Abstract
Lysine ε-acetylation is a post-translational modification that alters the biochemical properties of many proteins. The reaction is catalyzed by histone/protein acetyltransferases (HATs), and is reversed by histone/protein deacetylases (HDACs). As a result, HATs and HDACs constitute an important, though little recognized, set of proteins that control the functions of T-regulatory (Treg) cells. Targeting certain HDACs, especially HDAC6, HDAC9, and Sirtuin-1 (Sirt1), can augment Treg suppressive potency by several distinct and potentially additive mechanisms. These involve promoting Forkhead box p3 (Foxp3) gene expression and preserving Foxp3 lysine ε-acetylation, which infers resistance to ubiquitination and proteasomal degradation, and increases DNA binding. Moreover, depleting certain HDAC can enhance the heat shock response, which increases the tenacity of Treg to survive under stress, and helps preserve a suppressive phenotype. As a result, HDAC inhibitor therapy can be used to enhance Treg functions in vivo and have beneficial effects on allograft survival and autoimmune diseases.
Introduction
The ability to control the immune response is an important therapeutic goal in the management of many diseases. However, doing so requires finding a delicate balance between activation and attenuation. Unfortunately, most current therapeutic strategies targeting the immune system have relatively limited antigen specificity and therefore notoriously lack precision. For example, immune modulation after solid organ transplantation faces the challenge of achieving enough suppression to limit graft rejection without impairing the host's ability to protect against infections [1] and malignancy [2]. This comes in addition to numerous nonimmune toxicities [3]. Treatments for autoimmune conditions like inflammatory bowel disease face very similar problems [4]. Conversely, at the opposite end of the spectrum, cancer immunotherapy, while very promising and increasingly effective against a wide range of tumors, can predispose to autoimmunity [5]. Throughout the search for more specific approaches, T-regulatory (Treg) cells have been recognized as an important T cell subset able to limit immune responses in an antigen-specific manner, and are crucial to maintaining self-tolerance [6,7•]. Treg-based therapies, such as ex-vivo expansion or efforts to enhance in vivo suppressive function offer a potential avenue towards more antigen directed immunosuppression [8•], and Tregs are now recognized as an obstacle and therapeutic target in anti-neoplastic treatments [9•]. The best established and most studied type of Treg cells are characterized by expression of the transcription factor forkheadbox-p3 (Foxp3), which plays a key role in their development and functions [10,11]. HDAC inhibitor (HDACi) use can augment Foxp3+ Treg production and induce various molecular changes that enhance their phenotype [12]. As a result, the suppressive capacity of murine [13], non-human primate [14] and human [15••] Tregs can be increased by treatment with histone/protein deacetylase inhibitors (HDACi) [16,17•], with therapeutic consequences in models of autoimmunity and transplantation [18,19••,20••,21••]. At present, several histone/protein acetyltransferases (HATs) and histone/protein deacetylases (HDACs) have been implicated in Treg biology, and the relevant HDAC biology is summarized Figure 1. Aspects of this work are summarized in the following sections, with an emphasis on the lead HDAC and HAT molecular pathways that are known to influence Treg function in vivo as well as in vitro.
Figure 1. HDACs and potential target proteins in Treg cells.
Summary of HDAC biology and Tregs, using a schematic adapted from Bush and McKinsey [71]. Note that * class IIa HDACs are currently thought to act primarily through protein/protein interactions and their deacetylase activity is mediated via recruitment of a class I HDAC, such as HDAC3; and **HDACi acting on Zn-dependent HDACs (class I, IIa and IV) are distinct from HDACi that act on NAD-dependent sirtuins (Class III). There are HDAC specific inhibitors for HDAC6 and Sirt1 (20••, 21••). Abbreviations are HDAC, histone/protein deacetylase; HSF, heat shock factor; HSP, heat shock protein; MEF-2, myocyte enhancer factor-2; NAD, nicotinamide adenine dinucleotide; and. Sirt1, Sirtuin-1.
Acetylation of Foxp3 prevents proteasomal degradation and increases Treg potency
Post-translational modifications expand the regulatory potential of proteins vastly beyond mere gene expression. Lysine (K) provides one of the most reactive residues that can engage in a myriad of biochemical alterations [22]. ε-amino acetylation can neutralize lysine’s positive charge (Figure 2) and profoundly alter the biological functions of affected proteins [23,24••]. Historically, lysine acetylation was first appreciated in regard to post-translational modifications of histone tail residues that promoted chromatin accessibility and RNA synthesis [25]. Therefore, enzymes facilitating lysine acetylation were named histone acetyltransferases and those reversing it as histone deacetylases. Identification of an increasing number of biologically important non-histone targets prompted lysine acetyltransferase (KAT) and lysine deacetylase (KDAC) as an alternative nomenclature. In the current review, we use the terms histone/protein acetyltransferases (HATs) and histone/protein deacetylases (HDACs) so as to reflect these developments, and given a focus on the development of new therapeutic strategies for autoimmunity and transplantation, we emphasize consideration of HDACs and their inhibitors (HDACi).
