Abstract
Colour vision is found in many invertebrate and vertebrate species. It is the ability to discriminate objects based on the wavelength of emitted light independent of intensity. As it requires the comparison of at least two photoreceptor types with different spectral sensitivities, this process is often mediated by a mosaic made of several photoreceptor types. In this review, we summarize the current knowledge about the formation of retinal mosaics and the regulation of photopigment (opsin) expression in the fly, mouse and human retina. Despite distinct evolutionary origins, as well as major differences in morphology and phototransduction machineries, there are significant similarities in the stepwise cell-fate decisions that lead from progenitor cells to terminally differentiated photoreceptors that express a particular opsin. Common themes include i) the use of binary transcriptional switches that distinguish classes of photoreceptors, ii) the use of gradients of signaling molecules for regional specializations, iii) stochastic choices that pattern the retina and iv) the use of permissive factors with multiple roles in different photoreceptor types.
Keywords: photoreceptor, opsin, colour vision, retinal mosaic, transcription factors
INTRODUCTION
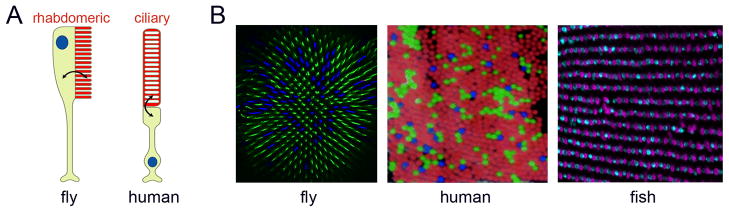
Visual processing begins in photoreceptors (PRs) with the conversion of photon energy into an electrical signal that is transmitted to the brain. This signal transduction process is mediated by photopigments and the phototransduction machinery that are located in specialized subcellular compartments (Hardie and Raghu, 2001). Based on their morphology and characteristic molecular components, two main types of PRs can be distinguished (Fig. 1A):Rhabdomeric PRs with tightly packed microvilli are exemplified by the Drosophila PRs and mainly found in protostome invertebrates. The deuterostome lineage typically uses ciliary PRs that have a modified cilium from which stacks of membranous discs emerge (Arendt, 2003; Yau and Hardie, 2009). The discovery that the marine ragworm Platynereis has both, rhabdomeric PRs in the eyes and ciliary PRs in the brain, suggests that a common ancestor of the protostome and deuterostome lineages probably had both types (Arendt et al., 2004). This view received additional support from the discovery that a subset of retinal ganglion cells in the mammalian retina expresses a rhabdomeric-type opsin, melanopsin, and appears to use a rhabdomeric phototransduction cascade (Graham et al., 2008; Bailes and Lucas, 2010; Do and Yau, 2010). This suggests that the mammalian retina has both ciliary-type and rhabdomeric-type PRs.
Figure 1.
Photoreceptor morphology and retinal mosaics in invertebrates and vertebrates. A) Light is absorbed by specialized photoreceptor compartments (red), called rhabdomeres in invertebrates and discs in vertebrates. Adapted from (Arshavsky, 2003) with permission from AAAS. B) Stochastic (fly, human) or highly organized (fish) opsin expression in retinal mosaics. The fly R8 photoreceptors express either the short wavelength Rh5 (blue) or the medium wavelength sensitive Rh6 (green). Humans have three types of cones for short (blue), medium (green) and long wavelengths (red). Adapted from (Roorda and Williams, 1999). The zebrafish retina differs from the previous two mosaics, as it has a precisely arranged distribution of cones (UV and blue; adapted from (Allison et al., 2010)).
Rhabdomeric and ciliary PRs contain light-sensitive photopigments that consist of an opsin protein, which is covalently linked by a lysine residue to a vitamin A-derived chromophore (retinal or 2-dehydroretinal in vertebrates, 3-hydroxyretinal in flies (Hardie, 2001)). As retinal is found in all photopigments and responds maximally to UV light, additional spectral tuning renders opsins sensitive to specific wavelengths. This is achieved by the interaction of retinal with particular amino acid residues in each opsin. Changing these critical amino acids can shift the absorption properties of an opsin (Salcedo et al., 2009).
In both rhabdomeric and ciliary PRs, photon absorption causes a cis-trans isomerization of retinal. This induces a conformational change of the opsin protein that activates an associated G protein cascade (Gq in invertebrates, Gt in vertebrates). This ultimately leads to opening (invertebrates) or closing (vertebrates) of cation channels and changes in the membrane potential of the PR: Light depolarizes invertebrate rhabdomeric PRs as well as ‘melanopsin ganglion cells’ (Graham et al., 2008), whereas it hyperpolarizes vertebrate ciliary PRs. Phototransduction, especially in flies, is one of the fastest known signal transduction cascades (Hardie, 2001). It provides high sensitivity due to remarkable signal amplification that allows the detection of single photons (Fain et al., 2010) with a very broad dynamic range (~ 7 orders of magnitude in human rods (Arshavsky, 2003)).
Despite the obvious morphological differences between vertebrate single lens eyes and insect compound eyes (Erclik et al., 2009), the arrangement of multiple PR subtypes in beautiful spatial mosaics is similar (Fig. 1B). Photoreception by these PR arrays represents the first processing step for a number of vital visual functions including image formation, motion detection and colour vision, the ability to distinguish objects based on their wavelength composition and independent of light intensity. The retina also mediates ‘non-image’ functions like circadian entrainment and pupillary reflexes. In this review, we focus on the expression of opsinsin defined subtypes of PRs that underlies colour vision.
PHOTORECEPTOR TYPES AND OPSIN EXPRESSION IN THE FLY, MOUSE AND HUMAN RETINA
Colour vision requires the presence of at least two PR subtypes with different wavelength sensitivity and a neuronal circuit for the comparison of their outputs to extract chromatic information (Pichaud et al., 1999; Conway, 2009). First, we describe the different PR subtypes that are found in the fly, mouse and human retina. Then, we present an overview of the regulatory mechanisms and key factors that guide their development. Finally, we highlight similarities and differences between the three species.
Flies have two main photoreceptor types(R1-6, R7/8)that express different rhodopsins
The compound eye of the fly consists of about 750 single unit eyes (ommatidia) that contain eight different rhabdomeric PRs, which fall into two main types: The six ‘outer’ PRs R1-6 form a trapezoid and the two ‘inner’ R7/8 PRs are positioned in its center (Fig. 2A). R1-6 and R7/8 differ from each other in anatomical features as well as functional aspects (Heisenberg and Wolf, 1984; Hardie, 1985; Fischbach and Dittrich, 1989). R1-6 PRs have large-diameter rhabdomeres that express the broad-spectrum, blue-green-sensitive Rhodopsin, Rh1 (O’Tousa et al., 1985; Zuker et al., 1985; O’Tousa et al., 1989; Huber et al., 1990). An additional UV-sensitizing pigment expands their photon absorption to the UV (Kirschfeld and Franceschini, 1977; Minke and Kirschfeld, 1979; Stavenga, 2004). R1-6 PRs are characterized by high sensitivity even at low light levels. They feed into four postsynaptic pathways in the first-order neuropil of the optic lobe, the lamina, which extract information about directional motion and the position of objects (Heisenberg and Buchner, 1977; Rister et al., 2007; Yamaguchi et al., 2008).
Figure 2.
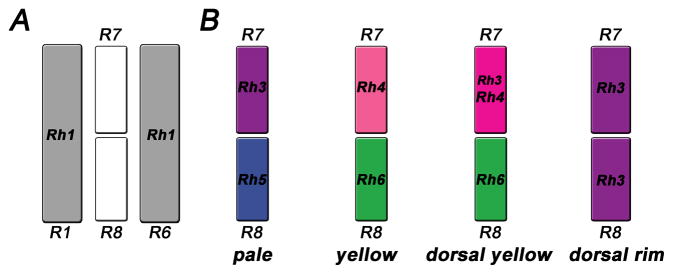
(A) ‘Outer’ photoreceptors (grey, only R1 and R6 are shown) express Rh1 and surround the ‘inner’ R7/8, which occur in four subtypes (B) that express different rhodopsins: pale (Rh3/Rh5), yellow (Rh4/Rh6), dorsal yellow (Rh3+Rh4/Rh6) and dorsal rim (Rh3/Rh3). Note that dorsal rim R7/8 are monochromatic and have enlarged rhabdomeres.
The ‘inner’ PRs are arranged in tandem, with R7 being more distal and on top of R8. R7/8 have smaller rhabdomere diameters than R1-6 and project to two different layers of the second neuropil, the medulla (Fischbach and Dittrich, 1989; Morante and Desplan, 2008). In contrast to R1-6 PRs that have high sensitivity at low light levels (Heisenberg and Buchner, 1977), R7/8 only operate at higher light intensities. They mediate phototaxis, colour vision and the detection of the vector of polarized light (Menne and Spatz, 1977; Wernet et al., 2003; Gao et al., 2008; Yamaguchi et al., 2010).
