2000). We discuss the role of these mosquitoes in the transmission of arboviruses including a review of host feeding patterns from blood meal analyses. We also discuss patterns of increased association between humans and these mosquitoes and the epidemiological consequences. Our aim is to highlight the role of vector ecology in transmission and its influence on the evolution of vector-borne pathogens, and integrate both these factors in determining the best approaches for control.
2. Cx. pipiens complex mosquitoes
2.1. Taxonomy of the Cx. pipiens complex
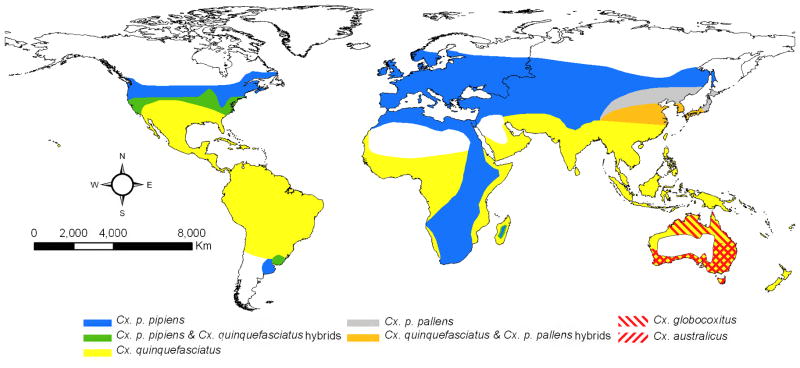
The current taxonomy in the Catalog of the Mosquitoes of the World (Knight, 1978) maintained by the Walter Reed Biosystematics Unit at the Smithsonian Institution (wrbu.si.edu), recognizes the following species as members of the Cx. pipiens complex: Cx. pipiens, Cx. quinquefasciatus, Cx. australicus, and Cx. globocoxitus (Figure 1). A species complex is usually defined as a group of evolutionarily closely related species that consequently are often difficult to separate morphologically (Collins and Paskewitz, 1996). This taxonomy is still controversial because of the historical dependence of taxonomy on morphological differences, the lack of such differences among many of the members of the Cx. pipiens complex, and especially the presence of hybrids (Harbach et al., 1985; Mattingly, 1965; Mattingly et al., 1951; Vinogradova, 2000; Zhao and Lu, 1999). Although all species in the Cx. pipiens complex are identifiable by the shape of the male genitalia (Barr, 1957; Dobrotworsky, 1967), this trait cannot be used to identify females, the primary target of surveillance efforts. Nonetheless their close evolutionary association has been repeatedly supported by genetic analyses (Kent et al., 2007; Miller et al., 1996) as well as by the relative transferability of genetic markers across species (Smith et al., 2005) . Further, Cx pipiens has two recognized subspecies, Cx. pipiens pipiens, an Old World taxa originally distributed from Northern Europe to the highlands of South Africa (Harbach et al., 1985) , and Cx. p. pallens, distributed east of the Urals across temperate Asia (Fonseca et al., 2009). Culex p. pipiens also has two recognized forms “pipiens” and “molestus”, which differ dramatically in ecology (see more details under “Behavior and physiology” below). Culex p. pipiens (possibly both forms or their hybrids, (Fonseca et al., 2004)) have been accidentally introduced to temperate zones in North America and South America, while only Cx. p. pipiens form molestus has been introduced to cities in Japan, Republic of South Korea, and Australia (Vinogradova, 2000).
Figure 1.
Global distribution of the Cx. pipiens complex mosquitoes. Geographic range for Cx. p. pipiens may include both forms (pipiens and molestus) and in temperate Asia and Australia although Cx. p. pipiens form molestus can be found in urban environments we omitted it for clarity. Note that Cx. australicus and Cx. globocoxitus are restricted to Australia. (Adapted from (Mattingly, 1965; Smith and Fonseca, 2004; Vinogradova, 2000).
Culex quinquefasciatus thrives in tropical and sub-tropical regions, including the African lowlands, Americas, Asia, and Australia (Fonseca et al., 2006). Together Cx. pipiens and Cx. quinquefasciatus occur across most inhabited areas globally and are often closely associated with humans, earning them the names of northern and southern house mosquitoes, respectively. Where their ranges overlap, Cx. pipiens (both subspecies) and Cx. quinquefasciatus can hybridize extensively as repeatedly shown by genitalia analysis, allozyme polymorphisms and more recently, microsatellite (nuclear DNA) analysis. There is extensive introgression between populations of Cx. pipiens and Cx. quinquefasciatus in North America, Argentina, Madagascar, Japan and Republic of South Korea (Barr, 1957; Cornel et al., 2003; Fonseca et al., 2009; Humeres et al., 1998; Kothera et al., 2009; Urbanelli et al., 1997; Urbanelli et al., 1995; Wang et al., 2000).
This introgression is in stark contrast to the sympatric, but non-hybridizing populations of Cx. pipiens and Cx. quinquefasciatus in South Africa (Cornel et al., 2003). The lack of hybridization in South Africa between Cx. pipiens and Cx. quinquefasciatus is supported by the fact that there, only Cx. quinquefasciatus is infected with Wolbachia pipientis, a rickettsian intracellular parasite that can limit reproduction between insect populations (Dobson 2003). Of further interest, throughout most of North America Cx. pipiens is considered the primary vector of WNV (Kilpatrick et al., 2005; Kramer et al., 2008; Turell et al., 2002), while in Africa it is not (McIntosh et al., 1967).
2.2. Behavior and physiology across the complex
The Cx.pipiens complex includes populations with distinct behaviors and physiologies that greatly influence their vectorial capacity, or the efficiency of pathogen transmission. In addition to their preferred larval habitat (underground hypogeous versus above-ground epigeous, rural versus urban) and geographic range distribution, members of the Cx. pipiens complex also exhibit wide urban variations in host feeding patterns, gonotrophic development (autogeny versus anautogeny), and means or presence of adult female hibernation (quiescence versus diapause). Hibernation of Cx. pipiens complex mosquitoes involves the harmonization of many behavioral, biochemical, and physiological pathways within the mosquito and is often initiated by environmental signals (e.g. photoperiod, temperature, nutrient availability, and moisture) that result in significant physiological or behavioral changes.
Under the influence of a short photoperiod, Culex p. pipiens form pipiens and Cx. p. pallens females will mate but will not seek a blood meal (Eldridge, 1987). Indeed, their ability to digest blood under short photoperiods is severely hampered by the downregulation of lipases, enzymes that digest blood (Robich and Denlinger, 2005). Instead, females raised under short photoperiod accumulate fat by feeding on nectar and other carbohydrate rich sources, a task aided by the simultaneous upregulation of proteins involved in carbohydrate digestion (Robich and Denlinger, 2005). Mated but not blood fed females retreat to cold and moist secluded/safe areas usually partly underground, such as basements and caves (Eldridge, 1987). Inside these hibernaculae, they survive freezing winters in partial torpor or diapause. In contrast, Cx. quinquefasciatus, Cx. globocoxitus, Cx. australicus, and Cx. p. pipiens form molestus do not diapause and will develop continuous cohorts across the seasons, although lower temperatures will slow down development (Dobrotworsky, 1967; Eldridge, 1987). For example, Cx. australicus in more temperate southern regions in Australia will retreat to protected areas but does not exhibit gonotrophic dissociation (also called ovarian arrest) and therefore does not undergo true diapause (Dobrotworsky, 1967). The propensity to enter diapause appears to be relatively consistent within taxa in the complex, although this may be a circular argument as that ability is often used to differentiate the taxa. One exception are populations of Cx. p. pipiens from South Africa that appear to be incapable of true diapause (Jupp, 1987) a pattern that deserves to be further explored. If Cx. pipiens mosquitoes enter diapause in late fall and cease blood feeding, this ends their contribution to transmission, whereas in areas with similar climate but where Cx. quinquefasciatus is present, the transmission season might be extended.
