INTRODUCTION
Nephron-sparing surgery (NSS) has become the definitive standard of care for treatment of most small renal masses.1 Laparoscopic partial nephrectomy (LPN) has been the traditional approach to minimally invasive NSS and has demonstrated decreased morbidity and equivalent long term oncologic outcomes compared to open surgery for T1 lesions.2, 3 However, the technical and ergonomic challenge of laparoscopic suturing has limited the dissemination of LPN and has led to overuse of laparoscopic radical nephrectomy when NSS may be feasible.4 Robotic technology has recently been applied to minimally invasive partial nephrectomy (MIPN) with the goal of facilitating renorrhaphy and reducing the learning curve (LC) for intracorporeal suturing.
Initial outcomes of robot-assisted laparoscopic partial nephrectomy (RALPN) have been promising as reported by several centers of excellence. Several retrospective comparisons of RALPN and LPN have shown comparable morbidity as well as early oncological outcomes.5–10 Other possible advantages including improvement in warm ischemia time (WIT) have been proposed and demonstrated in these retrospective comparisons.5, 9
We present a large comparative series of RALPN and LPN by a high volume minimally invasive surgeon at a tertiary care center who performs both types of procedure. We present both comparative outcomes and LC of the two approaches.
METHODS
Study Design and Statistical Analysis
The Johns Hopkins Minimally Invasive Urological Surgery Database (1994-present) was queried for men undergoing LPN. 150 men were identified who underwent LPN or RALPN for a renal mass containing an enhancing solid component on cross sectional imaging, for a single surgeon since 2006 and represent his initial experience with MIPN following residency training. Preoperative patient and operative data were evaluated among LPN and RALPN using appropriate comparative tests (t-test, chi-squared, ANOVA). Glomerular filtration rate (GFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) estimation.11 Pre-operative GFR was calculated using a serum creatinine measurement within 30 days prior to surgery, post-operative GFR using creatinine at discharge (median 2 days, range 1–8) and last GFR using the most recent serum creatinine, at least one month following surgery (n=57, median 7 months, range 1–43). Operative time was considered from initial incision to surgery stop to avoid bias of setup time or anesthesia time. Patients underwent a robotic approach in lieu of a laparoscopic one if they were assigned to a robotic room by our scheduler or if they indicated a preference for robotic surgery after being counseled. Selection bias was evaluated by comparing patient and tumor characteristics.
LC for LPN and RALPN was investigated by examining operative times, warm ischemia times (WIT) and estimated blood loss (EBL) in groups of 25 consecutive patients. To account for laparoscopic LC, perioperative outcomes of patients who underwent surgery in 2009 and later were compared. This surgeon started performing RALPN in 2009 and this marks the time period in which both operations were performed concurrently and a period in which effects of the LC could be minimized.
Surgical Technique
All patients underwent transperitoneal LPN/RALPN and were positioned in the modified flank position using a standard technique. After pneumoperitoneum was achieved three trocars were placed with a periumbilical camera port. For LPN a fourth 5mm trocar was placed in the ipsilateral lower quadrant to facilitate retraction and suction by the assistant. Occasionally a fifth 5mm trocar was placed for liver retraction in right sided cases. The da Vinci Surgical System was used for RALPN with a standard 3-arm approach with a fourth trocar for the assistant and fifth trocar as needed for right-sided cases. The renal hilum was first identified to locate the renal artery and vein. Perinephric fat surrounding the tumor was circumcised to allow clear visualization of the dissection margin and provide a clear view of the capsule for subsequent reconstruction. Fat overlying the tumor was left intact to act as a handle for retraction. A laparoscopic ultrasound probe was used to determine the line of incision and depth of tumor involvement if the lesion and dissection plane were not readily identifiable. The renal parenchyma was scored using electrocautery, maintaining a visual 0.5 cm tumor margin. The renal vessels were clamped individually or en bloc depending on patient anatomy. Laparoscopic 10 mm or robotic scissors were used to excise the renal mass. Frozen section biopsies were obtained from the resection margin. The excised tumor was placed in an entrapment sack and extracted. The collecting system was closed with 2-zero polyglactin sutures if entry was observed and large vessels were oversewn with 2-zero polyglactin suture as well. The renal parenchyma was approximated by placing sutures through the capsule and reapproximating the edges using the sliding-clip technique.12 Bolsters made of polyglactin mesh were used sporadically to ensure hemostasis in large parenchymal defects. Fourteen patients undergoing LPN underwent clampless excision using a radiofrequency ablation device 13 and were excluded from analyses of WIT; 11 patients who underwent clampless tumor excision without radiofrequency coagulation were included (7 LPN, 4 RALPN). After the sutures were secured the vascular clamps were removed to ensure adequate hemostasis. Laparoscopic exit was accomplished in standard fashion with a suction drain left in the retroperitoneum.