The fly retina has two main ommatidial subtypes called ‘pale’ (p) and ‘yellow’ (y) in which R7/8 express distinct combinations of four rhodopsins (Fig. 2B; Rh3 or Rh4 in R7, Rh5 or Rh6 in R8). In p ommatidia, R7 expresses UV-sensitive Rh3, while the underlyingR8 contains blue-sensitive Rh5 (Fig. 2B). In the remaining y ommatidia, R7 hasRh4 that is sensitive to longer UV-wavelengths and R8 contains the green-sensitive Rh6. Functionally, the p and y subtypes are involved in discriminating either short (p) or longer (y) wavelengths (Gao et al., 2008; Yamaguchi et al., 2010). They are stochastically distributed in a 30:70 ratio (Chou et al., 1996; Papatsenko et al., 1997; Chou et al., 1999) that is conserved even in evolutionarily distant (>120 Million years) species like the blowfly Calliphora and the housefly Musca (Kirschfeld and Franceschini, 1977; Schmitt et al., 2005).
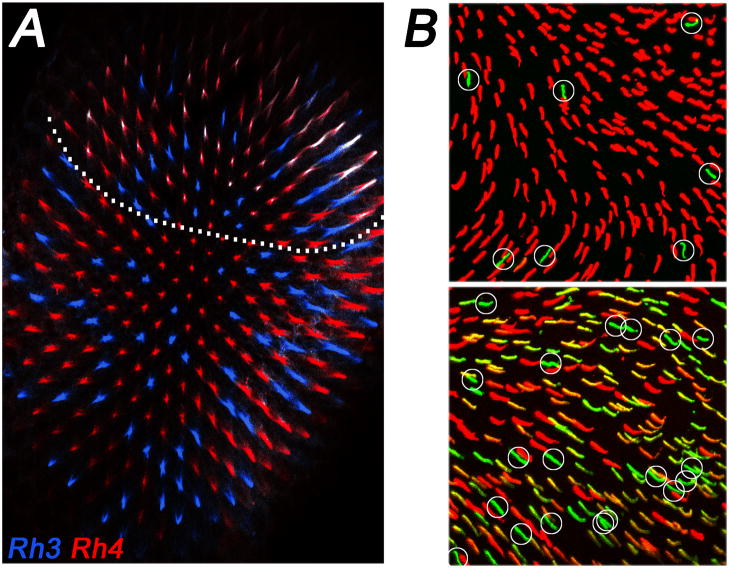
Two additional subtypes of ommatidia were discovered in the dorsal part of the eye. First, in ‘dorsal y’ ommatidia that span the dorsal third of the retina, Rh3 is coexpressed with Rh4 in yR7; the corresponding yR8 cells maintain Rh6 expression (Fig. 3A) (Mazzoni et al., 2008). The coexpression of Rh3 and Rh4 is surprising, as the choice for the expression of a particular receptor protein usually leads to the exclusion of all others to prevent sensory confusion (Mazzoni et al., 2004; Serizawa et al., 2004). The functional relevance of the regional specialization in dorsal y ommatidia is unclear, but it probably allows the fly to detect a broader UV range in R7s and to compare it to the long wavelengths detected by Rh6 in R8. This may facilitate the discrimination of solar vs. non-solar skylight for navigation (Stavenga and Arikawa, 2008).
Figure 3.
Regional differences in opsin expression. (A) Fly dorsal yellow ommatidia coexpress (white) Rh3 (blue) and Rh4 (red). Note that these specialized ommatidia occur only in the dorsal third of the eye (indicated by dotted line). (B) The dorsal mouse retina (top) has mostly M opsin (red) and few S opsin (green, circled) positive cones. In the ventral retina (bottom), the majority of cones show variable levels of coexpression of M and S opsin. Cones that appear to predominantly express S opsin (green, circled) are much more frequent than in the dorsal part of the retina. Figure adapted from (Haverkamp et al., 2005).
Second, dorsal rim area (DRA) ommatidia are found in the dorsal-most one to two rows of ommatidia. They have significantly larger rhabdomeres with orthogonally oriented microvilli in R7 and R8. They both express rh3 and are therefore monochromatic (Fortini and Rubin, 1991; Wernet et al., 2003). This configuration serves the detection of the vector of polarized light, which is used for navigation (Labhart and Meyer, 1999). Taken together, the fly retina contains four types of ommatidia (with the respective R7/8 opsins): pale (Rh3/Rh5), yellow (Rh4/Rh6), dorsal yellow (Rh3+Rh4/Rh6), and DRA (Rh3/Rh3).
Rods and cones in the mammalian retina
Mammalian eyes have two types of ciliary PRs, rods and cones (Nathans, 1989; Kolb, 2003; Swaroop et al., 2010), whose function is reminiscent of ‘outer’ R1-6 and ‘inner’ R7/8 in flies, respectively. Rods, like R1-6, have high sensitivity and are involved in dim light vision. They express rod opsin (rhodopsin) with a broad spectral sensitivity centered about 500 nm. In addition, most mammals have two types of cone PRs that express opsins that allow wavelength discrimination under bright light conditions. A third type of photosensitive cells, the retinal ganglion cells that express the rhabdomeric melanopsin, are involved in circadian entrainment as well as the regulation of pupillary reflexes, and are able to convey information about ambient light intensity (Brown et al., 2010; Do and Yau, 2010).
Mammals typically have a dichromatic colour vision system. For instance, mice are nocturnal dichromats with a relatively simple retinal mosaic (Jacobs, 1993; Neitz and Neitz, 2001). Rod opsin is expressed in 97% of PRs while the two cone opsins, one UV/blue-sensitive (S) and one green-sensitive (M), are expressed in the remaining 3% (Szel et al., 1993; Rohlich et al., 1994). Immunohistochemical and electrophysiological studies revealed that M expression follows a dorso-ventral gradient and that there is widespread coexpression with S opsin (Rohlich et al., 1994; Applebury et al., 2000; Nikonov et al., 2006). Depending on their relative amounts and whether either S or M dominates the spectral sensitivity of a given cone, two main domains can be distinguished (Szel et al., 1992; Haverkamp et al., 2005; Nikonov et al., 2006). In the dorsal third of the eye, the cone cell types predominantly express either S (3–5%) or high levels of M pigment (Fig. 3B). In the ventral two thirds, almost all cones express S opsin, which appears to be the only opsin in a few of them (genuine S cones), while the majority coexpresses modest to low levels of M opsin (Applebury et al., 2000; Haverkamp et al., 2005; Nikonov et al., 2006). This coexpression of S and M opsins resembles the coexpression of opsins in the dorsal fly eye (see above); it has the same advantage of broadening the absorption range, but it also compromises colour vision. Despite this problem, several lines of evidence suggest that mice are able to extract chromatic information and this is probably mediated by the genuine S or M cones mentioned above. Behavioural experiments revealed that mice are able to discriminate dichromatic stimuliin dependent of intensity (Jacobs et al., 2004)and recordings from ganglion cells detected spectrally opponent responses (Ekesten et al., 2000). Moreover, it has been shown that that genuine S cone cells in the ventral retina are connected to specialized ‘blue cone bipolar cells’ (Haverkamp et al., 2005). The function of widespread S/Mopsin coexpression remains unclear.
Humans, and other primates that are diurnal, have three cone opsins (Merbs and Nathans, 1992), one S (maximal absorption: 430nm) and two highly homologous M (530nm) and L (560nm) opsins that originated from a gene duplication specific to the primate lineage (Nathans, 1999). The density of M and L cones is highest in a central area known as fovea that mediates high acuity spatial and colour vision. L/M cones outnumber the regularly arranged S cones by tenfold (Marc and Sperling, 1977). The human cone mosaic differs from the mouse retina not only in the additional L opsin and the specialization of the fovea, but also in that it doesn’t exhibit widespread S/M opsin coexpression: Only a small number of cones coexpressing S and L/M was found in the adult human retina (Xiao and Hendrickson, 2000). However, during development there is a population of human cones that transiently coexpresses S and L/M (Cornish et al., 2004), and after the decision for either L or M is made, S appears to become switched off.
PHOTORECEPTOR CELL FATE DECISIONS AND THE MECHANISMS THAT REGULATE OPSIN EXPRESSION IN FLIES AND MAMMALS
In the next section, we summarize how retinal mosaics are generated by consecutive cell fate decisions. We also discuss the mechanisms that underlie the stochastic and mutually exclusive expression of opsins.