Figure 2. Lysine ε-amino deacetylation promotes protein turnover and shortens Foxp3 lifespan.
(1) HATs can acetylate lysine residues in Foxp3 at the ε-NH2 group. In the acetylated form, the lysine residue cannot enter the ubiquitination reaction. (2) HDACs can remove the acetyl-group from Foxp3, and make it susceptible to the ubiquitination reaction. (3) Activated ubiquitin is formed by binding of its c-terminus to a cysteine residue on ubiquitin-activating enzyme (E1) via a thiol bond. Next, E1 is replaced by ubiquitin-conjugating enzyme (E2). (4) The E2-ubiquitin complex can be linked to deacetylated lysine residues on Foxp3 via an ubiquitin-protein ligase (E3) forming an isopeptide bond (5). (6) Subsequently, other ubiquitins can bind to the ε-amino groups of lysine residues (K29 and K48) of ubiquitin already bound to Foxp3. (7) A chain of four or more ubiquitins is sufficient to indicate proteins for degradation in the proteasome.
Control of Foxp3 expression in Treg is not limited to regulation at the level of Foxp3 gene transcription. Instead, the speed at which the Foxp3 protein is degraded is equally important. Recently, van Loosdregt et al. showed, in non-immune cells, that the class III HDAC Sirtuin-1 (Sirt1) directly co-localizes with Foxp3 and mediates its deacetylation and poly-ubiquitination [26•]. In related studies involving genetic deletion of Sirt1 or its pharmacologic inhibition, we showed that loss of Sirt1 activity in Tregs led to increased Foxp3 protein expression and increased Treg suppressive function, which translated into prolonged allograft survival [20••]. Additional HDACs are almost certainly involved in deacetylating Foxp3, including HDAC9 [13]. In contrast, hyperacetylation of Foxp3 through p300 makes it less susceptible to ubiquitination, which in turn increases the overall Foxp3 protein amount [27••]. Tip60 is another HAT known to acetylate Foxp3 [28]. Thus, leaving Foxp3 in a more acetylated state either through activation of relevant HATs, or deactivation and various HDACs, can render Foxp3 resistant to proteasomal degradation, and thereby control its expression (Figure 2). To date, only p300 and Sirt1 were shown to regulate Foxp3 acetylation and thereby prevent Foxp3 ubiquitination and turnover [26•,27••]. However, beyond resistance to ubiquitination, acetylation of Foxp3 acetylation markedly increases its regulatory capability through improved DNA binding [13,29,30], e.g. at the interleukin-2 promoter when cooperating with nuclear factor of activated T-cells (NFAT) and competing with AP-1 for its DNA binding [31]. The biochemical details of Foxp3 acetylation were recently reviewed [32]. Therefore, acetylated Foxp3 persists longer and has higher function, depending on the lysine residues affected by acetylation.
HDACs alter transcription factors of the Foxp3 gene
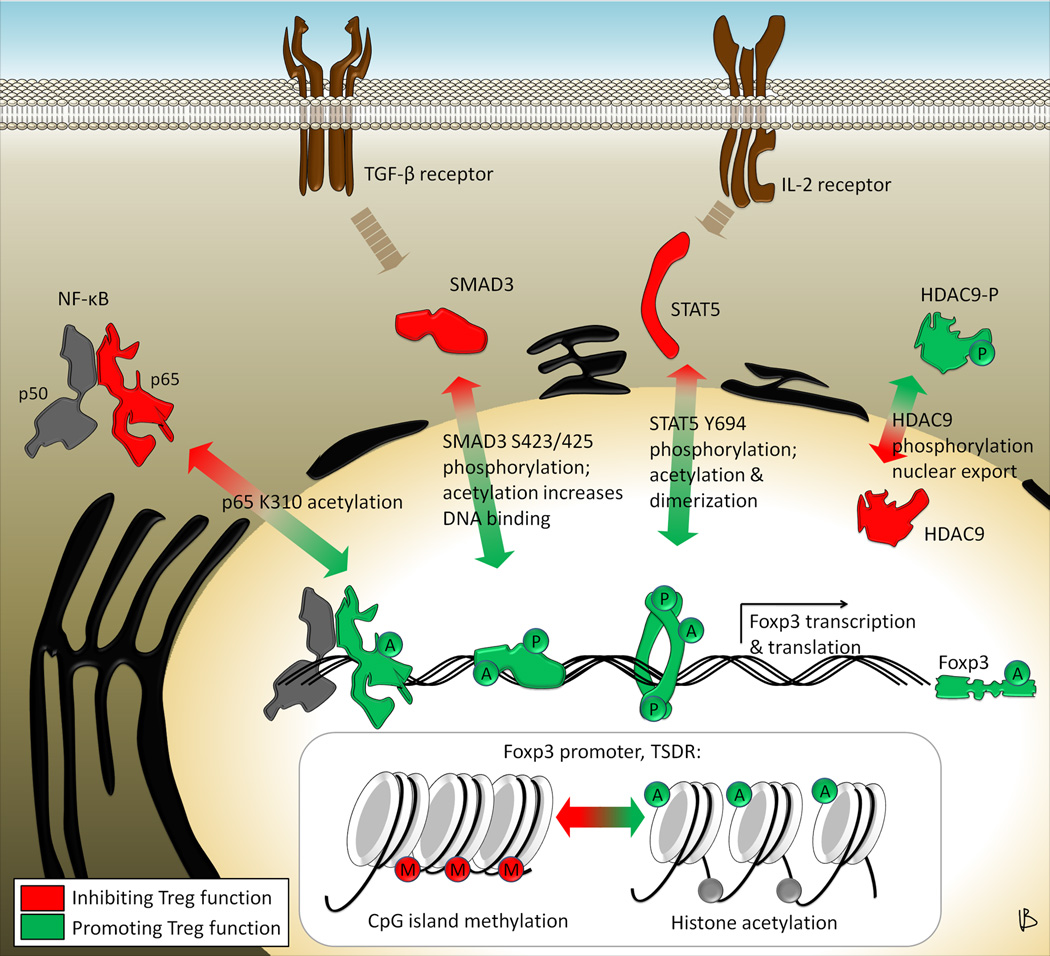