A second trait that varies across the Cx. pipiens complex is the expression of autogeny, or the ability to lay eggs without first obtaining vertebrate blood. Autogeny can increase mosquito abundance, especially if hosts for blood meals are limiting, but it could also decrease transmission of pathogens since mosquitoes would not need to feed to lay their first batch of eggs. Autogenous oviposition behavior may be influenced by larval overcrowding or diet: evidence suggests that genetically anautogenous mosquitoes cannot become autogenous by superabundant larval feeding, but autogenous development can be suppressed by the starving or overcrowding of genetically autogenous larvae (Spielman, 1971). Autogeny is a trait associated with Cx. p. pipiens form molestus, which in cold climates survives in underground sites such as sewage or subway systems in cities (Fonseca et al., 2004; Spielman, 2001), but autogeny can also be common in aboveground populations of Cx. p. pipiens form molestus in mild climates such as those in southern Europe (Gomes et al., 2009), northern Africa (Knight and Malek, 1951), and parts of northern California (Iltis, 1966).
A third trait that varies substantially within the complex is the propensity to feed on avian or mammalian blood for egg production. This is discussed in more detail in section 4, below.
2.3. Accurate identification of Culex species
The accurate identification of mosquitoes is critical for vector surveillance and control because the abundance and infection of different vectors frequently indicates different levels of risk of transmission. Accurate speciation of Cx. pipiens complex mosquitoes relies on a wide variety of methods for precise identification. Quantitative differences in the shape of the male genitalia (DV/D ratio), and quantitative characters in wing venation (cross vein index ratio) have been the gold standard to separate Cx. pipiens from Cx. quinquefasciatus (Barr, 1957). However, hybrids often show intermediate phenotypic and genotypic manifestations of the parent population, thus making reliance on some of the above mentioned morphological characters unreliable (Aspen et al., 2003; Aspen and Savage, 2003; Cornel et al., 2003; Sanogo et al., 2008; Urbanelli et al., 1997). Also, there are no known morphological differences between the two forms of Cx. pipiens (Harbach et al., 1984) and therefore their identification in temperate latitudes has been traditionally associated with differences in egg development (autogeny as frequently observed in Cx. p. pipiens form molestus) and/or preferred larval habitat - underground in areas of difficult access for Cx. p. pipiens form molestus or aboveground for Cx. p. pipiens form pipiens.
There are also several species whose females are often indistinguishable from those in the Cx. pipiens complex (especially if damaged during collection), but which are not members of the complex because they are genetically distinct. These include Culex restuans, Cx. nigripalpus and Culex salinarius in North America, Culex torrentium in northern Europe, Culex pervigilans in New Zealand, and Culex vagans in central and eastern Asia. To facilitate mosquito identification, several polymerase chain reaction-based assays that use species-specific primers targeting 12S-ribosomal (Crabtree et al., 1995), the acetylcholinesterase 2 locus (Aspen and Savage, 2003; Smith and Fonseca, 2004), or other nuclear sequences (Bahnck and Fonseca, 2006) have been developed (Table 1).
Table 1.
Summary of the available molecular assays to identify taxa within the Cx. pipiens complex and morphologically related species.
| Name | Locus | Taxa it targets | Reference |
|---|---|---|---|
| “Crabtree” | Ribosomal |
Cx. pipiens sl Cx. restuans Cx. salinarius |
(Crabtree et al., 1995) |
| Subtractive hybridization | Nuclear |
Cx. p. pipiens Cx. p. quinquefasciatus |
(Crabtree et al., 1997) |
| Aspen et al. | Ribosomal |
Cx. pipiens sl, Cx. nigripalpus |
(Aspen et al., 2003) |
| Smith & Fonseca | Nuclear (Ace2) |
Cx. p. pipiens Cx. quinquefasciatus Cx. p. pallens Cx. torrentium Cx. australicus Cx. pervigilans |
(Smith and Fonseca, 2004) |
| Ace.2 | Nuclear (Ace2) |
Cx. pipiens Cx. quinquefasciatus |
(Aspen and Savage, 2003) |
| HotAce | Nuclear (Ace2) |
Cx. pipiens Cx. quinquefasciatus |
(Savage et al., 2007) |
| Kasai et al. | Nuclear (Ace2) |
Cx. p. pallens Cx. p. pipiens f. molestus |
(Kasai et al., 2008) |
| Bahnck & Fonseca | Nuclear (CQ11) |
Cx. p. pipiens f. pipiens Cx. p. pipiens f. molestus |
(Bahnck and Fonseca, 2006) |
2.4. The two Cx. p. pipiens forms: recent developments
Recent work has shown that hybrizidation between the two forms of Cx. pipiens may have important implications for pathogen transmission. Genetic isolation exists between northern European populations of the two forms of Cx. p. pipiens, whereas extensive hybridization is present in the United States (Bahnck and Fonseca, 2006; Fonseca et al., 2004). Hybridization between bird biting and more mammalian biting forms of Cx. pipiens was hypothesized to make Cx. pipiens a superior bridge vector of WNV to humans. This is because mosquitoes would be frequently infected from feeding on birds, but could also transmit the virus to humans (Fonseca et al., 2004). Subsequently, two studies showed that North American Cx. pipiens mosquitoes with higher genetic ancestry from Cx. pipiens form molestus were in fact more likely to feed on humans (Kilpatrick et al., 2007) and mammals (Huang et al., 2009). This indicates that high “molestus” ancestry in a population may have led to increased transmission of WNV to humans. These results were somewhat surprising since recombination associated with hybridization would be expected to rapidly disassociate behavioral traits from the combination of neutral microsatellite markers that indicates species ancestry. Indeed, recombination may explain why US Cx. pipiens with a strong molestus ancestry (>80%) fed on birds only 60% of the time (Kilpatrick et al., 2007) (Cx. pipiens with little (<10%) molestus ancestry fed on birds >90% of the time). The strong association between behavioral and neutral markers may indicate an influx of genes from pure Cx. p. pipiens form molestus populations into the aboveground populations, possibly during the summer.
3. Culex pipiens mosquitoes and humans
Ancestral Cx. pipiens may have been an African species that colonized temperate northern European regions as well as the highlands of Africa after the last glaciations. More recently, possibly as early as the 16th century, it arrived in the New World and is now found in cities and suburbs in all temperate climates (Vinogradova 2000). In contrast, the ancestral distribution of Cx. quinquefasciatus was indubitably tropical, possibly in south-east Asia (Fonseca et al 2006), although further population genetic studies including extensive sampling in East Africa and Asia are necessary. The presence of Cx. quinquefasciatus in Western Africa is likely recent, as suggested by the early ecological observations of the species in the 1950’s (Mattingly 1951) and by more recent genetic analysis (Fonseca et al 2006). Thus, Cx. quinquefasciatus was likely not introduced into the New World with the slave trade as previously proposed (Vinogradova 2000) and instead may have reached western Africa in boats returning from the Americas.