OPSIN EXPRESSION IN FLIES
Binary cell fate decisions distinguishR1-6 fromR7/8 and R7 fromR8 photoreceptors in the fly retina
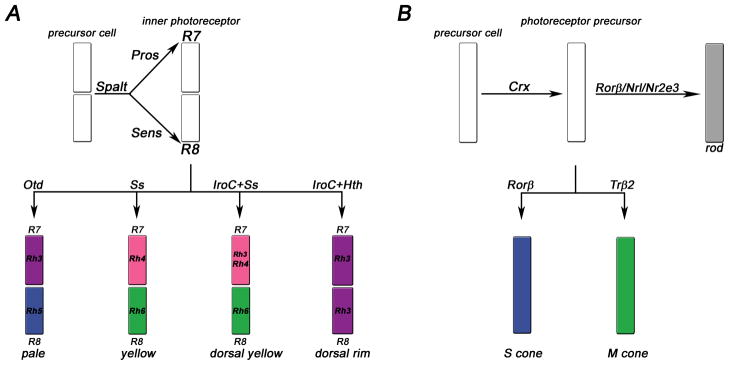
In the Drosophila eye, terminal PR differentiation begins with the distinction between ‘outer’ and ‘inner’ PRs (Fig. 4A) by the spalt gene complex. It encodes two zinc finger transcription factors (TF) that are expressed in R7 and R8 throughout development and play essential roles in their terminal differentiation (Mollereau et al., 2001); (Domingos et al., 2004): spalt mutant R7/8 PRs have enlarged, outer PR-liker habdomeres that also express Rh1 instead of R7/8 opsins (Fig. 4A). In R8, spalt is required for pupal expression of the zinc finger TF Senseless (Sens) (Frankfort et al., 2001) that promotes R8 features such as proximal cell bodies and rhabdomeres as well as R8 opsin expression (Domingos et al., 2004; Xie et al., 2007). Sens also negatively regulates R7 characteristics, for instance by repressing R7 opsins (Xie et al., 2007).
Figure 4.
Simplified schematic of major cell fate decisions that create the retinal mosaic in the fly (A) and mouse (B) eyes. A) In flies, Spalt specifies inner photoreceptor fate. Prospero (Pros) determines R7 fate and Senseless (Sens) R8 fate. Then, a choice is made for one of the four R7/8 subtypes. B) In the mouse retina, Crx is expressed in all photoreceptor precursor cells. Rods are distinguished from cones by the expression of Rorβ, Nrl and Nr2e3. Cones adopt either short (S) or medium (M) cone fate depending on the expression of either Rorβ or Trβ2, respectively. Note that Rorβ plays a dual role in the rod and S cone pathways. For details, see text.
In R7, spalt is required for the expression of several early R7 markers (Domingos et al., 2004) including the homeodomain TF Prospero (Pros) that promotes R7 fate and represses R8 features (Cook et al., 2003) together with the nuclear factor subunit NFY-C, which prevents Sens expression (Morey et al., 2008). Repression of R8 opsins in R7 is presumably mediated by direct binding of Prosto the rh5 and rh6 promoters (Cook et al., 2003). Pros mutant PRs are not completely transformed into R8 PRs, but display intermediate characteristics such as expression ofRh5 and Rh6 along with Rh4.
Stochastic and deterministic cell fate decisions in fly PRs give rise to different ommatidial subtypes
Differential Rh expression in the two types of ommatidia is established by a three-step process (Fig. 5). First, about 70% of R7 cells stochastically express the TF Spineless that inducesyR7 fate and rh4 expression (Wernet et al., 2006). The remaining R7s that do not express Spineless become pR7 and express rh3. Second, pR7 cells send a signal of unknown identity to the underlying R8, inducingpR8 fate and rh5 expression (Chou et al., 1996; Papatsenko et al., 1997). Third, the p/y fate choice is consolidated in R8 by a double-negative feedback loop that involves the tumour suppressor warts and the growth regulator melted (Fig. 5), which repress each other (Mikeladze-Dvali et al., 2005). Melted appears to receive the signal from pR7 and is necessary and sufficient for inducing pR8 by repressing warts. In yR8s that did not receive the signal, warts represses melted as well as rh5, and promotes Rh6 expression.
Figure 5.
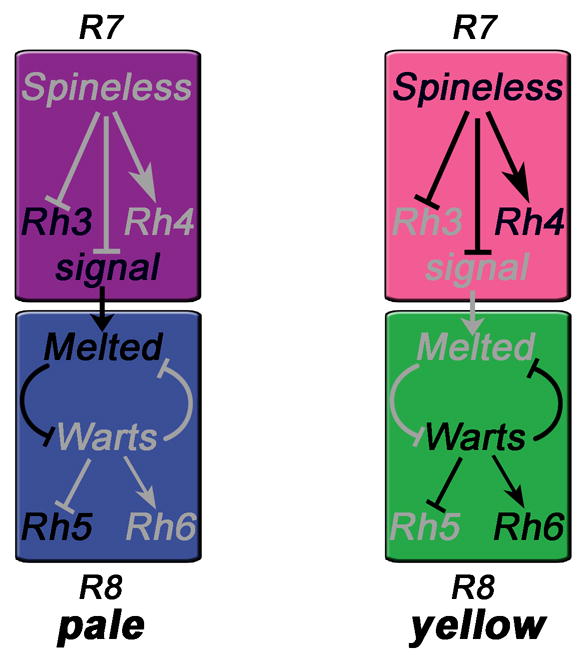
Pale vs. yellow cell fate decisions in R7 and instruction of R8 (Mikeladze-Dvali et al., 2005). Expression of the transcription factor Spineless in a subset of R7 cells induces yellow fate and Rh4 expression (right). The other R7s express Rh3 by default (left) and send a signal to the underlying R8, which appears to be received by Melted and leads to Rh5 expression. In yellow R8s, the signal is absent and Warts represses Melted as well as Rh5, promoting the default Rh6 fate. Grey indicates repressed genes and inactive regulatory pathways.
The terminal PR fate decisions described above are initiated by an unknown mechanism that regulates stochastic spineless expression, but there are also positional cues that guide the formation of two specialized types of ommatidia (DRA and dorsal y) in the dorsal eye region. DRA R7/8 PRs are specified by a combination of a graded signal mediated by the morphogen Wingless (Wg) that is secreted from the surrounding head cuticle, and an intrinsic signal from the dorsally expressed Iroquois complex (Iro-C) genes (Tomlinson, 2003; Wernet et al., 2003). Wg and Iro-C induce the expression of the homeodomain TF Homothorax (Hth) specifically in DRA R7/8 where it is necessary and sufficient for expression of rh3 in both R7 and R8 (Wernet et al., 2003). DRA R8 PRs do not express Sens, which would repress rh3 as it does in non-DRA R8 PRs (Xie et al., 2007). Iro-C genes are also necessary for the co-expression of rh3 with rh4 in y R7 PRs in the dorsal third of the eye (Mazzoni et al., 2008).
Permissive factors that are expressed in all fly photoreceptors are involved in activation of particular rhodopsins
The analysis of the rhodopsin promoters led to the discovery that TFs that are expressed in all fly PRs may directly regulate specific p or y rhodopsins. For instance, the promoters of rh3, rh5 and rh6 contain binding sites for the homeodomain TF Orthodenticle (Otd) (Vandendries et al., 1996). Removal of Otd function affects rhabdomere morphogenesis, causes loss of Rh3 and Rh5 (Fig. 4A) as well as derepression of Rh6 in R1-6 (Tahayato et al., 2003). It is unclear how Otd can act in opposite ways in different subsets of PRs while being present in all PRs. One possible scenario is that this involves unidentified, subset specific cofactors, as was reported for other homedomain TFs (Wilson and Desplan, 1995; Mann et al., 2009).
A key motif that is found in all rhodopsin promoters and is required for their general activation is the rhodopsin core sequence I (RCSI) (Mismer et al., 1988; Fortini and Rubin, 1990; Papatsenko et al., 2001). The RCSI motif alone is not sufficient for expression in specific PR subtypes, but oligomerized RCSI sites drive expression of a reporter in all PRs (Sheng et al., 1997; Papatsenko et al., 2001). Two TFs have been identified that can bind the RCSI in vitro: The homeodomain TF Eyeless/Pax6 (Sheng et al., 1997), the master regulator in both invertebrate and vertebrate eye development (Halder et al., 1995; Pichaud et al., 2001), and the homeodomain TF Pph13 (Mishra et al., 2010; S. G. Sprecher, pers. comm.). It is therefore possible that the RCSI sequences of different rhodopsins are recognized by several factors, including Pax6 and Pph13.