RESULTS
Patient Characteristics
102 (68.0%) and 48 (32.0%) patients underwent LPN and RALPN respectively. Patient characteristics are demonstrated in Table 1. Of note, RALPN patients tended to be older, more often had a history of smoking and a greater proportion of patients in the RALPN (n=28, 58.3%) had an ASA (American Society of Anesthesiologists) score of 1–2 compared to 47 (46.1%) in the LPN group (p=0.02) despite similar ACE-27 (Adult Comorbidity Evaluation-27)14 scores (p=0.38). There were no significant differences in tumor characteristics among groups including size, laterality, RENAL nephrometry complexity or its components.15
TABLE 1.
Patient characteristics of 102 and 48 patients undergoing laparoscopic partial nephrectomy (LPN) and robot-assisted laparoscopic partial nephrectomy (RALPN) respectively.
| LPNx | RALPNx | |||||
|---|---|---|---|---|---|---|
| no./value | (%/range) | no./value | (%/range) | p-value | ||
| Male Sex | 63 | (61.8%) | 27 | (56.3%) | 0.52 | |
| Age (median) | 56 | (25–81) | 62 | (27–77) | 0.006 | |
| Incidental | 73 | (71.6%) | 40 | (83.3%) | 0.53 | |
| Comorbidities | Hypertension | 64 | (62.7%) | 30 | (62.5%) | 0.98 |
| Diabetes | 25 | (24.5%) | 7 | (14.6%) | 0.17 | |
| Smoking History | 38 | (37.3%) | 27 | (56.3%) | 0.03 | |
| Family History RCC | 3 | (2.9%) | 2 | (4.2%) | 0.68 | |
| BMI (median) | 30.3 | (18.7–46.5) | 28.2 | (17.8–40.5) | 0.053 | |
| Race | White | 83 | (81.4%) | 41 | (85.4%) | 0.46 |
| Black | 14 | (13.7%) | 5 | (10.4%) | ||
| Other | 5 | (4.9%) | 2 | (4.2%) | ||
| ACE | 0 (none) | 18 | (17.6%) | 7 | (14.6%) | 0.38 |
| 1 (mild) | 51 | (50.0%) | 22 | (45.8%) | ||
| 2–3 (mod-severe) | 33 | (32.4%) | 19 | (39.6%) | ||
| ASA | 1 to 2 | 47 | (46.1%) | 28 | (58.3%) | 0.02 |
| 3+ | 52 | (51.0%) | 20 | (41.7%) | ||
| Prior Abdominal Surgery | 56 | (54.9%) | 23 | (47.9%) | 0.4 | |
| Tumor | Median Clincal Size (cm) | 2.5 | (1.0–6.4) | 2.2 | (1.0–7.5) | 0.17 |
| Characteristics | Median Pathologic Size (cm) | 2.2 | (0.5–7.7) | 2.0 | (0.9–6.) | 0.1 |
| Laterality | Left | 47 | (46.1%) | 26 | (54.2%) | 0.36 |
| Right | 55 | (53.9%) | 22 | (45.8%) | ||
| Solitary Kidney | 0 | (0.0%) | 2 | (4.2%) | 0.04 | |
| RENAL | Mean Score | 6.5 | (4–10) | 5.9 | (4–10) | 0.06 |
| nephrometry | Low Complexity | 39 | (52.0%) | 28 | (65.1%) | 0.4 |
| Moderate Complexity | 34 | (45.3%) | 14 | (32.6%) | ||
| High Complexity | 2 | (2.7%) | 1 | (2.3%) | ||
| Exophytic | >50% | 37 | (49.3%) | 24 | (55.8%) | 0.3 |
| <50% | 35 | (46.7%) | 15 | (34.9%) | ||
| Endophytic | 3 | (4.0%) | 4 | (9.3%) | ||
| Abut Collecting System | 31 | (41.3%) | 13 | (30.2%) | 0.04 | |
| Coronal Plane | Anterior | 25 | (33.3%) | 20 | (46.5%) | 0.25 |
| Posterior | 27 | (36.0%) | 15 | (34.9%) | ||
| Polar Location | Entirely polar | 39 | (52.0%) | 23 | (53.5%) | 0.8 |
| Crosses polar or midline | 36 | (48.0%) | 20 | (46.5%) | ||
| Pathology | RCC | 74 | (72.5%) | 33 | (68.8%) | 0.64 |