The success of the Cx. pipiens complex mosquitoes is partly due to their ability to exploit the large amounts of “food” found in standing water generated by humans and livestock. Unlike most other species of mosquitoes, Culex complex species commonly thrive in aquatic habitats with a high organic content (Bockarie et al., 2009, Vinogradova 2000). Many researchers have also attributed the worldwide distribution and abundance of Culex p. pipiens and Cx. quinquefasciatus to their ability to exploit several modes of human transportation (Barr, 1957; Kilpatrick et al., 2004). Filthy bilges of large ships may have provided habitat for juvenile mosquitoes, and the abundant human and animal occupants may have provided a suitable blood source for mosquitoes to undergo several generations, particularly during long voyages. In addition, these journeys may have selected for mosquitoes ability to mate in confined spaces and survival on ships likely required feeding on mammals.
The traits of the types of mosquitoes that have spread across the world is demonstrated by recent worldwide population genetic analysis of the yellow fever mosquito, Aedes aegypti (Brown et al., 2011), a species that currently exhibits a pantropical distribution. All populations of Ae. aegypti, outside Africa appear to derive from a single African population, and potentially a single domestication event from which they spread across the world through human commerce of slaves and goods. Little evidence of secondary expansion of Ae. aegypti from Africa was found, underscoring the stringent requirements of life associated with humans and the rarity of such events.
In the Cx. pipiens complex, however, there were two separate domestication events. The advent of agriculture in North Africa may have led to Cx. p. pipiens form molestus (Fonseca et al., 2004) whereas the advent of organized agriculture and high density civilizations in southeast Asia likely resulted in the domestic forms of Cx. quinquefasciatus (Fonseca et al 2006)(Kenoyer, 1998)
Cx. p. pipiens form molestus fits the stereotype of the “domestic” mosquito: it thrives in highly polluted sewers, mates in confined spaces often entering houses, and feeds readily on mammals, especially humans as evidenced by their role as principal vectors of lymphatic filariasis in Egypt (Abdel-Hamid et al 2011). Likewise, the existence of domestic populations of Cx. quinquefasciatus is supported by the critical role of this species in the transmission of lymphatic filariasis in China and Southeast Asia (Liu et al 1991, Sudomo et al 2010). Without a highly specialized vector this parasite may not have become exclusively human (Michael and Gambhir, 2010).
4. Pathogens transmitted by Cx. pipiens complex mosquitoes
Cx. pipiens complex mosquitoes play important roles in the transmission of several pathogens that infect humans including WNV, St. Louis encephalitis virus, and filarial worms (Bogh et al., 1998; Reisen et al., 1992; Turell et al., 2002) as well as wildlife pathogens such as bird malaria (Plasmodium spp, Lapointe et al 2005). This results partly from the wide variety of hosts on which they feed and also their very high abundances in developed areas. Their exact role and importance in different aspects of transmission (e.g. among avian hosts vs. between avian hosts and humans or other mammals such as horses) has sometimes been debated, but is becoming increasingly clear.
Variation in feeding between the different species and different populations within a species plays an important role in the pathogens they transmit. For example, in southeast Asia Cx. quinquefasciatus feeds predominantly on humans and is the principal vector of human lymphatic filariasis whereas in Hawaii Cx. quinquefasciatus likely feeds predominantly on birds because it is the most efficient vector of the local species of avian malaria (Plasmodium relictum) and avian pox among the endemic endangered birds (Fonseca et al., 1998; Van Riper et al., 1986).
In contrast, for human zoonotic pathogens with avian hosts, it is the mixed feeding patterns of species in the Cx. pipiens complex result in them playing key roles. For example, in the northeastern and north central US, the predominant vector of WNV is Cx. pipiens (Andreadis et al., 2004; Hamer et al., 2008; Kilpatrick et al., 2005; Turell et al., 2002), which transmits virus among a variety of avian hosts, and also is important in transmission of virus to humans (Hamer et al., 2008; Kilpatrick et al., 2005), especially later in the transmission season (Kilpatrick et al., 2006b). Evidence for the importance of Cx. pipiens mosquitoes in the transmission of WNV comes from the large number of virus isolations from field collected individuals (Andreadis et al., 2004; Lukacik et al., 2006), their moderately efficient vector competence for WNV (Sardelis et al., 2001; Tiawsirisup et al., 2005; Turell et al., 2005), their abundance in urban environments (Andreadis et al., 2004; Kilpatrick et al., 2005; Lukacik et al., 2006; Ruiz et al., ; Savage et al., 2006), their mixed host feeding behavior (Apperson et al., 2004; Hamer et al., 2008; Kilpatrick et al., 2006b), their ability to vertically pass the virus from an infected female to her offspring (Dohm et al., 2002), and their capacity to serve as an overwintering reservoir of WNV (Farajollahi et al., 2005; Nasci et al., 2001). In addition, their higher abundance in urban environments has been hypothesized as a key factor in increasing WNV transmission rates in urbanized areas (Bowden et al., ; Brown et al., 2008; Gomez et al., 2008).
5. Host feeding
Many questions still remain on the exact roles of different mosquito vectors in arbovirus transmission cycles. This has partly stemmed from recent research that has challenged previous characterizations of the feeding patterns exhibited by Culex mosquitoes, and the level of transmission risk to humans associated with these vectors (Fonseca et al. 2004, Kilpatrick et al. 2005, Kilpatrick et al. 2007, Hamer et al. 2008, 2009)(Kilpatrick et al., 2006b). Culex pipiens mosquitoes are known to be enzootic vectors for several arboviruses, and historically had been classified as ornithophilic mosquitoes. However, they are increasingly recognized as important bridge vectors based on comprehensive integrated studies that examine host preferences, vector/host abundance, virus infection rates, and vector competence. Here we review variation in feeding patterns of Cx. pipiens and Cx. quinquefasciatus mosquitoes in the context of arbovirus transmission. Further, we attempt to provide a broader perspective by comparing them to two other Culex species from North America, Cx. tarsalis and Cx. restuans, which are also important in arboviral transmission.
We found seven studies of the feeding patterns of Cx. pipiens mosquitoes in North America, nine studies of Cx. quinquefasciatus (six from North America, two from Australia, and one from Mexico), seven studies of feeding patterns of Cx. restuans, and ten studies of Cx. tarsalis, all from North America (Figure 2; Supplemental Online Table 1). All these studies determined the fraction of blood meals derived from mammals and birds (and usually from other vertebrate classes), and all determined the fraction that had fed on humans. This enables an examination of each species’ role and efficiency in the transmission of avian pathogens to humans, as well as their efficiency in transmitting both human and non-human mammal pathogens.
Figure 2.