Since the discovery of Foxp3+ Tregs and appreciation of their significance, investigators have sought to understand the mechanisms of Foxp3 gene regulation. In 2006, Mantel et al. identified the Foxp3 promoter region and reported several binding sites for the transcription factors NFAT and AP-1 [33]. Subsequently, Zorn et al. reported IL-2 dependent STAT5 as another transcription factor relevant to Foxp3 gene expression [34]. Additional transcription factors were implicated based on insights from Treg cell biology. For example, since transforming growth factor (TGF)-β has long been reported as a factor inducing Treg from conventional T cells [35], its downstream targets were expected to be involved in Foxp3 gene regulation. Indeed, Tone et al. showed that Smad3, in conjunction with NFAT, can enhance Foxp3 gene expression [36]. Other transcription factors affecting Foxp3 gene expression include the NF-κB family members RelA (p65) and c-rel [37], NOTCH1 [38], Id3 [39], Runx [40], and others [41•]. Opposing these are negative regulators, notably GATA-3 [42] and STAT3 [43], which are involved in transitioning the differentiation from a Treg to Th2 or Th17 cell fate, respectively. Importantly, Ruan et al. reported that NFAT, SMAD, CREB, c-Rel and Rel-A form the so-called c-rel enhanceosome, which is now understood to be central to Treg induction and lineage commitment [44].
Not surprisingly, HATs and HDACs are important to the functions of many of these transcription factors, e.g. through direct protein acetylation/deacetylation, or indirectly through modification of their own expression, DNA binding affinity, nuclear translocation or transcriptional activity, or by likewise affecting regulators of these transcription factors, providing many possibilities for complex biologic effects and for therapeutic intervention (Figure 3). For example, Sirt1 has been shown to deacetylate NF-κB, more specifically the RelA/p65 subunit at K310 [45]. Acetylation of p65 can augment gene transcription through several mechanisms; e.g. acetylation of K314/315 increases promoter selectivity, acetylation of K221 increases DNA binding, acetylation of K221/K218 decreases its binding to the IκBα inhibitor, and acetylation of K310 can increase the transcriptional activity of p65 [46]. Of note, loss of Sirt1 can augment RelA-dependent transcription in macrophages [47]. In our own studies, T cell or Treg-specific deletion of Sirt1 increased RelA K310 acetylation [20], which is relevant in regard to the concept of RelA being an important part of the c-rel enhanceosome [44], and could be one mechanism by which Sirt1 targeting can increase Foxp3 gene expression.
Figure 3. HDACs control Foxp3 gene expression.
Targeting certain HDACs can increase Foxp3 gene expression by aiding nuclear translocation of transcription factors and promoting histone acetylation at the Foxp3 promoter and the Treg-specific-demethylated region (TSDR). The p65 subunit of NF-κB can be deacetylated at K310 through Sirtuin-1. Acetylation of p65 at K310 is important for NF-κB nuclear translocation, and p65 is an integral part of the c-rel enhanceosome promoting Foxp3 expression. Likewise, SMAD3 and STAT5, both effectors of TGF-β and IL-2 receptor signaling, respectively, depend upon nuclear translocation as well, which is facilitated by phosphorylation. Proteasomal degradation of nuclear phosphorylated SMAD3 can be favored by HDAC6 activity (see text), whereas STAT5 dimerization is enhanced by acetylation. Abbreviations are: A, acetylated; P, phosphorylated; M, methylated CpG island.