| RCC(clear cell) | 44 | (59.5%) | 22 | (50.0%) | ||
| RCC(papillary) | 22 | (29.7%) | 7 | (15.9%) | ||
| RCC(chromophobe) | 7 | (9.5%) | 3 | (6.8%) | ||
| RCC(mixed) | 1 | (1.4%) | 1 | (2.3%) | ||
| AML | 10 | (9.8%) | 1 | (2.1%) | ||
| Oncocytoma | 10 | (9.8%) | 6 | (12.5%) | ||
| Benign (other) | 8 | (7.8%) | 6 | (12.5%) | ||
| Sarcoma Mets | 0 | (0.0%) | 2 | (4.2%) | ||
| Fuhrman | 1 to 2 | 48 | (47.1%) | 22 | (45.8%) | 0.4 |
| Grade | 3 to 4 | 22 | (21.6%) | 9 | (18.8%) | |
ACE: adult comorbidity evaluation, ASA: American Society of Anesthesiologists, BMI: body mass index, RCC: renal cell carcinoma.
The polar location is defined as the tumor location relative to the polar lines (the plane of the kidney above or below which the medial lip of parenchyma is interrupted by the renal sinus fat, vessels or the collecting system).15
Peri-operative Outcomes
Operative outcomes (Table 2) favored RALPN versus LPN: mean operative time was 152 vs. 193 minutes (p<0.001), WIT was 14.0 vs. 18.0 minutes (p<0.001) and EBL was 122 vs. 245cc (p=0.001) respectively.
TABLE 2.
Operative parameters and perioperative outcomes for 102 and 48 patients undergoing laparoscopic partial nephrectomy (LPN) and robot-assisted laparoscopic partial nephrectomy (RALPN) respectively.
| LPNx | RALPNx | |||||
|---|---|---|---|---|---|---|
| no./value | (%/range) | no./value | (%/range) | p-value | ||
| Operative | Mean Operative Time (min) | 193 | (100–420) | 152 | (108–265) | <0.001 |
| Parameters | Mean WIT (min)* | 18 | (8–65) | 14 | (8–30) | <0.001 |
| Mean EBL (mL) | 245 | (50–1700) | 122 | (0–500) | 0.001 | |
| Transfusions | 5 | (4.9%) | 0 | (0.0%) | 0.1 | |
| Median LOS (days) | 2 | (1–8) | 2 | (1–8) | 0.58 | |
| Collecting System Entry | 43 | (42.2%) | 18 | (37.5%) | 0.59 | |
| Vascular | Clampless | 7 | (8.0%) | 4 | (8.3%) | <0.001 |
| Clamping** | Artery Only Clamp | 27 | (30.7%) | 35 | (72.9%) | |
| Artery and Vein Clamp | 54 | (61.4%) | 9 | (18.8%) | ||
| Positive Margins | 1 | (1.0%) | 2 | (4.2%) | 0.06 | |
14 patients who underwent clampless resection with radiofrequency electrocoagulation device were excluded from warm ischemia time (WIT) and Vascular Clamping analyses.
Mean pre-operative GFR (mL/min/1.73m2) was 83.3 (26.1–139.9) and 79.3 (34.8–107.6) for LPN and RALPN patients respectively (p=0.3); mean post-operative GFR at discharge was 77.1 (22.1–131.3) and 78.0 (30.6–110.2) respectively (p=0.8). Patients undergoing LPN had a greater decrease in GFR immediately following surgery (−6.0 vs. −1.3 mL/min/1.73m2, p=0.046), however no difference in GFR was detected in 57 patients with a post-operative creatinine measurement greater than one month following surgery (mean GFR 73.5 and (8.8–123.1) and 66.2 (32.2–99.3) for LPN and RALPN respectively, p=0.45). One patient undergoing LPN with pre-operative renal dysfunction (GFR 43.6) due to focal segmental glomerulosclerosis progressed to need renal replacement therapy 14 months after surgery.