OPSIN EXPRESSION IN MAMMALS
Binary cell fate decisions distinguish rods from cones in the mammalian retina
In flies, the Spalt complex distinguishes R7/8PRs that are involved in colour vision from R1-6PRs that are achromatic and provide sensitivity at low light intensities (Mollereau et al., 2001). A comparable switch is made in the mammalian retina (Fig. 4B) by the neural leucine zipper TF Nrl (Mears et al., 2001; Oh et al., 2007). Nrl is expressed in developing and mature rods where it promotes rod fate and suppresses cone fate (Swain et al., 2001). Nrl mutant retinas have no rods, but only cone-like PRs with disorganized outer segments that slowly degenerate (Bessant et al., 1999; Mears et al., 2001; Daniele et al., 2005). Mutations in the human Nrl gene lead to autosomal dominant retinitis pigmentosa, a degeneration of rods, which is followed by secondary, non-cell autonomous degeneration of cones (DeAngelis et al., 2002).
Nrl is required for expression of the PR-specific orphan nuclear receptor Nr2e3 (Mears et al., 2001) that primarily represses cone genes in rods, but also coactivates rod phototransduction genes (Cheng et al., 2004). Nr2e3 thereby ensures the commitment to rod fate (Cheng et al., 2004; Chen et al., 2005; Cheng et al., 2006). Retinal degeneration 7 (rd7) mice, which carry a mutation in Nr2e3, show a slow retinal degeneration (Akhmedov et al., 2000). Mutation of NR2E3 in humans also causeslate-onset retinal degeneration, but, in addition, exhibits an enhanced S cone syndrome (ESCS) (Haider et al., 2000; Milam et al., 2002; Wright et al., 2004; Corbo and Cepko, 2005). ESCS is characterized by night blindness due to the loss of rods and an increased sensitivity towards short wavelengths due to an increase in S cones (Haider et al., 2000). ‘Rods’in the Nr2e3 mutant retina are hybrid PRs, as they coexpress both rod and cone specific genes (Chen et al., 2005; Corbo and Cepko, 2005). In the absence of Nr2e3, Nrl is still able to prevent cone development to some extent and the transformation of rods into cones appears more severe in Nrl mutants than in Nr2e3 mutants. This suggests that another unknown repressor downstream of Nrl is involved in mediating Nrl function (Chen et al., 2005; Corbo and Cepko, 2005; Corbo et al., 2007; Oh et al., 2008). Moreover, overexpression of Nrl, but not of Nr2e3, is sufficient to induce rod fate (Cheng et al., 2006; Oh et al., 2007).
A recent publication identified the retinoid-related orphan nuclear receptor RORβ as an upstream activator of the Nrl/Nr2e3 pathway (Jia et al., 2009). Rorβ mutant mice lose Nrl expression and lack rods; they also show an excess of non-functional S cone-like PRs, indicating that cone fate may be the default state, which is switched to rod fate by the Rorβ/Nrl/Nr2e3 pathway (Fig. 4B). In line with Rorβ being epistatic to Nrl, ectopic expression of Nrl in a Rorβ mutant background reprograms these abnormal cones into rod-like cells (Jia et al., 2009).
Cell fate decisions in mammalian PRs distinguish short from medium wavelength sensitive cones
After a developing PR is committed to the cone fate by the absence of Nrl, how are S and M subtypes distinguished from each other? It was suggested that all cones are initially of the S type while the M fate is an induced state (Cepko, 2000; Ng et al., 2001). M opsin expression requires the thyroid hormone receptor TRβ2 (Fig. 4B), a ligand-regulated TF that is expressed in cones (Ng et al., 2001; Harpavat and Cepko, 2003; Applebury et al., 2007; Ng et al., 2009). In TRβ2-deficient mice, cone cells develop, but they all express S opsin. TRβ2 therefore mediates the switch from S to M cone fate and inhibits S opsin expression (Ng et al., 2001), possibly as a heterodimer with the retinoic acid receptor RXRγ (Roberts et al., 2005). It is not clear, however, whether it acts directly or indirectly on the opsin promoters and the source of thyroid hormone is also unknown.
A major question is how the complex expression pattern of cone opsins in the mouse retina (Fig. 3B) is developmentally regulated. It is known that the nuclear receptor, RORβ, activates S opsin expression (Srinivas et al., 2006). As mentioned above, however, RORβ has an earlier function as a major switch to rod fate and its mutation leads to an excess of S-cone like PRs (see above). This suggests that interactions with rod and cone specific cofactors are required to explain this dual regulatory role (Jia et al., 2009). It is tempting to speculate that the two nuclear receptors, TRβ2 and RORβ also play a role in establishing the dorso-ventral M opsin gradient in the mouse eye. However, they are expressed uniformly across the retina and might therefore require gradients of their ligands. Their precise mechanism of action still needs to be resolved. A recent study suggested that dorsal suppression of S opsin is mediated by transient bone morphogenetic protein (BMP) signalling, which acts through the two orphan nuclear receptors COUP-TF I and II. In the ventral eye, only COUP-TF I acts to inhibit M opsin expression (Satoh et al., 2009). It remains to be determined how BMP is linked to the two COUP-TFs, how COUP-TF II activity is restricted to part of the retina and how COUP-TF I can specifically inhibit S or Mopsin despite being expressed both dorsally and ventrally (Satoh et al., 2009).
The permissive factor Crx is expressed in all mammalian photoreceptors and provides general activation of opsins
The ‘cone rod homeobox’ protein Crx belongs, like its fly ortholog Otd, to the Crx/Otx family of K50 homeodomain TFs that are characterized by a lysine at position 50 of their DNA binding domain. Crx is expressed in both rod and cone PRs with a critical function in PR differentiation and outer segment morphogenesis (Chen et al., 1997; Furukawa et al., 1997; Swain et al., 1997). Crx mutations lead to a variety of retinopathies in humans including cone rod dystrophy and Leber’s congenital amaurosis (Freund et al., 1997), as well as retinopathy in mice (Furukawa et al., 1999). Microarray analysis in fly and mouse retina (Livesey et al., 2000; Hsiau et al., 2007; Ranade et al., 2008) suggest that Otd/Crx controls the expression of opsins and other components of the phototransduction cascade, as well as PR morphogenesis, by binding to the consensus site TAATCC (Chen et al., 1997; Tahayato et al., 2003; Lee et al., 2010). The precise mechanism of Crx-mediated activation is unclear, but there is evidence based on in vitro and ChIP assays that Crx mediates chromatin remodeling via recruitment of coactivators that have histone-acetyltransferase activity (Peng and Chen, 2007). Crx, like Otd, is expressed in all PRs and appears to provide general activation of opsins. For instance, it activates S opsin expression in a synergistic manner with RORβ (Srinivas et al., 2006). In cotransfection studies, as well as in electroporated rod PRs in ex vivo retinas, Crx also positively regulates rod opsin promoters synergistically with Nrl (Chen et al., 1997; Mitton et al., 2000; Whitaker and Knox, 2004; Hsiau et al., 2007), emphasizing its suggested role as a general activator.
Stochastic M vs L cone fate decisions in the primate retina
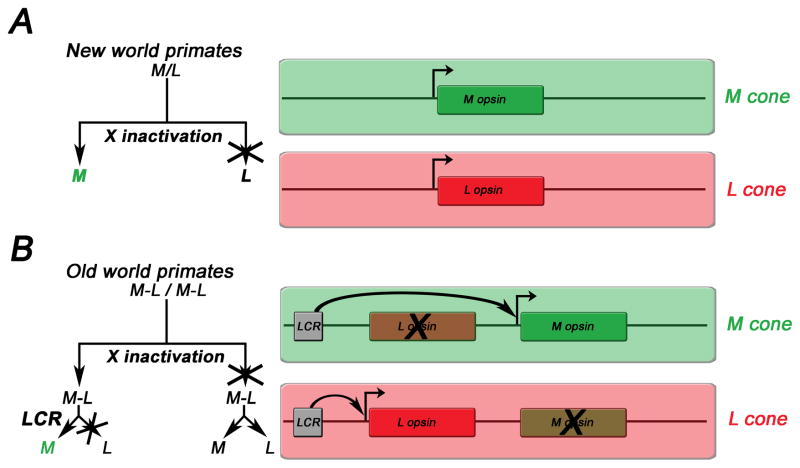
Two different mechanisms control trichromatic colour vision in primates (Fig. 6) (Jacobs, 2008; Jacobs, 2009). The first one is found in some new world (Central and South American) primates like squirrel monkeys. These have different alleles of an X-linked L/M opsin that are tuned differently to long (L) and medium (M) wavelengths, in addition to an S opsin. In a given population, heterozygous females that carry two different L/M alleles on their X-chromosomes have trichromatic colour vision (Fig. 6A) as stochastic X-chromosomal inactivation generates a retinal mosaic that shows random expression of one (M) or the other (L) allele in a given cone. In contrast, homozygous females that have two copies of the same allele or hemizygous males (that can only have one allele) are dichromats.
Figure 6.