Another transcription factor of interest is the TGFβ-dependent SMAD3. Its transcriptional activity is, in part, regulated through proteasomal degradation, which is induced by estrogen receptor (ER)-α signaling [48•]. At the same time, activation of ERα by estrogen binding promotes the expression of HDAC6 [49]. We hypothesize, that HDAC6 may be involved in mediating ERα dependent SMAD3 degradation, and that thus, loss of HDAC6, could preserve SMAD3, and transcriptionally active phospho-SMAD3 (Figure 3). However, the role of HDAC6 in controlling TGFβ-dependent signaling in Treg cells currently remains poorly defined. From the epithelial-mesenchymal transition literature, Shan et al reported, that HDAC6 is actually required for phosphorylating SMAD3 [50]. However, such regulatory functions of HDAC6 could differ significantly between different cell types. We are currently investigating the effects of HDAC6 on SMAD3-regulated events at the TGFβ-dependent Foxp3 enhancer. It is noteworthy that Sirt1 can regulate ERα expression and Sirt1 inhibition can thus impair ERα signaling pathways [51]. Moreover, Sirt1 has also been shown to deacetylate SMAD3, which reduces its DNA binding capacity [52]. Disrupting the SMAD3/p300 interaction also diminished SMAD3 acetylation and led to a similar decline in DNA binding [53]. Thus, targeting Sirt1 may lead to (a) more preserved (phospho)-SMAD3 through diminished ERα signaling, and b) to a more transcriptionally active SMAD3 through acetylation.
A third transcription factor potentially subject to HDAC/HAT manipulation is IL-2-dependent STAT5. STAT5 is transported into the nucleus after tyrosine phosphorylation in the C-terminal region [54,55]. In addition, STAT5 is acetylated (K694, K701 and K359) by the HAT, CREB-binding protein (CBP), which promotes formation of a stable STAT5 dimer though acetylation at K696 and K701 [56]. Importantly, sustained phospho-STAT5 dimer binding to DNA favors histone acetylation and chromatin remodeling, which further augments transcription [57]. It is therefore possible that manipulation of either CBP, or a yet unidentified HDAC, could influence stability of the phospho-STAT5 dimer through acetylation, and thus influence Foxp3 gene transcription.
Foxp3 gene methylation and histone acetylation
Over the past few years, increasing attention was dedicated to understanding methylation of the Foxp3 promoter and conserved non-coding sequences (CNS) within the Foxp3 gene [58,59]. Methylation of CpG islands and acetylation of histones determine accessibility of the DNA through chromatin remodeling. Of note, Zheng et al. found that three CNS within the Foxp3 gene convey lineage stability of the Treg phenotype through their methylation state and responsiveness to transcription Foxp3 factors [60••]. Therefore, the Foxp3 promoter and CNS regions are targets for epigenetic regulation of Foxp3 expression [61]. Liu et al. observed that the sumoylation ligase PIAS1 can recruit DNA methyltransferases and other factors promoting heterochromatin formation to the Foxp3 promoter and thus restrict mRNA transcription [62]. Since histone acetylation uncoils the DNA structure (euchromatin) making it more accessible to transcription factors, HATs and HDACs have become a subject of interest in epigenetic regulation of Foxp3 expression (Figure 3) [63•].