There were 17 (16.7%) and 5 (10.4%) complications in the LPN and RALPN respectively (p=0.3). The most common complication was urine leak requiring stent placement occurring in 6 (5.9%) and 2 (4.2%) of the patients respectively (p=0.7). Clavien grade I, II, III and IV complications occurred in 3 (2.9%), 2 (2.0%), 8 (7.8%) and 4 (3.9%) of LPN and 2 (4.2%), 0 (0%), 3 (6.3%) and 0 (0%) of RALPN patients (p=0.4). Two LPN were converted to open PN early in the experience and one RALPN was converted to standard LPN due to intraoperative bleeding. The patient did not require a blood transfusion and recovered without complications.
Learning Curve
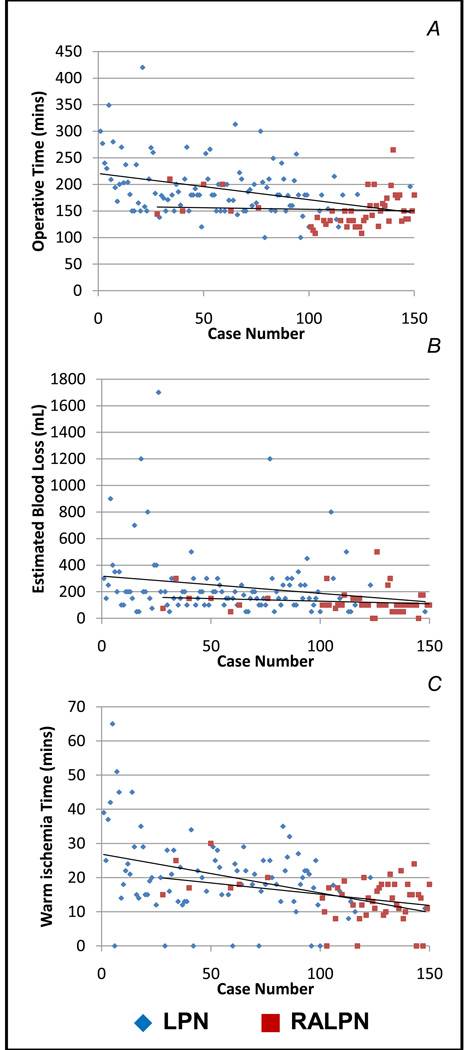
Improvements in operative time (p=0.01), WIT (p=0.006) and EBL (0.01) were noted as experience increased in the LPN cohort (Table 3; Figure 1). This difference was most pronounced when comparing the first 25 LPN patients to the more recent experience groups; there was no difference, however, noted in any parameter between the early and late experience of RALPN. Additionally, if surgical experience cohorts were considered irrespective of use of the robot; WIT, EBL and operative time were significantly longer in the first cohort of 25 patients when compared to every subsequent cohort and no differences were noted between cohorts once the first 25 cases were completed (data not shown). To account for tumor complexity, RENAL nephrometry scores were evaluated by experience cohorts (Table 3). There was a trend to less-complex tumors by nephrometry score in both the LPN (p=0.97) and RALPN (p=0.1), however the majority of tumors were low-to-moderate complexity in each cohort and no statistical difference was observed.
TABLE 3.
Learning curve for laparoscopic partial nephrectomy (LPN) and robot-assisted laparoscopic partial nephrectomy (RALPN).
| OR Time (mins) | WIT (mins) | EBL (mL) | Nephrometry | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| value | (range) | p-value | value | (range) | p-value | value | (range) | p-value | value | (range) | p-value | |||
| LPN Experience | A | 0–25 | 224* | (150–420) | 0.01 | 27.1* | (0–65) | 0.006 | 323* | (50–1200) | 0.01 | 6.1 | (4–9) | 0.97 |
| B | 26–50 | 188 | (120–270) | 19.2 | (0–34) | 254 | (50–1700) | 7.2 | (4–9) | |||||
| C | 51–75 | 185 | (100–313) | 17.7 | (0–29) | 189 | (50–1200) | 6.6 | (4–10) | |||||
| D | 75–102 | 175 | (100–257) | 17.4 | (0–35) | 217 | (50–800) | 6.2 | (4–10) | |||||
| RALPN Experience | X | 0–24 | 141 | (108–210) | 0.99 | 14.2 | (0–30) | 0.4 | 119 | (0–300) | 0.6 | 6.3 | (4–10) | 0.1 |
| Y | 25–48 | 163 | (121–265) | 13.9 | (0–24) | 125 | (0–500) | 5.6 | (4–9) | |||||
Mean values are demonstrated in each column. The p-value to the right of each column describes the difference in learning curve trend using analysis of variance (ANOVA).