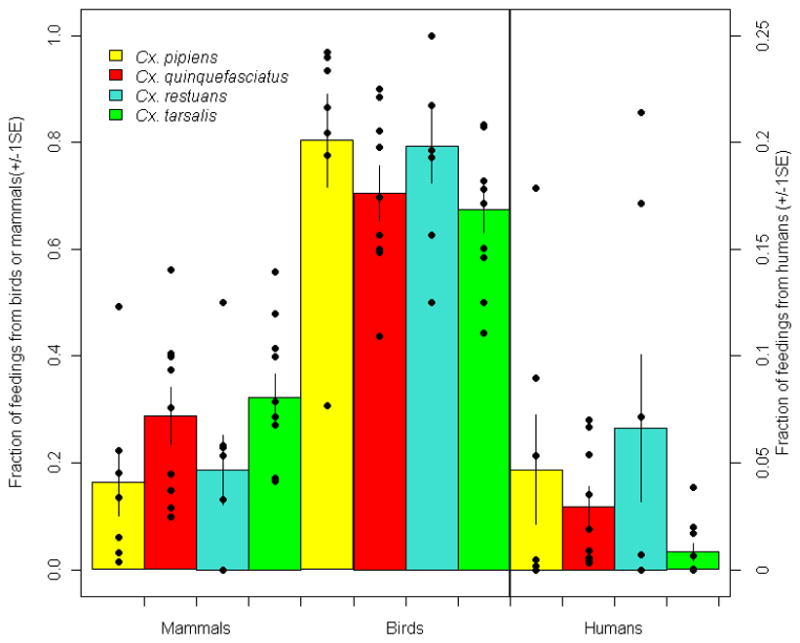
Feeding patterns of four species of Culex mosquitoes. Left axis shows the fraction of feedings from mammals and birds (first eight columns) represented by the mean (column height), 95% confidence interval (whiskers), and raw data (points). Right axis and last four columns show the fraction of feedings from humans (a subset of the mammals) on a different scale. See Supplemental Online Table 1 for sources and raw data.
Somewhat surprisingly, across all populations studied, there was no significant difference in the fraction of feedings taken from birds or humans between Cx. pipiens and Cx. quinquefasciatus (Figure 2; all three 95% confidence intervals overlap; ANOVA on arc-sin square root transformed data to normalize residuals: all p’s>0.1) Interestingly, populations of Cx. quinquefasciatus from Australia, while genetically different from North American populations, did not show widely disparate feeding patterns. Perhaps even more surprising, there were no significant differences between the mammal, human, or avian fraction of feedings among any of the four Culex species (Figure 2; ANOVAs on transformed data, all p’s>0.07).
These surprising results stem, in part, from substantial spatial variability in feeding patterns among populations as is clear from Figure 2. The causes of this variability are not well known, but likely reflect variation in the abundance of different hosts, and variation in genetic predisposition of the mosquitoes at different sites that influence feeding patterns. Although several recent studies simultaneously examined feeding patterns and estimated the local abundance of at least part of the host community (usually birds) (Hamer et al., 2009; Hassan et al., 2003; Kent et al., 2009; Kilpatrick et al., 2006a), we are unaware of any studies that have estimated the abundance of all avian and mammalian hosts simultaneously with data on mosquito feeding patterns. This would be necessary to determine the influence of host abundance on mammal vs. bird feeding. Futher, no study has estimated the abundance of amphibian or reptile hosts which sometimes appear to make up a non-trivial fraction of Cx. pipiens feedings (Apperson et al., 2004). Finally, some studies have estimated the densities of domestic mammals and birds (usually chickens, cows, pigs, etc.) while examining vector feeding, but these studies provide limited information for pathogens that circulate in wild animals.
The consequences of these feeding patterns for transmission of avian and mammalian pathogens to humans are profound. As noted earlier, the appreciable feeding (up to 30% of blood meals) by Cx. pipiens on humans (Figure 2) makes them likely to be the most important bridge vector of the primarily avian pathogen, WNV, to humans in several regions of North America (Hamer et al., 2008; Kilpatrick et al., 2005). It is worth noting that increased feeding on humans by any of these vectors decreases the enzootic intensity of transmission for WNV and SLEV, but simultaneously increases the “force-of-infection” experienced by humans (Kilpatrick et al., 2006b; Kilpatrick et al., 2007). The feeding pattern that would, in the worst case, maximize human incidence, is a complex function of the other factors that influence transmission, including vector abundance, survival, other hosts fed on, etc. However, it can be stated that transmission of avian pathogens to humans will initially increase with increasing feedings on humans until the fraction of feedings on humans (which are dead-end hosts for WNV) is so large that transmission is inefficient. In contrast, if humans can serve as an amplifying host for a pathogen (e.g. filiariasis, dengue virus), increasing feeding on humans will both increase enzootic transmission and the force-of-infection experienced by humans in a monotonic fashion. As a result, for these human-amplified pathogens, any control strategies that decrease feeding on humans, without increasing other factors (e.g. vector abundance) should reduce transmission. This is the logic behind zooprophylaxis, placing non-human animal hosts near humans to divert vector feeding. As has been noted before, this technique is likely to be most effective when the placement of animal hosts near humans decreases human feeding but does not increase vector density, which is a likely scenario only if larval habitats are limiting.
One of the next frontiers in determining the role of vectors in the transmission of zoonotic pathogens that infect multiple vertebrate classes (e.g. mammals and birds) will be assessing the under- or over-utilization (a smaller or larger fraction, respectively, of feedings coming from a species than expected from the fraction of the host community a species represents) of avian and mammalian hosts through simultaneous studies of local host abundance of both mammals and birds coincidently with feeding patterns. It is worth emphasizing that data on host abundance should be collected at the same locations where engorged mosquitoes are collected. Host abundances can vary by at least an order of magnitude between sites separated by only 1–3km, making “semi-local” host abundance data of limited utility in understanding mosquito feeding patterns. Finally, there has been relatively little work done in the last decade on the mechanistic causes of over- or under-utilization of host species. Over-utilization of a species can arise from a preference of biting vectors for that species, an overlap between mosquito microclimate selection and host roosting behavior (especially for nocturnal or crepuscular feeding vectors), or relatively lower host defensive behavior against biting vectors. The fact that any of these mechanisms can cause over-utilization makes the use of the term “preference” to describe raw feeding patterns is somewhat misleading. Thus, it is critical that the mechanisms underlying feeding patterns are distinguished to maximize the understanding gained and for implementing interventions such as alteration of host or mosquito microhabitats.
6. Variability in vector competence
Across members of the Cx. pipiens complex, there is evidence of genetic (heritable) control underlying feeding behavior and vector competence, although the identification of the actual genes that determine those traits is in its infancy (Bartholomay et al., 2010). For example, based on the analysis of neutral genomic DNA loci (microsatellites), Cx pipiens collected from distant locations in New York State were more genetically distinct and differed in vector competence for WNV more than mosquitoes collected from a single location (Kilpatrick et al., 2010). In addition, although temporal variation was evident in all locations, genetic ancestry was associated with differences in vector competence, with form pipiens mosquitoes more likely to become infected with WNV in one of two populations studied (Kilpatrick et al., 2010). This pattern was also replicated in recent studies of vector competence conducted with laboratory hybrids of colonized Cx pipiens form pipiens, form molestus, and Cx. quinquefasciatus that indicated significant differences in vector competence for WNV (Kramer, Kilpatrick and Fonseca, personal communication). Thus it appears that genetic variation of Cx pipiens complex mosquitoes can affect the ability of the mosquito to become infected, allow virus to disseminate, and/or transmit virus.
There is also evidence that the genetics of the virus influences the ecological cycle of WNV through dynamic interactions with Cx. pipiens and Cx. tarsalis mosquitoes. An evolved genotype of WNV that was first detected in 2001 (termed WN02) completely displaced the introduced 1999 genotype (termed NY99) throughout the United States by 2004 (Davis et al., 2005; Ebel et al., 2004). Subsequent research showed that the viral strains in the evolved genotype, WN02, increased vector competence (the fraction of mosquitoes transmitting the virus) in both Culex pipiens and Culex tarsalis mosquitoes (Ebel et al., 2004; Moudy et al., 2007), and the difference was especially pronounced at higher temperatures (Kilpatrick et al., 2008). This occurred despite only three consistent nucleotide differences between the NY99 clade of WNV and the strains in the WN02 clade, and only one of these differences leads to an amino acid change, a valine to alanine at position 159 (Davis et al., 2005; Ebel et al., 2004). Interestingly, there were no consistent differences in vector competence between the 1999 and WN02 isolates with Cx. quinquefasciatus (Vanlandingham et al., 2004).