HDAC influence on the heat shock response
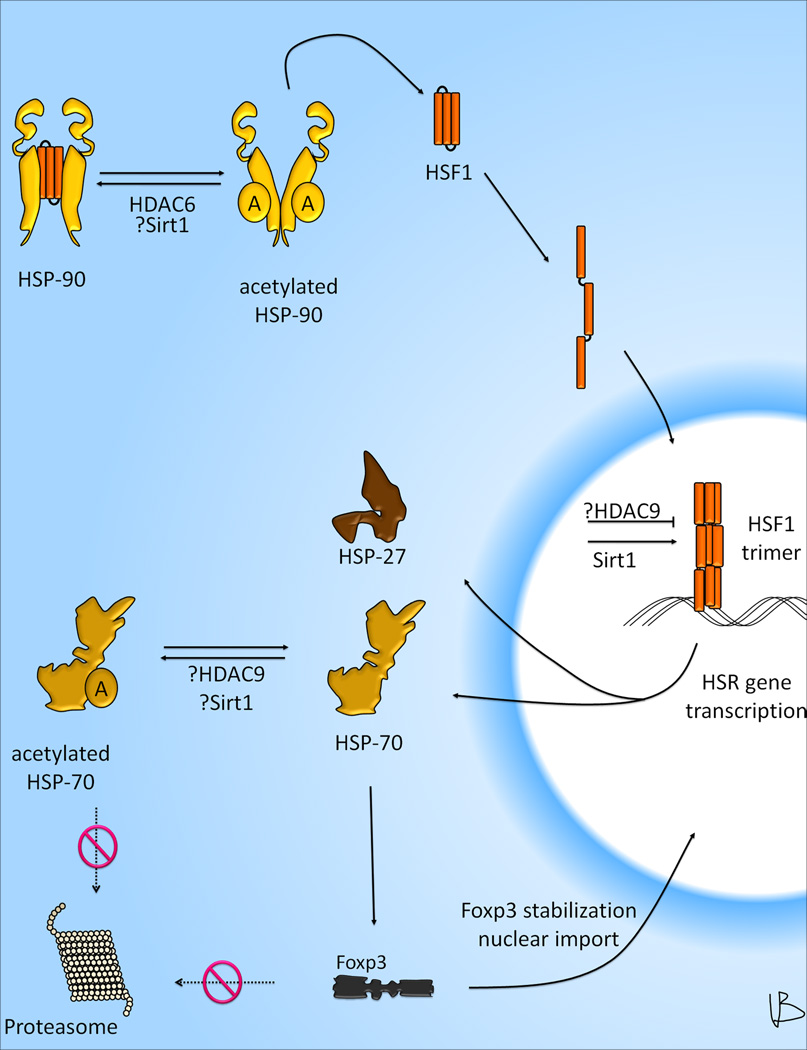
Pan-HDACi, but also HDAC6 and HDAC9 specific targeting, can augment activation of heat shock response (HSR) gene transcription in Treg cells [20,21]. The HSR enables the expression of chaperone proteins that alleviate and counter the sequelae of cellular stress, and is primarily induced through DNA binding of heat shock transcription factor-1 (HSF1) to heat shock elements in the regulatory regions of many genes. Under resting conditions, the majority of HSF1 monomer is inactive and bound to a complex of HSP90, HSP70, p23, and FK506 binding protein [64]. This complex can be disrupted by the emergence of reactive oxygen species and/or misfolded proteins, which leads to release of the HSF1 monomer. Furthermore, HSP90 hyperacetylation due to loss of HDAC6 can prompt dissociation from other proteins [65], including HSF1, which leads to activation of the HSR (Figure 4) [21]. The HSR is induced by nuclear translocation of HSF1, phosphorylation of serine residues, and formation of a competent HSF1 trimer capable of binding to the DNA [66]. Remarkably, unlike many other transcription factors, the DNA binding of HSF1 can be disrupted through acetylation, and stabilized by deacetylation through Sirt1 [67••]. Indeed, we found that Sirt1 deletion in Treg in vivo led to diminished induction of key HSR genes such as HSP70 and HSP27 [20••]. However, we also noted in preliminary studies, that Sirt1-deficient Treg show improved apoptosis free survival in response to heat shock challenge. We are currently investigating if Sirt1 might directly affect HSP70 acetylation and thereby influence the cellular HSR on a post-translational level. Of relevance, HSP70 acetylation has been shown to increase its chaperone activity [68]. While this may help undo protein misfolding and aid Treg surviving in acidotic and hypoxic inflammatory tissues, it may also be important for Treg function. We have previously shown, that HSP70 can co-localize with Foxp3 (19••). We therefore hypothesize, that HSP70 can aid in the correct folding of newly synthesized cytoplasmic Foxp3 and aid in its transport to the nucleus (Figure 4).
Figure 4. HDACs and the Treg heat shock response.
At rest, HSP-90 is bound to multiple client proteins, including HSF1. Stress or HSP90 acetylation (reversed by HDAC6) cause displacement of HSF1, which unfolds, translocates into the nucleus and forms transcriptionally active trimer. The HSF1 trimer is stabilized in its deacetylated state through Sirt1, and turns on heat shock response genes, such as HSP-27 and HSP-70. HSP-70 is deacetylated by HDAC9, and perhaps Sirt1, which confers resistance to proteasomal degradation. HSP-70 can also act as a chaperone for newly translated Foxp3 and aid in its nuclear import. Abbreviation used: A, acetylated.