The p-values described by each asterisk describe differences in each parameter when pairs of groups were compared using students t-test.
For OR time, A was significantly different from B (p=0.07), C (p=0.03) and D (p=0.003).
For WIT, A was significantly different from C (p=0.04) and D (p=0.02).
For EBL, no difference was observed between groups.
For RENAL nephrometry, no difference was observed between groups.
FIGURE 1.
Learning curve for operating time (A), warm ischemia time (WIT, B) and estimated blood loss (EBL, C) for 150 consecutive patients undergoing laparoscopic (LPN) or robot-assisted laparoscopic partial nephrectomy (RALPN).
55 and 44 patients underwent LPN and RALPN respectively since 2009. While absolute differences were less pronounced, operative time (150 vs. 182mins, p<0.001), WIT (13.3 vs. 18.1mins, p=0.003) and EBL (118 vs. 206cc, p=0.005) continued to favor RALPN in this most recent cohort. RENAL nephrometry scores were 6.7 and 5.7 for LPN and RALPN in this cohort since 2009 (p=0.01).
DISCUSSION
A number of recent studies demonstrate the safety and feasibility of RALPN while discussing potential advantages with regard to perioperative outcomes.5, 7, 9 Most of these studies have focused on RALPN, with only one focusing on the LC associated with the transition from LPN to RALPN.10 Studies of open and laparoscopic radical prostatectomy for prostate cancer have demonstrated the importance of surgeon experience in optimizing perioperative and oncological outcomes and demonstrate LCs on the order of 200–250 patients.16, 17 Most LC analyses focus on de novo learning of a new technique and little data exists demonstrating the transition, in any organ system, for standard laparoscopic to robot-assisted laparoscopy. We therefore investigated the perioperative outcomes of RALPN in the setting of the LC for minimally invasive PN.
Overall, RALPN seemed to afford significant improvements in operative time, WIT and EBL. These improvements have been demonstrated by other authors5, 7, 9, 18, 19 with the exception of one group who experienced longer WIT in the early experience of RALPN6 and one group with equivocal WIT differences.8 We believe that improvements in perioperative outcomes are related to the improved visualization and ease of tumor excision/reconstruction allowed by the articulating robotic instruments, as the technique for LPN and RALPN was otherwise very similar and performed by the same surgical team.
While there was no conscious effort to select cases for either technique in this series, it is important to consider selection bias and its effect on this data. Differences in perioperative parameters could be explained by a number of confounding factors including tumor size and complexity, patient characteristics, including body habitus and comorbidities, and differences in surgical technique. Importantly there were no differences in tumor size, laterality or complexity by nephrometry scoring system – although the majority of tumors in both groups were of low to intermediate complexity. Although there was a trend toward less complex tumors when RENAL nephrometry scores were analyzed per experience cohort, there was not a clear clinical or statistical difference between groups. Importantly, when considering components of the RENAL nephrometry score, a slightly higher proportion of patients undergoing LPN (41%) had tumors adjacent to the collecting system than did patients undergoing RALPN (30%, p=0.04). This may explain some of the differences in reconstruction of the tumor defect resulting in longer WIT and greater EBL. Additionally there were no significant differences in clinically meaningful patient characteristics including BMI, comorbidity scoring, or prior abdominal surgeries.