Cell-fate decisions (left) and opsin configurations (right) underlying trichromacy in new world (A) and old world primates (B). A) In new world primates (e.g. squirrel monkeys), X chromosomal inactivation leads to expression of each of two different alleles of an M/L opsin (green/red), in addition to an S opsin (not shown) in heteroallelic females that are therefore trichromats. Males and homoallelic females are dichromats. B) In old world primates (e.g. humans), X-chromosomal inactivation and a stochastic choice by a locus control region (LCR) ensure the exclusive expression of either an L or an M opsin that are arranged in a tandem on the X-chromosome. This leads to trichromacy in both males and females. The decision trees to the left exemplify how the choice for M is made in the two different contexts.
The second mechanism evolved in old world monkeys through duplication of the X-linked L/M gene and different spectral tuning of the two copies, one to L and the other to M wavelengths (Jacobs, 2009). This mechanism renders all males as well as females trichromats. In humans, the L and M opsin genes are arranged in tandem on the X-chromosome (Fig. 6B). Three consecutive steps give rise to a stochastic opsin distribution: First, a decision to either express S or L/M opsin is made in a given cone. Second, X-chromosomal inactivation results in only one X chromosome being active in females (males have only one X-chromosome). Third, on this active X chromosome, a single upstream locus control region (LCR) (Wang et al., 1992; Smallwood et al., 2002) can only activate one of the two opsin genes, either L or M. In other words, this mechanism ensures a stochastic, cell-autonomous choice between the two opsins. Females, who carry two different alleles of their M or L opsin gene on each of their X chromosomes may achieve tetrachromacy when X inactivation allows expression of each allele in different subsets of PRs (Conway, 2009).
Mechanistic similarities in primate and fish retina
A LCR mechanism analogous to the one that controls L/M opsin expression in the human retina (see above) is involved in opsin expression in the zebrafish retinal mosaic. However, it differs significantly from the human and fly retina in that it has a remarkably precise and non-random spatial arrangement of different cone types (Fig. 1B). Zebrafish have nine opsin genes, four of which encode green-sensitive opsins (RH2-1 to RH2-4) that are arranged in tandem similar to the human L/M opsins. A 500bp upstream LCR is both necessary and sufficient for their expression (Tsujimura et al., 2007). Therefore, both the human and zebrafish retina use an upstream LCR to ensure mutually exclusive expression of duplicated opsin genes. The mechanisms that lead to mutually exclusive, yet highly organized and repetitive patterns of the four types of fish opsins (red, green, blue and UV), remain to be identified.
COMPARISON OF THE DEVELOPMENTAL MECHANISMS IN INVERTEBRATE AND VERTEBRATE RETINAL MOSAICS
Common design principles of the vertebrate and invertebrate brain circuitry downstream of the PRs as well as shared genetic pathways in eye morphogenesis have been recently reviewed (Charlton-Perkins and Cook, 2010; Sanes and Zipursky, 2010). Are there also mechanistic similarities in the terminal differentiation events that lead to the PR mosaics? For one, both systems develop from generic progenitors that are restricted in their cell fate in a stepwise manner by a combinatorial code of TFs (Cook and Desplan, 2001; Wernet and Desplan, 2004; Swaroop et al., 2010). Genetic analysis revealed that both involve regulators that act as major cell-fate switches. In this respect, it stands out that two TFs of the same family, mouse Crx and Drosophila Otd, are used in a very similar way as permissive general activators of many terminal PR specific differentiation genes, including opsins. Additionally, they are required for proper outer segment and rhabdomere morphogenesis, respectively. However, Otd mutant rhabdomeres are abnormally shaped, but do not completely degenerate like outer segments in Crx mutant mice. This is because in flies, a partially redundant pathway of rhabdomere morphogenesis is mediated by Pph13 (Mishra et al., 2010) and, in fact, double mutation of Otd and Pph13 resembles the Crx mutant phenotype in mice (Mishra et al., 2010). Another difference is that the role of Otd in opsin regulation appears more specific, as it is required only for expression of rh3 and rh5 in the pale ommatidial subset and repression of rh6 in R1-6, whereas other opsins (rh1, rh4) are unaffected by loss of Otd.
The basic principles of sequential PR specification are also preserved from flies to mice. For instance, the rod-specific TF Nrl promotes rod fate and suppresses cone fate. A similar switch occurs in flies with the Spalt genes that are only expressed in R7 and R8: They prevent R1-6 (‘rod’-like) fate and lead to a generic R7/8 (‘cone’-like) fate that is then refined by the TFs Pros (R7) or Sens (R8). Spalt mutant PRs are not completely transformed into R1-6, as they still project to the R7/8 target layers in the optic lobes (Mollereau et al., 2001). They therefore have characteristics of both PR types. Moreover, pros mutant R7 PRs that derepress R8 opsins are not completely transformed into R8 PRs, as they lack the R8 marker Sens and retain some R7 features like Rh4 expression (Cook et al., 2003). These two examples of mixed PR identity in selector gene mutants resemble the Nr2e3 mutant retina that has rod-cone hybrid PRs that coexpress rod and cone specific genes and have an intermediate anatomy (Chen et al., 2005; Corbo and Cepko, 2005). As many factors involved in the terminal differentiation programs that promote a particular fate and exclude others are yet to be discovered (especially in vertebrates), future research will show how common the concept of dual switches is for the generation of retinal mosaics. Examples of mutant PRs that are hybrids of different cell types as well as the existence of transient opsin coexpression in humans during development suggest that the programs may not always follow simple binary paths and that the picture could turn out to be more complex.
Another conserved mechanism is the use of gradients of signalling molecules that are best known for their role in anterior/posterior and dorsal/ventral patterning (Tabata and Takei, 2004). In the fly, the morphogen Wingless (the ortholog of Wnt-1) is required for terminal differentiation of the specialized dorsal ommatidial subtype (DRA) while a regionalized expression of IroC genes directs the formation of yR7 that co-express rh3 and rh4 in the dorsal third of the retina. A similar dorso-ventral gradient is found in the mouse retina that affects the distribution of M opsin, although this involves different factors (TRβ2, RORβ and COUP TFs) and uses thyroid hormone and BMP rather than Wnt signalling. Nevertheless, it would be interesting to know what kind of signalling mediates the coexpression of opsins in mouse PRs. In general, the prominent use of nuclear receptors in the mammalian retina is striking and it remains an open question whether they also play a role in the adult fly eye. So far, the best studied example for a nuclear factor acting in fly eye development is the orphan receptor Seven-up that acts in early fate specification of a subset ofR1-6 PRs (Mlodzik et al., 1992; Miller et al., 2008); as well as in promoting Rh6 expression in the larval eye (Sprecher et al., 2007).
Stochastic cell-fate decisions as observed in the patterning of fly and human retinal mosaics (Fig. 1B) have also been reported in other developmental contexts (Losick and Desplan, 2008; Johnston and Desplan, 2010). It would therefore be important to gain a better understanding of their mechanistic requirements. X-chromosomal inactivation is not found in flies and a LCR mechanism is unlikely to account for the opsin distribution in Drosophila, as the rhodopsin genes are not clustered as L/M opsin in humans or green opsin in zebrafish. R7 opsins appear to be randomly distributed and nearest-neighbour evaluation showed that there is no cross talk between ommatidia (Bell et al., 2007). However, as there is a preference for having more of the long wavelength sensitive y ommatidia (70%), this requires some kind of biased mechanism. It is not known how the stochastic expression of Spineless is achieved in flies and how it controls the 30:70 ratio of ommatidial subtypes.
The origin and function of the L/M opsin distribution in humans is also not fully understood. One model suggests that it depends on the strength of the L and M promoters in competing for the association with the LCR, or on the distance between the LCR and the L or M promoters (Smallwood et al., 2002). The L/M ratio appears to be quite variable between individuals, as recent in vivo high resolution imaging studies found a broad range of distribution, from 1:1 to 17:1 (Roorda and Williams, 1999; Hofer et al., 2005). Moreover, the precise ratio of L and M does not appear to be relevant for colour processing, as the high inter-individual variability in opsin ratios seems to have no detectable functional impact, at least in standard colour vision tests (Hofer et al., 2005). However, it could well be that tests with higher sensitivity have to be developed in order to detect subtle differences in colour discrimination. Interestingly, the arrangement of human L and M cones appears not to be a completely random process, as clumping of the same type can be observed (Packer et al., 1996; Hofer et al., 2005). As this deviation from randomness appears to be rather small, it remains to be seen whether this represents a significant difference between fly and human mosaics.
CONCLUDING REMARKS
In this review, we summarized the current knowledge about the developmental mechanisms that underlie opsin expression in the fly, mouse and human retina and highlighted four main similarities: i) binary cell-fate decisions distinguish PR classes, ii) gradients of signaling molecules allow regional specializations in the fly and mouse retina, iii) stochastic mechanisms pattern the fly and human retina and iv) permissive factors play key roles in PR differentiation and opsin expression.