Additional considerations and conclusions
We are currently assembling evidence showing that several additional HDACs, beyond HDAC6, HDAC9 and Sirt1, have biologically relevant effects on Foxp3-dependent Treg biology. There are also, of course, numerous additional post-translational and epigenetic mechanisms beyond protein acetylation and DNA methylation that are relevant to modulation of Foxp3+ Treg function. We have recently reported evidence of the key role of proteolytic cleavage so as to generate a form of Foxp3 that appears most potent in terms of chromatin binding and control of Treg function [69], and identification of the mechanisms responsible for the nuclear translocation of Foxp3 [70]. Likewise, given limitations of space, the current review has not discussed Foxp3 phosphorylation, sumoylation or regulation via effects on mRNA stability. While these have intrinsic interest, we believe that the potential clinical application of our data most clearly relates to the use of selective HDACi so as to promote Foxp3+ Treg suppression in autoimmunity and post-transplantation. To that end, the availability of selective inhibitors of HDAC6 and Sirt1 has particular significance. Their testing in disease models, alone and in various combinations, is currently underway in our laboratory.
Highlights.
-
*
Histone/protein acetyltransferases (HATs) and histone/protein deacetylases (HDACs) control lysine acetylation, an important post-translational modification in histone and non-histone proteins.
-
*
Acetylation of Foxp3, the master transcription factor of T-regulatory (Treg) cells, promotes increased DNA binding and resistance to proteasomal degradation.
-
*
Several Foxp3 transcription factors can be regulated by acetylation (p65, STAT5), or indirectly though HDAC/HAT dependent mediators (SMAD3).
-
*
Targeting HDAC6 or HDAC9 increases heat shock response (HSR) gene expression, which protects Treg from stress and preserves a suppressive phenotype.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases (K08AI095353 to U.H.B., and AI073489 to W.W.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 2.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 3.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 4.Dayharsh GA, Loftus EV, Jr, Sandborn WJ, Tremaine WJ, Zinsmeister AR, Witzig TE, Macon WR, Burgart LJ. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72–77. doi: 10.1053/gast.2002.30328. [DOI] [PubMed] [Google Scholar]
- 5.Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK, Kershaw MH. Autoimmunity associated with immunotherapy of cancer. Blood. 2011 doi: 10.1182/blood-2011-01-325266. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. • One of several excellent reviews by this group on the developing literature around Treg subsets that are characterized by differences in their level of activation and expression of transcription factors, membrane molecules and homing receptors, as well as differences according to their anatomic site. Tregs present in adipose tissues appear especially distinct from other Treg cells.
- 8. McMurchy AN, Bushell A, Levings MK, Wood KJ. Moving to tolerance: Clinical application of T regulatory cells. Semin Immunol. 2011 doi: 10.1016/j.smim.2011.04.001. • Timely discussion of methods for Treg expansion and their application as a form of cellular therapy in transplant recipients, if and as concerns about Treg instability and reversion to an effector cell phenotype can be assuaged.
- 9. Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. • In this thoughtful perspective by one of the leading pioneers of Treg biology, Dr. Sakaguchi discusses evidence that Tregs not only protect against autoimmunity, but also dampen host anti-tumor immune responses. Finding a way to modulate Treg function without depletion or induction of autoimmunity is now seen as a major challenge to advances in the field of oncology.
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Tao R, de Zoeten EF, Ozkaynak E, Wang L, Li B, Greene MI, Wells AD, Hancock WW. Histone deacetylase inhibitors and transplantation. Curr Opin Immunol. 2007;19:589–595. doi: 10.1016/j.coi.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 14.Johnson J, Pahuja A, Graham M, Hering B, Hancock WW, Bansal-Pakala P. Effects of histone deacetylase inhibitor SAHA on effector and FOXP3+ regulatory T cells in rhesus macaques. Transplant Proc. 2008;40:459–461. doi: 10.1016/j.transproceed.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL, Hancock WW. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol. 2010;136:348–363. doi: 10.1016/j.clim.2010.04.018. •• Provides key evidence that various HDACi can enhance the suppressive functions of both freshly harvested and expanded human Tregs, and promote expression of key Treg-associated molecules such as CTLA-4. Beginning with use of raw data, the paper also gives detailed consideration to how to analyze and standardize data from Treg suppression assays.
- 16.Wang L, Tao R, Hancock WW. Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance. Immunol Cell Biol. 2009;87:195–202. doi: 10.1038/icb.2008.106. [DOI] [PubMed] [Google Scholar]
- 17. Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nat Rev Drug Discov. 2009;8:969–981. doi: 10.1038/nrd3031. • This review summarizes the effects of HDACi on multiple cell types and, looking beyond oncology, documents their use as powerful anti-inflammatory agents. Additional attention is given to the potential for isoform-selective HDACi to exhibit these effects without the toxicities associated in many cases with pan-HDACi use.