Mosquitoes may also shape the viral transmission cycle through their effect on the virus itself. Like all RNA viruses, WNV has a high mutation rate and replicates to high titers rapidly in competent hosts. Studies on field-collected Cx. pipiens indicated WNV isolated from mosquito pools demonstrated twice as much heterogeneity in nucleotide sequence as virus isolated from dead infected American crows from the same locations (Bertolotti et al., 2008; Jerzak et al., 2005). Experimental passage studies with both WNV and St. Louis encephalitis virus confirm that Cx. pipiens mosquitoes serve as a source for significant intrahost genetic diversity (Ciota et al., 2009; Jerzak et al., 2007). Despite this, the capacity to maintain such viral diversity in mosquitoes over time may be limited by species-specific differences in seasonal maintenance, vector competence, and/or within-host bottlenecks (Ciota and Kramer).
7. Conclusions and perspectives
We have provided a overview of the diverse Cx. pipiens complex of mosquitoes. The diversity in ecology, physiology, and behavior is somewhat surprising given the relatively close genetic relationships among members of the complex, but is partly explained by the intraspecific diversity in genetics, behavior, and vector competence that results in steep spatial and temporal discontinuities in disease transmission. This diversity, especially in feeding patterns, results in these mosquitoes being key vectors for pathogens ranging from avian malaria to strictly human filariasis.
Despite the substantial recent work many outstanding issues require further study. These include, but are not limited to: 1) the factors influencing hybridization and genetic introgression between Cx. pipiens and Cx. quinquefasciatus, as well as between the two forms of Cx. pipiens, form pipiens and form molestus; 2) the causes of variation in feeding patterns for all mosquitoes in the complex, including the role of mosquito attraction, host defense, and overlap in microhabitats of host-seeking mosquitoes and hosts, as well as availability-driven selection; and 3) the causes of variation in competence of Cx. pipiens complex mosquitoes for various pathogens, including the relative importance of genetic and environmental influences. The results of these studies will enable better mapping of the risk of infection in space and time, more efficient control and mosquito population management efforts, and insight into the evolutionary relationships underlying host-pathogen interactions.
Supplementary Material
Highlights.
Mosquitoes in the Culex pipiens complex play key roles in the transmission of a range of pathogens including several viruses such as West Nile and St. Louis encephalitis, avian malaria (Plasmodium spp.) and filarial worms that cause elephantiasis.
The advent of genetic tools has greatly enhanced our understanding of the history of speciation, domestication, dispersal and hybridization.
The adaptation of Cx. pipiens complex mosquitoes to human-altered environments combined with their mixed feeding patterns on birds and mammals (including humans) greatly increase the transmission of several avian pathogens to humans.
Acknowledgments
We thank Eric Williges for GIS assistance in creating the global distribution map. Funding was provided by CDC gr ant CCU220532, NIH grant 1RO1AI090159-01, NIH/NIAID Contract N01A125490, and NSF grant EF-0914866 from the NSF-NIH ecology of infectious disease program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen DM, Collett GC, Winget RN. Preliminary host preference studies of Culex tarsalis Coquillett and Culiseta inornata (Wiluston) in Utah. Mosquito News. 1967;27:12–15. [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: A five-year analysis of mosquito data 1999–2003. Vector-Borne and Zoonotic Diseases. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- Anosike JC, Nwoke BE, Okere AN, Oku EE, Asor JE, Emmy-Egbe IO, Adimike DA. Epidemiology of tree-hole breeding mosquitoes in the tropical rainforest of Imo State, south-east Nigeria. Ann Agric Environ Med. 2007;14:31–38. [PubMed] [Google Scholar]
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, Nasci RS. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector-Borne and Zoonotic Diseases. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspen S, Crabtree MB, Savage HM. Polymerase chain reaction assay identifies Culex nigripalpus: Part of an assay for molecular identification of the common Culex (Culex) mosquitoes of the eastern United States. Journal of the American Mosquito Control Association. 2003;19:115–120. [PubMed] [Google Scholar]
- Aspen S, Savage HM. Polymerase chain reaction assay identifies North American members of the Culex pipiens complex based on nucleotide sequence differences in the acetylcholinesterase gene Ace.2. Journal of the American Mosquito Control Association. 2003;19:323–328. [PubMed] [Google Scholar]
- Aubry P. Dengue outbreaks in the French West-Indies in a context of arbovirosis emergence and reemergence. Bull Acad Natl Med. 2008;192:781–793. [PubMed] [Google Scholar]
- Bahnck CM, Fonseca DM. Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: culicidae) and hybrid populations. Am J Trop Med Hyg. 2006;75:251–255. [PubMed] [Google Scholar]
- Barr AR. The distribution of Culex p. pipiens and Culex p. quinquefasciatus in North America. Am J Trop Med Hyg. 1957;6:153–165. doi: 10.4269/ajtmh.1957.6.153. [DOI] [PubMed] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong YM, Erickson SM, Karunaratne S, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MAT. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti L, Kitron UD, Walker ED, Ruiz MO, Brawn JD, Loss SR, Hamer GL, Goldberg TL. Fine-scale genetic variation and evolution of West Nile Virus in a transmission "hot spot" in suburban Chicago, USA. Virology. 2008;374:381–389. doi: 10.1016/j.virol.2007.12.040. [DOI] [PubMed] [Google Scholar]
- Bertsch ML, Norment BR. The host feeding patterns of Culex quinquefasciatus in Mississippi. Mosquito News. 1983;43:203–206. [Google Scholar]
- Bogh C, Pedersen EM, Mukoko DA, Ouma JH. Permethrin-impregnated bednet effects on resting and feeding behaviour of lymphatic filariasis vector mosquitoes in Kenya. Medical and Veterinary Entomology. 1998;12:52–59. doi: 10.1046/j.1365-2915.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- Bowden SE, Magori K, Drake JM. Regional differences in the association between land cover and West Nile virus disease incidence in humans in the United States. Am J Trop Med Hyg. 2011;84:234–238. doi: 10.4269/ajtmh.2011.10-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HE, Childs JE, Diuk-Wasser MA, Fish D. Ecological factors associated with west nile virus transmission, northeastern United States. Emerging Infectious Diseases. 2008;14:1539–1545. doi: 10.3201/eid1410.071396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, McBride CS, Johnson P, Ritchie S, Paupy C, Bossin H, Lutomiah J, Fernandez-Salas I, Ponlawat A, Cornel AJ, Black WCt, Gorrochotegui-Escalante N, Urdaneta-Marquez L, Sylla M, Slotman M, Murray KO, Walker C, Powell JR. Worldwide patterns of genetic differentiation imply multiple 'domestications' of Aedes aegypti, a major vector of human diseases. Proceedings. Biological sciences/The Royal Society; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JA, Boreham PF, Highton RB, Hill MN. A study of the host selection patterns of the mosquitoes of the Kisumu area of Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1975;69:415–425. doi: 10.1016/0035-9203(75)90200-x. [DOI] [PubMed] [Google Scholar]