It is not clear why colour vision involves stochastic opsin expression in randomly organized PR mosaics. A parsimonious explanation could be that this is the simplest way to evolve such a system and that a precisely organized lattice of opsin expression would be either too costly to develop or not possible due to constraints in the regulatory networks. However, this is certainly not the case: Teleost fish, such as tetrachromatic zebrafish, have a nearly crystalline arrangement of four types of PRs organized in stereotyped arrays (Fig. 1B): Rows of red and green double cone pairs alternate with rows of blue and UV single cones (Allison et al., 2010). Moreover, tetrachromatic birds have a sophisticated and highly organized mosaic of four single cone types and one double cone (Kram et al., 2010). Disordered PR arrays are therefore not a prerequisite for colour vision. We conclude that in those species where stochastic distributions of PRs are found, such an arrangement is sufficient to meet the evolutionary demands (Ahnelt and Kolb, 2000) placed upon that species.
Acknowledgments
We thank Robert Johnston, Dominic Didiano, Joseph Corbo, Simon Sprecher, Maria Tsachaki and two anonymous reviewers for comments on the manuscript. We also thank Silke Haverkamp for providing images of the mouse retina. This work was supported by NIH EY13010-11 to C. D. and an EMBO long-term fellowship (ALTF 462-2008) to J. R.
References
- Ahnelt PK, Kolb H. The mammalian photoreceptor mosaic-adaptive design. Prog Retin Eye Res. 2000;19:711–777. doi: 10.1016/s1350-9462(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Akhmedov NB, Piriev NI, Chang B, Rapoport AL, Hawes NL, Nishina PM, Nusinowitz S, Heckenlively JR, Roderick TH, Kozak CA, Danciger M, Davisson MT, Farber DB. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc Natl Acad Sci U S A. 2000;97:5551–5556. doi: 10.1073/pnas.97.10.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison WT, Barthel LK, Skebo KM, Takechi M, Kawamura S, Raymond PA. Ontogeny of cone photoreceptor mosaics in zebrafish. J Comp Neurol. 2010;518:4182–4195. doi: 10.1002/cne.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Farhangfar F, Glosmann M, Hashimoto K, Kage K, Robbins JT, Shibusawa N, Wondisford FE, Zhang H. Transient expression of thyroid hormone nuclear receptor TRbeta2 sets S opsin patterning during cone photoreceptor genesis. Dev Dyn. 2007;236:1203–1212. doi: 10.1002/dvdy.21155. [DOI] [PubMed] [Google Scholar]
- Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 2003;47:563–571. [PubMed] [Google Scholar]
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- Arshavsky VY. Protein translocation in photoreceptor light adaptation: a common theme in vertebrate and invertebrate vision. Sci STKE. 2003;2003:PE43. doi: 10.1126/stke.2003.204.pe43. [DOI] [PubMed] [Google Scholar]
- Bailes HJ, Lucas RJ. Melanopsin and inner retinal photoreception. Cell Mol Life Sci. 2010;67:99–111. doi: 10.1007/s00018-009-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Earl JB, Britt SG. Two types of Drosophila R7 photoreceptor cells are arranged randomly: a model for stochastic cell-fate determination. J Comp Neurol. 2007;502:75–85. doi: 10.1002/cne.21298. [DOI] [PubMed] [Google Scholar]
- Bessant DA, Payne AM, Mitton KP, Wang QL, Swain PK, Plant C, Bird AC, Zack DJ, Swaroop A, Bhattacharya SS. A mutation in NRL is associated with autosomal dominant retinitis pigmentosa. Nat Genet. 1999;21:355–356. doi: 10.1038/7678. [DOI] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. Giving in to the blues. Nat Genet. 2000;24:99–100. doi: 10.1038/72887. [DOI] [PubMed] [Google Scholar]
- Charlton-Perkins M, Cook TA. Building a fly eye: terminal differentiation events of the retina, corneal lens, and pigmented epithelia. Curr Top Dev Biol. 2010;93:129–173. doi: 10.1016/B978-0-12-385044-7.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- Cheng H, Aleman TS, Cideciyan AV, Khanna R, Jacobson SG, Swaroop A. In vivo function of the orphan nuclear receptor NR2E3 in establishing photoreceptor identity during mammalian retinal development. Hum Mol Genet. 2006;15:2588–2602. doi: 10.1093/hmg/ddl185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Khanna H, Oh EC, Hicks D, Mitton KP, Swaroop A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Genet. 2004;13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, Britt SG. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17:1101–1115. doi: 10.1016/s0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, Chadwell LV, Paulsen R, Britt SG. Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development. 1999;126:607–616. doi: 10.1242/dev.126.4.607. [DOI] [PubMed] [Google Scholar]
- Conway BR. Color vision, cones, and color-coding in the cortex. Neuroscientist. 2009;15:274–290. doi: 10.1177/1073858408331369. [DOI] [PubMed] [Google Scholar]
- Cook T, Desplan C. Photoreceptor subtype specification: from flies to humans. Semin Cell Dev Biol. 2001;12:509–518. doi: 10.1006/scdb.2001.0275. [DOI] [PubMed] [Google Scholar]
- Cook T, Pichaud F, Sonneville R, Papatsenko D, Desplan C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev Cell. 2003;4:853–864. doi: 10.1016/s1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Corbo JC, Cepko CL. A hybrid photoreceptor expressing both rod and cone genes in a mouse model of enhanced S-cone syndrome. PLoS Genet. 2005;1:e11. doi: 10.1371/journal.pgen.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo JC, Myers CA, Lawrence KA, Jadhav AP, Cepko CL. A typology of photoreceptor gene expression patterns in the mouse. Proc Natl Acad Sci U S A. 2007;104:12069–12074. doi: 10.1073/pnas.0705465104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish EE, Xiao M, Yang Z, Provis JM, Hendrickson AE. The role of opsin expression and apoptosis in determination of cone types in human retina. Exp Eye Res. 2004;78:1143–1154. doi: 10.1016/j.exer.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, Swaroop A, Williams DS, Pugh EN., Jr Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol Vis Sci. 2005;46:2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis MM, Grimsby JL, Sandberg MA, Berson EL, Dryja TP. Novel mutations in the NRL gene and associated clinical findings in patients with dominant retinitis pigmentosa. Arch Ophthalmol. 2002;120:369–375. doi: 10.1001/archopht.120.3.369. [DOI] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos PM, Brown S, Barrio R, Ratnakumar K, Frankfort BJ, Mardon G, Steller H, Mollereau B. Regulation of R7 and R8 differentiation by the spalt genes. Dev Biol. 2004;273:121–133. doi: 10.1016/j.ydbio.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Ekesten B, Gouras P, Yamamoto S. Cone inputs to murine retinal ganglion cells. Vision Res. 2000;40:2573–2577. doi: 10.1016/s0042-6989(00)00122-x. [DOI] [PubMed] [Google Scholar]
- Erclik T, Hartenstein V, McInnes RR, Lipshitz HD. Eye evolution at high resolution: the neuron as a unit of homology. Dev Biol. 2009;332:70–79. doi: 10.1016/j.ydbio.2009.05.565. [DOI] [PubMed] [Google Scholar]
- Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Curr Biol. 2010;20:R114–124. doi: 10.1016/j.cub.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach KF, Dittrich APM. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. 1989;258:441–475. [Google Scholar]
- Fortini ME, Rubin GM. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev. 1990;4:444–463. doi: 10.1101/gad.4.3.444. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Rubin GM. The optic lobe projection pattern of polarization-sensitive photoreceptor cells in Drosophila melanogaster. Cell Tissue Res. 1991;265:185–191. doi: 10.1007/BF00318153. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Nolo R, Zhang Z, Bellen H, Mardon G. senseless repression of rough is required for R8 photoreceptor differentiation in the developing Drosophila eye. Neuron. 2001;32:403–414. doi: 10.1016/s0896-6273(01)00480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund CL, Gregory-Evans CY, Furukawa T, Papaioannou M, Looser J, Ploder L, Bellingham J, Ng D, Herbrick JA, Duncan A, Scherer SW, Tsui LC, Loutradis-Anagnostou A, Jacobson SG, Cepko CL, Bhattacharya SS, McInnes RR. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997;91:543–553. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Gao S, Takemura SY, Ting CY, Huang S, Lu Z, Luan H, Rister J, Thum AS, Yang M, Hong ST, Wang JW, Odenwald WF, White BH, Meinertzhagen IA, Lee CH. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99:2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, Beck G, Hockey R, Hanna DB, Gorman S, Duhl D, Carmi R, Bennett J, Weleber RG, Fishman GA, Wright AF, Stone EM, Sheffield VC. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hardie RC. Functional organization of the fly retina. In: Ottoson D, editor. Ottoson Ds Sensory Physiology. Springer-Verlag; 1985. pp. 1–79. [Google Scholar]