- 18.Saouaf SJ, Li B, Zhang G, Shen Y, Furuuchi N, Hancock WW, Greene MI. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp Mol Pathol. 2009;87:99–104. doi: 10.1016/j.yexmp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010;138:583–594. doi: 10.1053/j.gastro.2009.10.037. •• HDAC9 appears to be the most Treg selective HDAC and its deletion or knockdown are shown to potentiate Treg functions in vitro and in vivo, including in models of inflammatory bowel disease. However, since class IIa HDACs such HDAC9 display little catalytic activity, the conundrum in pharmacology is how to take advantage of this potentially very useful target for immunomodulation. While knowledge of HDAC9 biology is in its infancy, this paper will doubtless stimulate investigators interested in the biochemical foundations of Foxp3+ Treg functions.
- 20. Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G, Hancock WW. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol. 2011;31:1022–1029. doi: 10.1128/MCB.01206-10. •• Lots attention have been given to the roles that Sirt1 may play in caloric restriction and efforts to promote longevity, though such data have yet to be convincingly translated into mammalian systems. This study provides pivotal evidence that Sirt1 neutralization or Treg-specific deletion can enhance Treg functions and promote allograft survival. While sirtuin biology is multi-faceted and highly complex, the use of Sirt1-selective inhibitors, alone or in conjunction with HDAC6-selective inhibitors, is just beginning to be explored and appears to have much potential.
- 21. de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, Bradner JE, Mazitschek R, Kozikowski AP, Matthias P, et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol. 2011;31:2066–2078. doi: 10.1128/MCB.05155-11. •• HDAC6−/− mice are essentially normal and yet their Tregs have enhanced suppressive functions in vitro and in vivo. Moreover, the catalytic activity of HDAC6 can be blocked by highly selective and non-toxic HDAC6 inhibitors, and monoclonal antibodies are available to monitor HDAC6-specific deacetylation. Hence, HDAC6 currently appears a very attractive target for the enhancement of endogenous Foxp3+ Tregs in vivo, and as a practical and possibly superior alternative to Treg cellular therapy.
- 22.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? Embo J. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. •• Outstanding and comprehensive mass spectroscopy-based study identifying multiple new acetylation sites on more than 1750 proteins, reinforcing the suggestion by Tony Kouzarides (reference 23) that acetylation could come to be recognized as important as phosphorylation in the regulation of cellular process.
- 25.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Loosdregt J, Brunen D, Fleskens V, Pals CE, Lam EW, Coffer PJ. Rapid temporal control of Foxp3 protein degradation by sirtuin-1. PLoS ONE. 2011;6:e19047. doi: 10.1371/journal.pone.0019047. • Excellent biochemical analysis of the ability of Sirt1 to deacetylate Foxp3 and thereby promote its ubiquitination and proteasomal degradation. Along with reference 20, this study makes a strong case for the importance of Sirt1 in Treg biology.
- 27. van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–974. doi: 10.1182/blood-2009-02-207118. •• Important biochemical study identifying p300 as a HAT that acetylates Foxp3, and Sirt1 as a Foxp3 deacetylase. Foxp3 acetylation is shown to protect against ubiquitination and proteasomal degradation.
- 28.Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Rowell EA, Thomas RM, Hancock WW, Wells AD. Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J Biol Chem. 2006;281:36828–36834. doi: 10.1074/jbc.M608848200. [DOI] [PubMed] [Google Scholar]
- 30.Samanta A, Li B, Song X, Bembas K, Zhang G, Katsumata M, Saouaf SJ, Wang Q, Hancock WW, Shen Y, et al. TGF-beta and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3. Proc Natl Acad Sci U S A. 2008;105:14023–14027. doi: 10.1073/pnas.0806726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Y, Li B, Zhou Z, Hancock WW, Zhang H, Greene MI. Histone acetyltransferase mediated regulation of FOXP3 acetylation and Treg function. Curr Opin Immunol. 2010;22:583–591. doi: 10.1016/j.coi.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 34.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 36.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 37.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Samon JB, Champhekar A, Minter LM, Telfer JC, Miele L, Fauq A, Das P, Golde TE, Osborne BA. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood. 2008;112:1813–1821. doi: 10.1182/blood-2008-03-144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, Zhang P, Zamarron BF, Yu D, Wu Y, et al. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudra D, Egawa T, Chong MM, Treuting P, Littman DR, Rudensky AY. Runx-CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2009;10:1170–1177. doi: 10.1038/ni.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tone M, Greene MI. Cooperative regulatory events and Foxp3 expression. Nat Immunol. 2011;12:14–16. doi: 10.1038/ni0111-14. • Thoughtful commentary and update on the increasingly crowded literature concerning the transcriptional regulation of Foxp3, and including a fine graphic.