- Ciota AT, Jia YQ, Payne AF, Jerzak G, Davis LJ, Young DS, Ehrbar D, Kramer LD. Experimental Passage of St. Louis Encephalitis Virus In Vivo in Mosquitoes and Chickens Reveals Evolutionarily Significant Virus Characteristics. Plos One. 2009;4 doi: 10.1371/journal.pone.0007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciota AT, Kramer LD. Insights into Arbovirus Evolution and Adaptation from Experimental Studies. Viruses-Basel. 2010;2:2594–2617. doi: 10.3390/v2122594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinet T, Mourou JR, Pradines B, Toto JC, Jarjaval F, Amalvict R, Kombila M, Carnevale P, Pages F. First record of Aedes albopictus in Gabon. J Am Mosq Control Assoc. 2007;23:471–472. doi: 10.2987/5636.1. [DOI] [PubMed] [Google Scholar]
- Collins FH, Paskewitz SM. A review of the use of ribosomal DNA (rDNA) to differentiate among cryptic Anopheles species. Insect Mol Biol. 1996;5:1–9. doi: 10.1111/j.1365-2583.1996.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Cornel AJ, Mcabee RD, Rasgon J, Stanich MA, Scott TW, Coetzee M. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40:36–51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- Crabtree MB, Savage HM, Miller BR. Development of a species-diagnostic polymerase chain reaction assay for the identification of Culex vectors of St. Louis encephalitis virus based on interspecies sequence variation in ribosomal DNA spacers. Am J Trop Med Hyg. 1995;53:105–109. [PubMed] [Google Scholar]
- Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DWC, Novak RJ, Elizondo-Quiroga D, Green EN, Young DS, Stark LM, Drebot MA, Artsob H, Tesh RB, Kramer LD, Barrett ADT. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: Evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control. Parasite. 2008;15:3–13. doi: 10.1051/parasite/2008151003. [DOI] [PubMed] [Google Scholar]
- Dobrotworsky NV. The problem of the Culex pipiens complex in the South Pacific (including Australia) Bulletin of the World Health Organization. 1967;37:251–255. [PMC free article] [PubMed] [Google Scholar]
- Dohm DJ, Sardelis MR, Turell MJ. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera: Culicidae) J Med Entomol. 2002;39:640–644. doi: 10.1603/0022-2585-39.4.640. [DOI] [PubMed] [Google Scholar]
- Ebel GD, Carricaburu J, Young D, Bernard KA, Kramer LD. Genetic and phenotypic variation of West Nile virus in New York, 2000–2003. Am J Trop Med Hyg. 2004;71:493–500. [PubMed] [Google Scholar]
- Eldridge BF. 1. Diapause and related phenomena in Culex mosquitoes: Their telation to arbovirus disease ecology. In: Harris KF, editor. Current Topics in Vector Research. Springer-Verlag; New York: 1987. [Google Scholar]
- Farajollahi A, Crans WJ, Bryant P, Wolf B, Burkhalter KL, Godsey MS, Aspen SE, Nasci RS. Detection of West Nile viral RNA from an overwintering pool of Culex pipens pipiens (Diptera : Culicidae) in New Jersey, 2003. J Med Entomol. 2005;42:490–494. doi: 10.1093/jmedent/42.3.490. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Atkinson CT, Fleischer RC. Microsatellite primers for Culex pipiens quinquefasciatus, the vector of avian malaria in Hawaii. Molecular Ecology. 1998;7:1617–1619. [PubMed] [Google Scholar]
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, Wilkerson RC. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Smith JL, Kim HC, Mogi M. Population genetics of the mosquito Culex pipiens pallens reveals sex-linked asymmetric introgression by Culex quinquefasciatus. Infect Genet Evol. 2009;9:1197–1203. doi: 10.1016/j.meegid.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca DM, Smith JL, Wilkerson RC, Fleischer RC. Pathways of expansion and multiple introductions illustrated by large genetic differentiation among worldwide populations of the southern house mosquito. Am J Trop Med Hyg. 2006;74:284–289. [PubMed] [Google Scholar]
- Garcia-Rejon JE, Bradley BJ, Farfan-Ale JA, Lorono-Pino MA, Chim WAC, Flores-Flores LF, Rosado-Paredes E, Baak-Baak C, Perez-Mutul J, Suarez-Solis V, Fernandez-Salas I, Beaty BJ. Host-feeding preference of the mosquito, Culex quinquefasciatus, in Yucatan State, Mexico. Journal of Insect Science. 2010;10:32. doi: 10.1673/031.010.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes B, Sousa CA, Novo MT, Freitas FB, Alves R, Corte-Real AR, Salgueiro P, Donnelly MJ, Almeida AP, Pinto J. Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in the Comporta region, Portugal. BMC Evol Biol. 2009;9:262. doi: 10.1186/1471-2148-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Kilpatrick AM, Kramer LD, Dupuis AP, Jones MJ, Goetz SJ, Marra PP, Daszak P, Aguirre AA. Land use and West Nile virus seroprevalence in wild mammals. Emerging Infectious Diseases. 2008;14:962–965. doi: 10.3201/eid1406.070352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstream SE, Chew RM, Hagstrum DW, Tempelis CH. Feeding Patterns of 6 Species of Mosquitoes in Arid Southeastern California. Mosquito News. 1971;31:99–101. [Google Scholar]
- Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Walker ED. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol. 2008;45:125–128. doi: 10.1603/0022-2585(2008)45[125:cpdcab]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host selection by Culex pipiens mosquitoes and West Nile Virus amplification. Am J Trop Med Hyg. 2009;80:268–278. [PubMed] [Google Scholar]
- Harbach RE, Dahl C, White GB. Culex (Culex) pipiens Linnaeus (Diptera: Culicidae): concepts, type designations, and description. Proceedings of the Entomological Society of Washinton. 1985;87:1–24. [Google Scholar]
- Harbach RE, Harrison BA, Gad AM. Culex (Culex) molestus Forskal (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proc. Entomol. Soc. Wash. 1984;86:521–542. [Google Scholar]
- Hassan HK, Cupp EW, Hill GE, Katholi CR, Klingler K, Unnasch TR. Avian host preference by vectors of eastern equine encephalomyelitis virus. Am J Trop Med Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- Hayes RO, Tempelis CH, Hess AD, Reeves WC. Mosquito host preference studies in Hale County, Texas. Am J Trop Med Hyg. 1973;22:270–277. doi: 10.4269/ajtmh.1973.22.270. [DOI] [PubMed] [Google Scholar]
- Huang SM, Hamer GL, Molaei G, Walker ED, Goldberg TL, Kitron UD, Andreadis TG. Genetic variation associated with mammalian feeding in Culex pipiens from a West Nile virus epidemic region in Chicago, Illinois. Vector-Borne and Zoonotic Diseases. 2009;9:637–642. doi: 10.1089/vbz.2008.0146. [DOI] [PubMed] [Google Scholar]
- Humeres SG, Almiron WR, Sabattini MS, Gardenal CN. Estimation of genetic divergence and gene flow between Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in Argentina. Memorias do Instituto Oswaldo Cruz. 1998;93:57–62. doi: 10.1590/s0074-02761998000100011. [DOI] [PubMed] [Google Scholar]