Another potential explanation for the difference reported could be related to the general LC for partial nephrectomy since the majority of RALPNs were performed later in the surgeon’s experience. In order to adjust for this potential bias we compared LPN and RALPN cases performed since 2009. It should be noted that RENAL nephrometry score was significantly different between patients undergoing LPN and RALPN in this contemporary experience. However the absolute difference was one-point on the nephrometry score scale, questioning the clinical utility of this distinction. On further analysis, this one-point difference could not be attributed to an individual component on the nephrometry scores: tumor diameter, exophytic nature, approximation to the collecting system or anatomic location were not statistically different among LPN and RALPN. While the difference in EBL, WIT and operative time was less pronounced in this group of patients, the advantage persisted in favor of RALPN. Mottrie et al. discussed the RALPN LC in the context of a surgeon with extensive robotic experience and found the LC to be between 20–30 cases.10 Additionally, Haber et al. presented a comparison of LPN and RALPN for a surgeon experienced in LPN.8 However, neither of these groups presented the LC of LPN coincidental with RALPN. Nonetheless we found the LC for RALPN to be minimal for a surgeon experienced with LPN.
Recent literature has focused on the importance of minimizing ischemia times and preserving renal parenchyma in an attempt to avoid chronic renal disease and the associated morbidity.20, 21 For the experienced laparoscopic surgeon, RALPN may be a means to reduce WIT further and promote healthy, long-term renal function in patients in whom nephron-sparing surgery is indicated. Our data demonstrate a mean change of four minutes while DeLong et al. observed a reduction in WIT by 10 minutes, Wang and Bhayani demonstrated a decrease of six minutes and Mottrie et al. were able to reduce average WIT to less than 20 minutes in a short period of time.9, 10, 19 This may have implications beyond the general health and renal function of patients, as it is difficult to estimate the cost-savings of sparing functional nephrons over a lifetime. While it has been suggested that shorter WIT coincides with improved renal function,20 it is unclear whether a few additional minutes of warm ischemia as seen in the LPN group actually impacts long term renal function as most patients in this series had a normal contralateral kidney. In fact, our data suggest that the improvement experienced in WIT does not translate into improved GFR in patients who have normal or mild renal dysfunction preoperatively. The implications of a few minutes of WIT for patients with moderate or severe renal dysfunction and a potentially malignant renal tumor may be more pronounced, though this is beyond the scope of our paper.
Additional, important considerations surrounding this data are the cost of the robotic system and associated disposables. However, the improvement in operative time by 40 minutes and WIT by 4 minutes seen in this study may counter these financial disincentives. Additionally, the need for a skilled bedside assistant is crucial during RALPN and not necessary for LPN. As noted by others,7 RALPN requires a competent and skilled bedside assistant to actively participate in renal hilar dissection, clamping and unclamping – portions of the case at greatest risk for causing significant injury and subsequent morbidity. These additional significant investments must be taken into consideration when starting a RALPN program and weighed against the improved perioperative outcomes suggested by our data and that of others.
These data demonstrate no improvement in RALPN parameters through the initial robotic experience while perioperative parameters seemed to plateau following the initial 25 LPN patients. This relatively fast LC should be viewed in the appropriate context and with caution. Unlike radical prostatectomy experiences where most surgeons transition from open to laparoscopy, both LPN and RALPN exist in a laparoscopic environment and thus transition from LPN to RALPN may indeed occur with a minimal LC. Also the LPN LC in our study results largely from the surgeon’s training in a high-volume, center-of-expertise for LPN and robotic prostatectomy, with exposure to hundreds of LPN and robotic prostatectomy procedures during training, albeit without an advanced laparoscopic fellowship. As previously noted, additional factors such as patient and tumor selection bias may have also influenced this LC data. What may be inferred from this data is that the transition from LPN to RALPN is facile and can be associated with immediate improvements in perioperative parameters for surgeons with a solid baseline experience with LPN. It is also possible that further improvements will occur with additional RALPN experience.
This analysis is not without limitations. As previously mentioned it is a single-surgeon experience; the addition of multiple surgeons with different training experiences could improve the knowledge gained from examination of such a LC. Selection bias may also impact the differences seen. Finally, our analysis does not take into account financial costs; robotic surgery comes with many cost premiums and has been demonstrated to be more expensive to the patient and health-care system than standard laparoscopic surgeries.22
This experience documents that outcomes of contemporary MIPN can be optimized within a short time period and are associated with acceptable WIT, surgical times, and complication rates even in the early experience of a single surgeon. This is likely due to the experience gained by the pioneers of LPN who defined and standardized contemporary methods. Data from additional centers with LPN and RALPN experience are ultimately required to determine their relative role in nephron sparing surgery.