- 42.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory T cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghizzoni M, Haisma HJ, Maarsingh H, Dekker FJ. Histone acetyltransferases are crucial regulators in NF-kappaB mediated inflammation. Drug Discov Today. 2011;16:504–511. doi: 10.1016/j.drudis.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ito I, Hanyu A, Wayama M, Goto N, Katsuno Y, Kawasaki S, Nakajima Y, Kajiro M, Komatsu Y, Fujimura A, et al. Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J Biol Chem. 2010;285:14747–14755. doi: 10.1074/jbc.M109.093039. • Interesting study showing that ERα signaling can dampen TGF-β-dependent Smad signaling. This interaction may help explain differences in the suppressive functions of female vs. male Tregs.
- 49.Saji S, Kawakami M, Hayashi S, Yoshida N, Hirose M, Horiguchi S, Itoh A, Funata N, Schreiber SL, Yoshida M, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24:4531–4539. doi: 10.1038/sj.onc.1208646. [DOI] [PubMed] [Google Scholar]
- 50.Shan B, Yao TP, Nguyen HT, Zhuo Y, Levy DR, Klingsberg RC, Tao H, Palmer ML, Holder KN, Lasky JA. Requirement of HDAC6 for transforming growth factor-beta1-induced epithelial-mesenchymal transition. J Biol Chem. 2008;283:21065–21073. doi: 10.1074/jbc.M802786200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao Y, Li H, Gu Y, Davidson NE, Zhou Q. Inhibition of SIRT1 deacetylase suppresses estrogen receptor signaling. Carcinogenesis. 2010;31:382–387. doi: 10.1093/carcin/bgp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim JY, Oh MA, Kim WH, Sohn HY, Park SI. AMP-activated protein kinase inhibits TGF-beta-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator p300. J Cell Physiol. 2011 doi: 10.1002/jcp.22824. [DOI] [PubMed] [Google Scholar]
- 54.Zeng R, Aoki Y, Yoshida M, Arai K, Watanabe S. Stat5B shuttles between cytoplasm and nucleus in a cytokine-dependent and -independent manner. J Immunol. 2002;168:4567–4575. doi: 10.4049/jimmunol.168.9.4567. [DOI] [PubMed] [Google Scholar]
- 55.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–612. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 56.Ma L, Gao JS, Guan Y, Shi X, Zhang H, Ayrapetov MK, Zhang Z, Xu L, Hyun YM, Kim M, et al. Acetylation modulates prolactin receptor dimerization. Proc Natl Acad Sci U S A. 2010;107:19314–19319. doi: 10.1073/pnas.1010253107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J Cell Biol. 2009;184:57–66. doi: 10.1083/jcb.200807021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H, Huehn J. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 60. Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. •• Superb dissection of the roles of various conserved non-coding sequences in the regualtion of Foxp3 transcription.
- 61.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B, Tahk S, Yee KM, Fan G, Shuai K. The ligase PIAS1 restricts natural regulatory T cell differentiation by epigenetic repression. Science. 2010;330:521–525. doi: 10.1126/science.1193787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–3735. doi: 10.1182/blood-2009-05-219584. • Useful overview of the literature around Foxp3 methyaltion and related epigenetic events.
- 64.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 65.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 66.Anckar J, Sistonen L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol. 2007;594:78–88. doi: 10.1007/978-0-387-39975-1_8. [DOI] [PubMed] [Google Scholar]
- 67. Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. •• Demonstration that HSF1 is inducibly acetylated at a critical residue that negatively regulates DNA binding activity, and how Sirt1 reverses this acetylation and prolongs HSF1 binding to DNA This finding provides mechanistic insights into the HSR and may have relevance to efforts to increase longevity.
- 68.Lee JH, Park JH, Jung Y, Kim JH, Jong HS, Kim TY, Bang YJ. Histone deacetylase inhibitor enhances 5-fluorouracil cytotoxicity by down-regulating thymidylate synthase in human cancer cells. Mol Cancer Ther. 2006;5:3085–3095. doi: 10.1158/1535-7163.MCT-06-0419. [DOI] [PubMed] [Google Scholar]
- 69.de Zoeten EF, Lee I, Wang L, Chen C, Ge G, Wells AD, Hancock WW, Ozkaynak E. Foxp3 processing by proprotein convertases and control of regulatory T cell function. J Biol Chem. 2009;284:5709–5716. doi: 10.1074/jbc.M807322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hancock WW, Ozkaynak E. Three distinct domains contribute to nuclear transport of murine Foxp3. PLoS ONE. 2009;4:e7890. doi: 10.1371/journal.pone.0007890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bush EW, McKinsey TA. Protein acetylation in the cardiorenal axis: the promise of histone deacetylase inhibitors. Circ Res. 2010;106:272–284. doi: 10.1161/CIRCRESAHA.109.209338. • Set in the context of cardiovascular and renal diseases, this excellent review of HDACs and their inhibitors provides an up-to-date analysis of much data that is integrated into useful concepts and principles that have broad relevance in HDAC biology.