- Iltis WG. Biosystematics of the Culex pipiens Complex in Northern California. University of California; Davis: 1966. [Google Scholar]
- Jansen CC, Webb CE, Graham GC, Craig SB, Zborowski P, Ritchie SA, Russell RC, van den Hurk AF. Blood Sources of Mosquitoes Collected from Urban and Peri-Urban Environments in Eastern Australia with Species-Specific Molecular Analysis of Avian Blood Meals. Am J Trop Med Hyg. 2009;81:849–857. doi: 10.4269/ajtmh.2009.09-0008. [DOI] [PubMed] [Google Scholar]
- Jerzak G, Bernard KA, Kramer LD, Ebel GD. Genetic variation in West Nile virus from naturally infected mosquitoes and birds suggests quasispecies structure and strong purifying selection. Journal of General Virology. 2005;86:2175–2183. doi: 10.1099/vir.0.81015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzak GVS, Bernard K, Kramer LD, Shi PY, Ebel GD. The West Nile virus-mutant spectrum is host-dependant and a determinant of mortality in mice. Virology. 2007;360:469–476. doi: 10.1016/j.virol.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen CA, Power SL, Broom AK. Determination of Mosquito (Diptera: Culicidae) Bloodmeal Sources in Western Australia: Implications for Arbovirus Transmission. J Med Entomol. 2009;46:1167–1175. doi: 10.1603/033.046.0527. [DOI] [PubMed] [Google Scholar]
- Jupp PG. Comparative studies on morphology and laboratory biology of Cuiex (Culex) pipiens Linnaeus (Diptera: Culicidae) from South Africa and England. J Entomol Soc South Africa. 1987;50:455–461. [Google Scholar]
- Kasai S, Komagata O, Tomita T, Sawabe K, Tsuda Y, Kurahashi H, Ishikawa T, Motoki M, Takahashi T, Tanikawa T, Yoshida M, Shinjo G, Hashimoto T, Higa Y, Kobayashi M. PCR-Based identification of Culex pipiens complex collected in Japan. Japanese Journal of Infectious Diseases. 2008;61:184–191. [PubMed] [Google Scholar]
- Kenoyer JM. Ancient cities of the Indus Valley Civilization. Oxford University Press; Oxford: 1998. [Google Scholar]
- Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- Kent RJ, Harrington LC, Norris DE. Genetic differences between Culex pipiens f. molestus and Culex pipiens f. pipiens (Diptera: Culicidae) in New York. J Med Entomol. 2007;44:50–59. doi: 10.1603/0022-2585(2007)44[50:gdbcpf]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn L, Murphy KE, Yudin MH, Loeb M. Self-reported protective behaviour against West Nile Virus among pregnant women in Toronto. J Obstet Gynaecol Can. 2008;30:1103–1109. doi: 10.1016/S1701-2163(16)34019-1. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proceedings of the Royal Society B: Biological Sciences. 2006a;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MR, Kramer LD. Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. Am J Trop Med Hyg. 2010;77:667–671. doi: 10.4269/ajtmh.2010.10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Gluzberg Y, Burgett J, Daszak P. A quantitative risk assessment of the pathways by which West Nile virus could reach Hawaii. Ecohealth. 2004;1:205–209. [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell S, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerging Infectious Diseases. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biology. 2006b;4:606–610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P, Fonseca DM. Genetic influences on mosquito feeding behavior and the emergence of zoonotic pathogens. Am J Trop Med Hyg. 2007;77:667–671. [PubMed] [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathogens. 2008;4:e1000092. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight KL. Supplement to the Catalog of the mosquitoes of the World (Diptera: Culicidae) Entomological Society of America; 1978. [Google Scholar]
- Knight KL, Malek AAA. A morphological and biological study of Culex pipiens in the Cairo Area of Egypt. Bull Soc Fouad I Entomol. 1951;35:175–185. [Google Scholar]
- Kothera L, Zimmerman EM, Richards CM, Savage HM. Microsatellite characterization of subspecies and their hybrids in Culex pipiens complex (Diptera: Culicidae) mosquitoes along a north-south transect in the central United States. J Med Entomol. 2009;46:236–248. doi: 10.1603/033.046.0208. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annual Review of Entomology. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- Lukacik G, Anand M, Shusas EJ, Howard JJ, Oliver J, Chen H, Backenson PB, Kauffman EB, Bernard KA, Kramer LD, White DJ. West Nile virus surveillance in mosquitoes in New York State, 2000–2004. Journal of the American Mosquito Control Association. 2006;22:264–271. doi: 10.2987/8756-971X(2006)22[264:WNVSIM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mackay AJ, Kramer WL, Meece JK, Brumfield RT, Foil LD. Host feeding patterns of Culex mosquitoes (Diptera: Culicidae) in East Baton Rouge Parish, Louisiana. J Med Entomol. 2010;47:238–248. doi: 10.1603/me09168. [DOI] [PubMed] [Google Scholar]
- Magnarelli LA. Host feeding patterns of Connecticut mosquitoes (Diptera: Culicidae) Am J Trop Med Hyg. 1977;26:547–552. doi: 10.4269/ajtmh.1977.26.547. [DOI] [PubMed] [Google Scholar]
- Mattingly PF. The systematics of the Culex pipiens complex. Bulletin of the World Health Organization. 1965;37:257–261. [PMC free article] [PubMed] [Google Scholar]
- Mattingly PF, Rozeboom LE, Knight KL, Laven H, Drummond FH, Christophers SR, Shute PG. The Culex pipiens complex. Transactions of the Royal Entomological Society of London. 1951;102:331–382. [Google Scholar]
- McIntosh BM, Jupp PG, Dickinson DB, McGillivray GM, Sweetnam J. Ecological studies on Sindbis and West Nile viruses in South Africa. I. Viral activity as revealed by infection of mosquitoes and sentinel fowls. South African Journal of Medical Science. 1967;32:1–14. [PubMed] [Google Scholar]
- Michael E, Gambhir M. Vector transmission heterogeneity and the population dynamics and control of lymphatic filariasis. Adv Exp Med Biol. 2010;673:13–31. doi: 10.1007/978-1-4419-6064-1_2. [DOI] [PubMed] [Google Scholar]
- Miller BR, Crabtree MB, Savage HM. Phylogeny of fourteen Culex mosquito species, including the Culex pipiens complex, inferred from the internal transcribed spacers of ribosomal DNA. Insect Mol Biol. 1996;5:93–107. doi: 10.1111/j.1365-2583.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis T, Armstrong P, Anderson J, Vossbrinck C. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerging Infectious Diseases. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis T, Armstrong P, Bueno R, Dennett J, Real S, Sargent C, Bala AA, Randle Y, Guzman H, Travassos da Rosa A, Wuithiranyagool T, Tesh RB. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile Virus in Harris County, Texas. Am J Trop Med Hyg. 2007;77:73–81. [PubMed] [Google Scholar]
- Molaei G, Cummings RF, Su TY, Armstrong PM, Williams GA, Cheng ML, Webb JP, Andreadis TG. Vector-host interactions governing epidemiology of West Nile virus in Southern California. Am J Trop Med Hyg. 2010;83:1269–1282. doi: 10.4269/ajtmh.2010.10-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudy RM, Meola MA, Morin LL, Ebel GD, Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Trop Med Hyg. 2007;77:365–370. [PubMed] [Google Scholar]
- Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, Kerst AJ, Bennett P, Gottfried K, Lanciotti RS. West Nile Virus in Overwintering Culex Mosquitoes, New York City, 2000. Emerging Infectious Diseases. 2001;7:742–744. doi: 10.3201/eid0704.010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Milby MM, Presser SB, Hardy JL. Ecology of Mosquitos and St. Louis Encephalitis Virus in the Los Angeles Basin of California, 1987–1990. J Med Entomol. 1992;29:582–598. doi: 10.1093/jmedent/29.4.582. [DOI] [PubMed] [Google Scholar]
- Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc Natl Acad Sci U S A. 2005;102:15912–15917. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Chaves LF, Hamer GL, Sun T, Brown WM, Walker ED, Haramis L, Goldberg TL, Kitron UD. Parasites & Vectors 3. 2010. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanogo YO, Kim CH, Lampman R, Halvorsen JG, Gad AM, Novak RJ. Identification of male specimens of the Culex pipiens complex (Diptera: Culicidae) in the hybrid zone using morphology and molecular techniques. J Med Entomol. 2008;45:203–209. doi: 10.1603/0022-2585(2008)45[203:iomsot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Dohm DJ, O'Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerging Infectious Diseases. 2001;7:1018–1022. doi: 10.3201/eid0706.010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, McMillen L, Unnasch EA, Unnasch TR. Host choice and West Nile virus infection rates in blood fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee 2002–2003. Vector-Borne Zoonotic Diseases. 2007;7:365–386. doi: 10.1089/vbz.2006.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Anderson M, Gordon E, McMillen L, Colton L, Charnetzky D, Delorey M, Aspen S, Burkhalter K, Biggerstaff BJ, Godsey M. Oviposition activity patterns and West Nile virus infection rates for members of the Culex pipiens complex at different habitat types within the hybrid zone, Shelby County, TN, 2002 (Diptera : Culicidae) J Med Entomol. 2006;43:1227–1238. doi: 10.1603/0022-2585(2006)43[1227:oapawn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Siriyasatien P, Pengsakul T, Kittichai V, Phumee A, Kaewsaitiam S, Thavara U, Tawatsin A, Asavadachanukorn P, Mulla MS. Identification of blood meal of field caught Aedes aegypti (L.) by multiplex PCR. Southeast Asian J Trop Med Public Health. 2010;41:43–47. [PubMed] [Google Scholar]
- Smith JL, Fonseca DM. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: culicidae) Am J Trop Med Hyg. 2004;70:339–345. [PubMed] [Google Scholar]
- Smith JL, Keyghobadi N, Matrone MA, Escher R, Fonseca DM. Cross-species comparison of microsatellite loci in the Culex pipiens complex and beyond. Molecular Ecology Notes. 2005;5:697–700. [Google Scholar]
- Spielman A. Bionomics of autogenous mosquitoes. Annu Rev Entomol. 1971;16:231–248. doi: 10.1146/annurev.en.16.010171.001311. [DOI] [PubMed] [Google Scholar]
- Spielman A. Structure and seasonality of nearctic Culex pipiens populations. Annals of the New York Academy of Sciences. 2001;951:220–234. doi: 10.1111/j.1749-6632.2001.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Tarantola A, Quatresous I, Ledrans M, Lassel L, Krastinova E, Cordel H, Lapidus N, Debruyne M, Poveda JD, Boude-Chevalier M, Schuffenecker I, Zeller H, Grandadam M, Tolou H, Paquet C. Imported cases of dengue fever diagnosed in metropolitan France, from January 2001 to December 2006. Med Mal Infect. 2009;39:41–47. doi: 10.1016/j.medmal.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Tempelis CH, Francy DB, Hayes RO, Lofy MF. Variations in feeding patterns of 7 culcine mosquitoes on vertebrate hosts in Weld and Larimer counties Colorado. Am J Trop Med Hyg. 1967;16:111–119. doi: 10.4269/ajtmh.1967.16.111. [DOI] [PubMed] [Google Scholar]
- Tempelis CH, Reeves WC, Bellamy RE, Lofy MF. A 3-Year study of feeding habits of Culex tarsalis in Kern County California. Am J Trop Med Hyg. 1965;14:170–177. doi: 10.4269/ajtmh.1965.14.170. [DOI] [PubMed] [Google Scholar]
- Tempelis CH, Washino RK. Host-feeding patterns of Culex tarsalis in Sacramento Valley California with notes on other species. J Med Entomol. 1967;4:315–318. doi: 10.1093/jmedent/4.3.315. [DOI] [PubMed] [Google Scholar]
- Tiawsirisup S, Platt KB, Evans RB, Rowley WA. A comparision of West Nile virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse) Vector-Borne and Zoonotic Diseases. 2005;5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- Townson H, Nathan MB. Resurgence of chikungunya. Trans R Soc Trop Med Hyg. 2008;102:308–309. doi: 10.1016/j.trstmh.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera : Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, O'Guinn ML, Dohm DJ. Potential vectors of West Nile virus in North America. In: Mackenzie J, Barrett A, Deubel V, editors. Japanese Encephalitis and West Nile Viruses Vol. 267 Current Topics in Microbiology and Immunology. Springer-Verlag; Berlin: 2002. pp. 241–252. [DOI] [PubMed] [Google Scholar]
- Urbanelli S, Silvestrini F, Reisen WK, De Vito E, Bullini L. Californian hybrid zone between Culex pipiens pipiens and Cx. p. quinquefasciatus revisited (Diptera:Culicidae) J Med Entomol. 1997;34:116–127. doi: 10.1093/jmedent/34.2.116. [DOI] [PubMed] [Google Scholar]
- Urbanelli S, Silvestrini F, Sabatinelli G, Raveloarifera F, Petrarca V, Bullini L. Characterization of the Culex pipiens complex (Diptera: Culicidae) in Madagascar. J Med Entomol. 1995;32:778–786. doi: 10.1093/jmedent/32.6.778. [DOI] [PubMed] [Google Scholar]
- Van Riper C, III, Van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian (USA) land birds. Ecological Monographs. 1986;56:327–344. [Google Scholar]
- Vanlandingham DL, Schneider BS, Klingler K, Fair J, Beasley D, Huang J, Hamilton P, Higgs S. Real-time reverse transcriptase-polymerase chain reaction quantification of West Nile virus transmitted by Culex pipiens quinquefasciatus. Am J Trop Med Hyg. 2004;71:120–123. [PubMed] [Google Scholar]
- Vazeille M, Moutailler S, Pages F, Jarjaval F, Failloux AB. Introduction of Aedes albopictus in Gabon: what consequences for dengue and chikungunya transmission? Trop Med Int Health. 2008;13:1176–1179. doi: 10.1111/j.1365-3156.2008.02123.x. [DOI] [PubMed] [Google Scholar]
- Vinogradova EB. Culex pipiens pipiens mosquitoes: taxonomy, distribution, ecology, physiology, genetics, applied importance and control. Pensoft; Sofia: 2000. [Google Scholar]
- Wang E, Ni H, Xu R, Barrett AD, Watowich SJ, Gubler DJ, Weaver SC. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol. 2000;74:3227–3234. doi: 10.1128/jvi.74.7.3227-3234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekesa JW, Yuval B, Washino RK. Multiple blood feeding by Anopheles freeborni and Culex tarsalis (Diptera: Culicidae): Spatial and temporal variation. J Med Entomol. 1997;34:219–225. doi: 10.1093/jmedent/34.2.219. [DOI] [PubMed] [Google Scholar]
- Zhao TY, Lu BL. The clasdics of Culex pipiens complex. Acta Zootaxonomica Sinica. 1999;24:206–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.