- Hardie RC. Phototransduction in Drosophila melanogaster. J Exp Biol. 2001;204:3403–3409. doi: 10.1242/jeb.204.20.3403. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Harpavat S, Cepko CL. Thyroid hormone and retinal development: an emerging field. Thyroid. 2003;13:1013–1019. doi: 10.1089/105072503770867183. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M, Buchner E. The role of retinula cell types in visual behavior of Drosophila melanogaster. J Comp Physiol. 1977;117:127–162. [Google Scholar]
- Heisenberg M, Wolf R. Genetics of Microbehaviour. Heidelberg New York Tokyo: Springer; 1984. Vision in Drosophila. [Google Scholar]
- Hofer H, Carroll J, Neitz J, Neitz M, Williams DR. Organization of the human trichromatic cone mosaic. J Neurosci. 2005;25:9669–9679. doi: 10.1523/JNEUROSCI.2414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiau TH, Diaconu C, Myers CA, Lee J, Cepko CL, Corbo JC. The cis-regulatory logic of the mammalian photoreceptor transcriptional network. PLoS ONE. 2007;2:e643. doi: 10.1371/journal.pone.0000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Smith DP, Zuker CS, Paulsen R. Opsin of Calliphora peripheral photoreceptors R1-6. Homology with Drosophila Rh1 and posttranslational processing. J Biol Chem. 1990;265:17906–17910. [PubMed] [Google Scholar]
- Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev Camb Philos Soc. 1993;68:413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Primate color vision: a comparative perspective. Vis Neurosci. 2008;25:619–633. doi: 10.1017/S0952523808080760. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Evolution of colour vision in mammals. Philos Trans R Soc Lond B Biol Sci. 2009;364:2957–2967. doi: 10.1098/rstb.2009.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs GH, Williams GA, Fenwick JA. Influence of cone pigment coexpression on spectral sensitivity and color vision in the mouse. Vision Res. 2004;44:1615–1622. doi: 10.1016/j.visres.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Jia L, Oh EC, Ng L, Srinivas M, Brooks M, Swaroop A, Forrest D. Retinoid-related orphan nuclear receptor RORbeta is an early-acting factor in rod photoreceptor development. Proc Natl Acad Sci U S A. 2009;106:17534–17539. doi: 10.1073/pnas.0902425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Jr, Desplan C. Stochastic mechanisms of cell fate specification that yield random or robust outcomes. Annu Rev Cell Dev Biol. 2010;26:689–719. doi: 10.1146/annurev-cellbio-100109-104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschfeld K, Franceschini N. Evidence for a sensitising pigment in fly photoreceptors. Nature. 1977;269:386–390. doi: 10.1038/269386a0. [DOI] [PubMed] [Google Scholar]
- Kolb H. How the retina works. American Scientist. 2003;91:28–35. [Google Scholar]
- Kram YA, Mantey S, Corbo JC. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS ONE. 2010;5:e8992. doi: 10.1371/journal.pone.0008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart T, Meyer EP. Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech. 1999;47:368–379. doi: 10.1002/(SICI)1097-0029(19991215)47:6<368::AID-JEMT2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Lee J, Myers CA, Williams N, Abdelaziz M, Corbo JC. Quantitative fine-tuning of photoreceptor cis-regulatory elements through affinity modulation of transcription factor binding sites. Gene Ther. 2010;17:1390–1399. doi: 10.1038/gt.2010.77. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Furukawa T, Steffen MA, Church GM, Cepko CL. Microarray analysis of the transcriptional network controlled by the photoreceptor homeobox gene Crx. Curr Biol. 2000;10:301–310. doi: 10.1016/s0960-9822(00)00379-1. [DOI] [PubMed] [Google Scholar]
- Losick R, Desplan C. Stochasticity and cell fate. Science. 2008;320:65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann RS, Lelli KM, Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol. 2009;88:63–101. doi: 10.1016/S0070-2153(09)88003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Sperling HG. Chromatic organization of primate cones. Science. 1977;196:454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Celik A, Wernet MF, Vasiliauskas D, Johnston RJ, Cook TA, Pichaud F, Desplan C. Iroquois complex genes induce co-expression of rhodopsins in Drosophila. PLoS Biol. 2008;6:e97. doi: 10.1371/journal.pbio.0060097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni EO, Desplan C, Celik A. ‘One receptor’ rules in sensory neurons. Dev Neurosci. 2004;26:388–395. doi: 10.1159/000082281. [DOI] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- Menne D, Spatz HC. Colour vision in Drosophila melanogaster. Journal of Comparative Physiology. 1977;114:301–312. [Google Scholar]
- Merbs SL, Nathans J. Absorption spectra of human cone pigments. Nature. 1992;356:433–435. doi: 10.1038/356433a0. [DOI] [PubMed] [Google Scholar]
- Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Milam AH, Rose L, Cideciyan AV, Barakat MR, Tang WX, Gupta N, Aleman TS, Wright AF, Stone EM, Sheffield VC, Jacobson SG. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc Natl Acad Sci U S A. 2002;99:473–478. doi: 10.1073/pnas.022533099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AC, Seymour H, King C, Herman TG. Loss of seven-up from Drosophila R1/R6 photoreceptors reveals a stochastic fate choice that is normally biased by Notch. Development. 2008;135:707–715. doi: 10.1242/dev.016386. [DOI] [PubMed] [Google Scholar]
- Minke B, Kirschfeld K. The contribution of a sensitizing pigment to the photosensitivity spectra of fly rhodopsin and metarhodopsin. J Gen Physiol. 1979;73:517–540. doi: 10.1085/jgp.73.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Oke A, Lebel C, McDonald EC, Plummer Z, Cook TA, Zelhof AC. Pph13 and orthodenticle define a dual regulatory pathway for photoreceptor cell morphogenesis and function. Development. 2010;137:2895–2904. doi: 10.1242/dev.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mismer D, Michael WM, Laverty TR, Rubin GM. Analysis of the promoter of the Rh2 opsin gene in Drosophila melanogaster. Genetics. 1988;120:173–180. doi: 10.1093/genetics/120.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton KP, Swain PK, Chen S, Xu S, Zack DJ, Swaroop A. The leucine zipper of NRL interacts with the CRX homeodomain. A possible mechanism of transcriptional synergy in rhodopsin regulation. J Biol Chem. 2000;275:29794–29799. doi: 10.1074/jbc.M003658200. [DOI] [PubMed] [Google Scholar]
- Mlodzik M, Hiromi Y, Goodman CS, Rubin GM. The presumptive R7 cell of the developing Drosophila eye receives positional information independent of sevenless, boss and sina. Mech Dev. 1992;37:37–42. doi: 10.1016/0925-4773(92)90013-a. [DOI] [PubMed] [Google Scholar]
- Mollereau B, Dominguez M, Webel R, Colley NJ, Keung B, de Celis JF, Desplan C. Two-step process for photoreceptor formation in Drosophila. Nature. 2001;412:911–913. doi: 10.1038/35091076. [DOI] [PubMed] [Google Scholar]
- Morante J, Desplan C. The color-vision circuit in the medulla of Drosophila. Curr Biol. 2008;18:553–565. doi: 10.1016/j.cub.2008.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey M, Yee SK, Herman T, Nern A, Blanco E, Zipursky SL. Coordinate control of synaptic-layer specificity and rhodopsins in photoreceptor neurons. Nature. 2008;456:795–799. doi: 10.1038/nature07419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J. The genes for color vision. Sci Am. 1989;260:42–49. doi: 10.1038/scientificamerican0289-42. [DOI] [PubMed] [Google Scholar]
- Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312. doi: 10.1016/s0896-6273(00)80845-4. [DOI] [PubMed] [Google Scholar]
- Neitz M, Neitz J. The uncommon retina of the common house mouse. Trends Neurosci. 2001;24:248–250. doi: 10.1016/s0166-2236(00)01773-2. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Ng L, Ma M, Curran T, Forrest D. Developmental expression of thyroid hormone receptor beta2 protein in cone photoreceptors in the mouse. Neuroreport. 2009;20:627–631. doi: 10.1097/WNR.0b013e32832a2c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- O’Tousa JE, Leonard DS, Pak WL. Morphological defects in oraJK84 photoreceptors caused by mutation in R1-6 opsin gene of Drosophila. J Neurogenet. 1989;6:41–52. doi: 10.3109/01677068909107099. [DOI] [PubMed] [Google Scholar]