CONCLUSIONS
Outcomes of contemporary MIPN can be optimized within a short time period and are associated with acceptable WIT, surgical times, and complications rates even in the early experience of a single surgeon. RALPN appears to have shorter operative and ischemia times, and less blood loss when compared to LPN. For a surgeon trained in a minimally invasive rich environment, the LC for LPN appeared to be reached following 25 cases. Transition from LPN to RALPN can be undertaken without a significant LC and may be associated with immediate benefits.
Acknowledgement
Phillip M. Pierorazio is supported by award Number T32DK007552 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
REFERENCES
- 1.Touijer K, Jacqmin D, Kavoussi LR, et al. The expanding role of partial nephrectomy: a critical analysis of indications, results, and complications. Eur Urol. 2010;57:214. doi: 10.1016/j.eururo.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Lane BR, Gill IS. 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol. 2010;183:473. doi: 10.1016/j.juro.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Allaf ME, Bhayani SB, Rogers C, et al. Laparoscopic partial nephrectomy: evaluation of long-term oncological outcome. J Urol. 2004;172:871. doi: 10.1097/01.ju.0000134292.36152.fa. [DOI] [PubMed] [Google Scholar]
- 4.Miller DC, Hollingsworth JM, Hafez KS, et al. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853. doi: 10.1016/S0022-5347(05)00422-2. [DOI] [PubMed] [Google Scholar]
- 5.Benway BM, Bhayani SB, Rogers CG, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 6.Aron M, Koenig P, Kaouk JH, et al. Robotic and laparoscopic partial nephrectomy: a matched-pair comparison from a high-volume centre. BJU Int. 2008;102:86. doi: 10.1111/j.1464-410X.2008.07580.x. [DOI] [PubMed] [Google Scholar]
- 7.Kural AR, Atug F, Tufek I, et al. Robot-assisted partial nephrectomy versus laparoscopic partial nephrectomy: comparison of outcomes. J Endourol. 2009;23:1491. doi: 10.1089/end.2009.0377. [DOI] [PubMed] [Google Scholar]
- 8.Haber GP, White WM, Crouzet S, et al. Robotic versus laparoscopic partial nephrectomy: single-surgeon matched cohort study of 150 patients. Urology. 2010;76:754. doi: 10.1016/j.urology.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 9.DeLong JM, Shapiro O, Moinzadeh A. Comparison of laparoscopic versus robotic assisted partial nephrectomy: one surgeon's initial experience. Can J Urol. 2010;17:5207. [PubMed] [Google Scholar]
- 10.Mottrie A, De Naeyer G, Schatteman P, et al. Impact of the learning curve on perioperative outcomes in patients who underwent robotic partial nephrectomy for parenchymal renal tumours. Eur Urol. 2010;58:127. doi: 10.1016/j.eururo.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benway BM, Wang AJ, Cabello JM, et al. Robotic partial nephrectomy with sliding-clip renorrhaphy: technique and outcomes. Eur Urol. 2009;55:592. doi: 10.1016/j.eururo.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Andonian S, Adebayo A, Okeke Z, et al. Habib laparoscopic bipolar radiofrequency device: a novel way of creating an avascular resection margin in laparoscopic partial nephrectomy. J Laparoendosc Adv Surg Tech A. 2008;18:853. doi: 10.1089/lap.2008.0050. [DOI] [PubMed] [Google Scholar]
- 14.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 17.Secin FP, Savage C, Abbou C, et al. The learning curve for laparoscopic radical prostatectomy: an international multicenter study. J Urol. 2010;184:2291. doi: 10.1016/j.juro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deane LA, Lee HJ, Box GN, et al. Robotic versus standard laparoscopic partial/wedge nephrectomy: a comparison of intraoperative and perioperative results from a single institution. J Endourol. 2008;22:947. doi: 10.1089/end.2007.0376. [DOI] [PubMed] [Google Scholar]
- 19.Wang AJ, Bhayani SB. Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of >100 consecutive procedures. Urology. 2009;73:306. doi: 10.1016/j.urology.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RH, Lane BR, Lohse CM, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010;58:340. doi: 10.1016/j.eururo.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 22.Mir SA, Cadeddu JA, Sleeper JP, et al. Cost comparison of robotic, laparoscopic, and open partial nephrectomy. J Endourol. 2011;25:447. doi: 10.1089/end.2010.0510. [DOI] [PubMed] [Google Scholar]