- Oh EC, Cheng H, Hao H, Jia L, Khan NW, Swaroop A. Rod differentiation factor NRL activates the expression of nuclear receptor NR2E3 to suppress the development of cone photoreceptors. Brain Res. 2008;1236:16–29. doi: 10.1016/j.brainres.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Khan N, Novelli E, Khanna H, Strettoi E, Swaroop A. Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc Natl Acad Sci U S A. 2007;104:1679–1684. doi: 10.1073/pnas.0605934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer OS, Williams DR, Bensinger DG. Photopigment transmittance imaging of the primate photoreceptor mosaic. J Neurosci. 1996;16:2251–2260. doi: 10.1523/JNEUROSCI.16-07-02251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatsenko D, Nazina A, Desplan C. A conserved regulatory element present in all Drosophila rhodopsin genes mediates Pax6 functions and participates in the fine-tuning of cell-specific expression. Mech Dev. 2001;101:143–153. doi: 10.1016/s0925-4773(00)00581-5. [DOI] [PubMed] [Google Scholar]
- Papatsenko D, Sheng G, Desplan C. A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development. 1997;124:1665–1673. doi: 10.1242/dev.124.9.1665. [DOI] [PubMed] [Google Scholar]
- Peng GH, Chen S. Crx activates opsin transcription by recruiting HAT-containing co-activators and promoting histone acetylation. Hum Mol Genet. 2007;16:2433–2452. doi: 10.1093/hmg/ddm200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichaud F, Briscoe A, Desplan C. Evolution of color vision. Curr Opin Neurobiol. 1999;9:622–627. doi: 10.1016/S0959-4388(99)00014-8. [DOI] [PubMed] [Google Scholar]
- Pichaud F, Treisman J, Desplan C. Reinventing a common strategy for patterning the eye. Cell. 2001;105:9–12. doi: 10.1016/s0092-8674(01)00292-6. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Yang-Zhou D, Kong SW, McDonald EC, Cook TA, Pignoni F. Analysis of the Otd-dependent transcriptome supports the evolutionary conservation of CRX/OTX/OTD functions in flies and vertebrates. Dev Biol. 2008;315:521–534. doi: 10.1016/j.ydbio.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister J, Pauls D, Schnell B, Ting CY, Lee CH, Sinakevitch I, Morante J, Strausfeld NJ, Ito K, Heisenberg M. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Vis Sci. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Rohlich P, van Veen T, Szel A. Two different visual pigments in one retinal cone cell. Neuron. 1994;13:1159–1166. doi: 10.1016/0896-6273(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Roorda A, Williams DR. The arrangement of the three cone classes in the living human eye. Nature. 1999;397:520–522. doi: 10.1038/17383. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Farrell DM, Zheng L, Phistry M, Bagg EE, Britt SG. The green-absorbing Drosophila Rh6 visual pigment contains a blue-shifting amino acid substitution that is conserved in vertebrates. J Biol Chem. 2009;284:5717–5722. doi: 10.1074/jbc.M807368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Tang K, Iida A, Inoue M, Kodama T, Tsai SY, Tsai MJ, Furuta Y, Watanabe S. The spatial patterning of mouse cone opsin expression is regulated by bone morphogenetic protein signaling through downstream effector COUP-TF nuclear receptors. J Neurosci. 2009;29:12401–12411. doi: 10.1523/JNEUROSCI.0951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Vogt A, Friedmann K, Paulsen R, Huber A. Rhodopsin patterning in central photoreceptor cells of the blowfly Calliphora vicina: cloning and characterization of Calliphora rhodopsins Rh3, Rh5 and Rh6. J Exp Biol. 2005;208:1247–1256. doi: 10.1242/jeb.01527. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2004;20:648–653. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Sheng G, Thouvenot E, Schmucker D, Wilson DS, Desplan C. Direct regulation of rhodopsin 1 by Pax-6/eyeless in Drosophila: evidence for a conserved function in photoreceptors. Genes Dev. 1997;11:1122–1131. doi: 10.1101/gad.11.9.1122. [DOI] [PubMed] [Google Scholar]
- Smallwood PM, Wang Y, Nathans J. Role of a locus control region in the mutually exclusive expression of human red and green cone pigment genes. Proc Natl Acad Sci U S A. 2002;99:1008–1011. doi: 10.1073/pnas.022629799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher SG, Pichaud F, Desplan C. Adult and larval photoreceptors use different mechanisms to specify the same Rhodopsin fates. Genes Dev. 2007;21:2182–2195. doi: 10.1101/gad.1565407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Ng L, Liu H, Jia L, Forrest D. Activation of the blue opsin gene in cone photoreceptor development by retinoid-related orphan receptor beta. Mol Endocrinol. 2006;20:1728–1741. doi: 10.1210/me.2005-0505. [DOI] [PubMed] [Google Scholar]
- Stavenga DG. Visual acuity of fly photoreceptors in natural conditions--dependence on UV sensitizing pigment and light-controlling pupil. J Exp Biol. 2004;207:1703–1713. doi: 10.1242/jeb.00949. [DOI] [PubMed] [Google Scholar]
- Stavenga DG, Arikawa K. One rhodopsin per photoreceptor: Iro-C genes break the rule. PLoS Biol. 2008;6:e115. doi: 10.1371/journal.pbio.0060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain PK, Chen S, Wang QL, Affatigato LM, Coats CL, Brady KD, Fishman GA, Jacobson SG, Swaroop A, Stone E, Sieving PA, Zack DJ. Mutations in the cone-rod homeobox gene are associated with the cone-rod dystrophy photoreceptor degeneration. Neuron. 1997;19:1329–1336. doi: 10.1016/s0896-6273(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Swain PK, Hicks D, Mears AJ, Apel IJ, Smith JE, John SK, Hendrickson A, Milam AH, Swaroop A. Multiple phosphorylated isoforms of NRL are expressed in rod photoreceptors. J Biol Chem. 2001;276:36824–36830. doi: 10.1074/jbc.M105855200. [DOI] [PubMed] [Google Scholar]
- Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010;11:563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szel A, Rohlich P, Caffe AR, Juliusson B, Aguirre G, Van Veen T. Unique topographic separation of two spectral classes of cones in the mouse retina. J Comp Neurol. 1992;325:327–342. doi: 10.1002/cne.903250302. [DOI] [PubMed] [Google Scholar]
- Szel A, Rohlich P, Mieziewska K, Aguirre G, van Veen T. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J Comp Neurol. 1993;331:564–577. doi: 10.1002/cne.903310411. [DOI] [PubMed] [Google Scholar]
- Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- Tahayato A, Sonneville R, Pichaud F, Wernet MF, Papatsenko D, Beaufils P, Cook T, Desplan C. Otd/Crx, a dual regulator for the specification of ommatidia subtypes in the Drosophila retina. Dev Cell. 2003;5:391–402. doi: 10.1016/s1534-5807(03)00239-9. [DOI] [PubMed] [Google Scholar]
- Tomlinson A. Patterning the peripheral retina of the fly: decoding a gradient. Dev Cell. 2003;5:799–809. doi: 10.1016/s1534-5807(03)00326-5. [DOI] [PubMed] [Google Scholar]
- Tsujimura T, Chinen A, Kawamura S. Identification of a locus control region for quadruplicated green-sensitive opsin genes in zebrafish. Proc Natl Acad Sci U S A. 2007;104:12813–12818. doi: 10.1073/pnas.0704061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandendries ER, Johnson D, Reinke R. orthodenticle is required for photoreceptor cell development in the Drosophila eye. Dev Biol. 1996;173:243–255. doi: 10.1006/dbio.1996.0020. [DOI] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J, Gearhart J, Nathans J. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- Wernet MF, Desplan C. Building a retinal mosaic: cell-fate decision in the fly eye. Trends Cell Biol. 2004;14:576–584. doi: 10.1016/j.tcb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267–279. doi: 10.1016/s0092-8674(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker SL, Knox BE. Conserved transcriptional activators of the Xenopus rhodopsin gene. J Biol Chem. 2004;279:49010–49018. doi: 10.1074/jbc.M406080200. [DOI] [PubMed] [Google Scholar]
- Wilson DS, Desplan C. Homeodomain proteins. Cooperating to be different. Curr Biol. 1995;5:32–34. doi: 10.1016/s0960-9822(95)00010-8. [DOI] [PubMed] [Google Scholar]
- Wright AF, Reddick AC, Schwartz SB, Ferguson JS, Aleman TS, Kellner U, Jurklies B, Schuster A, Zrenner E, Wissinger B, Lennon A, Shu X, Cideciyan AV, Stone EM, Jacobson SG, Swaroop A. Mutation analysis of NR2E3 and NRL genes in Enhanced S Cone Syndrome. Hum Mutat. 2004;24:439. doi: 10.1002/humu.9285. [DOI] [PubMed] [Google Scholar]
- Xiao M, Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. J Comp Neurol. 2000;425:545–559. [PubMed] [Google Scholar]
- Xie B, Charlton-Perkins M, McDonald E, Gebelein B, Cook T. Senseless functions as a molecular switch for color photoreceptor differentiation in Drosophila. Development. 2007;134:4243–4253. doi: 10.1242/dev.012781. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Desplan C, Heisenberg M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc Natl Acad Sci U S A. 2010;107:5634–5639. doi: 10.1073/pnas.0809398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci U S A. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985;40:851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]