Abstract
Introduction
The detection of circulating tumor cells (CTC) is prognostic in several cancer types. This trial examines the incidence and prognostic value of CTCs in urothelial cancer (UC).
Materials and Methods
44 subjects with UC were assessed for CTCs using CellSearch® Technology (Veridex, LLC, Raritan, NJ), using 7.5 ml of peripheral blood, sorted by magnetic separation (EpCaM positive, and immunofluorescent staining, (Cytokeratin 8, 18, or 19 positive, CD 45 negative, DAPI positive cells) to identify CTCs.
Results
Five of 30 (17%) subjects with clinically localized and 7 of 14 (50%) patients with metastatic UC had at least 1 detectable CTC, with a range of 1–177 CTCs. Six subjects had 5 or more CTCs. FISH analysis was performed in 20 samples from 18 unique subjects, using the UroVysion probe set. Copy number gains consistent with neoplasm were observed in cases with measurable CTCs, but not in any of the CTC-negative samples tested. With a median follow-up of 337 days, all 7 metastatic subjects with detectable CTCs had died, versus 3 of 7 (43%) metastatic subjects without detectable CTCs.
Conclusions
CTCs were observed in 50% of the metastatic UC patients tested. FISH analysis confirmed the aneusomic chromosomal content in the CTCs. The findings suggest measurable CTCs may be prognostic for shortened survival in metastatic UC patients, although the optimal threshold for a “positive” finding is unknown. CTCs were also detected in a subset of patients with clinically-localized disease, identifying a potential high-risk, pre-operative group for future study.
Keywords: Urinary Bladder Neoplasms Neoplastic Cells, Circulating Tumor Markers, Biological In Situ Hybridization, Fluorescence
Introduction
Accurate and robust disease staging is crucial for the appropriate care of cancer patients, guiding the use of both systemic and local therapy. Current staging approaches rely primarily on radiographic and direct pathologic assessments to determine the extent of neoplastic spread. The existence of circulating tumor cells (CTC) has been appreciated for a long period of time; however, the assessment of CTCs in routine clinical decision-making has only recently been realized, with the wide availability of commercial products for this detection. The CellSearch® system utilizes magnetic isolation of epithelial cells in peripheral blood based on the presence of the epithelial cell adhesion molecule (EpCam).1 On this basis, CTCs may now be accurately and reproducibly detected in the general practice of oncology.
The immunomagnetic assessment method of CTCs is currently used in several cancer types, including breast,2,3 colon4 and prostate cancer.5 The presence of significant levels of CTCs has been inversely associated with progression-free and overall survival.2,4,6 Additionally, isolated cells may be used to assess for the presence of tumor specific targets of therapy. For example, the detection of mutated epidermal growth factor receptor (EGFR) has been demonstrated in the CTCs of lung cancer patients, allowing for the appropriate use of an EGFR inhibitor on the basis of a minimally-invasive peripheral blood draw rather than a tissue biopsy.7 It has also been demonstrated that the serial assessment of CTCs gives ongoing information about an individual patient’s response to therapy.8 In prostate cancer patients, those with 5 or more CTCs in 7.5 ml of blood have a significantly worse overall survival than those with fewer than 5 CTCs. If the number of CTCs falls below 5 with therapy, then the initially poor prognosis patient assumes an survival similar to those patients in the good prognosis (< 5 CTC) group.6
While the existence of CTCs in bladder cancer patients has previously been reported via detection of epithelial markers in the peripheral circulation by RT-PCR,9,10 there are few reports using the newer method of immunomagnetic capture of CTCs in UC patients. Naoe et al first described the use of the CellSearch® System in a cohort of 26 urothelial cancer (UC) patients.11 None of the 12 non-metastatic, but 8 of the 14 metastatic subjects, had detectable CTCs ranging from 0–79 CTCs per sample. Researchers at the Memorial Sloan-Kettering Cancer Center more recently reported that 14 of 33 metastatic UC patients had detectable CTCs, with 10 of these having 5 or more CTCs in a 7.5 ml blood sample.12 A higher number of CTCs were noted in patients with 2 or more sites of metastases. One preliminary report from Germany presents an assessment of CTCs in 5 patients before radical cystectomy, with one of these patients observed with 2 CTCs in a 25ml sample of peripheral blood.13
The current study reports on the presence of CTCs in UC patients with either localized (muscle-invasive) or metastatic UC. In those with 5 or more CTCs, serial CTC measurements were made after treatment (cystectomy or chemotherapy). Additionally, an exploratory assessment of the survival of patients based on their CTC status was made.
Materials and Methods
50 subjects were enrolled from December of 2008 through January of 2010. All subjects provided written informed consent for their participation in this IRB-approved protocol, which was registered on ClinicalTrials.Gov (Identifier: NCT00829920). All subjects had either localized, muscle-invasive disease or had metastatic urothelial cancer and all were initiating therapy for their cancer, which could be either surgery or chemotherapy. Although 50 subjects consented, there were technical failures on the samples of 6 participants (inadequate blood volume, broken tube, and processing failure) so that baseline CTC levels were obtained on 44 bladder patients. Of these, 28 subjects were preoperative, 14 had known metastatic disease and 2 were post-operative and planning for adjuvant chemotherapy. The patient characteristics are given in TABLE 1.
Table 1.
Patient Characteristics
| Characteristics | No. |
|---|---|
| Total no. of patients | 50 |
| Evaluable patients (adequate samples) | 44 |
| Pre-operative | 28 |
| Post-operative | 2 |
| Metastatic | 14 |
| Age (years) | |
| Median | 61 |
| Range | 56–84 |
| Previous cytotoxic chemotherapy in metastatic patients | |
| No previous systemic | 5 |
| 1 previous systemic | 7 |
| 2 previous systemic | 1 |
| Pathologic T stage in those enrolled pre-operatively * | |
| T0 | 4 |
| T1 | 4 |
| T2 | 6 |
| T3 | 8 |
| T4 | 4 |
| Metastatic (determined at time of surgery | 2 |
| Sites of disease in the metastatic subjects** | |
| Bone | 6 |
| Lung | 4 |
| Pelvic mass | 3 |
| Liver | 2 |
T stage is from the time of surgery, except in 2 cases of T2 disease based on transurethral resection of the bladder tumor, in which one patient refused the recommended cystectomy and in another who pursued definitive radiation therapy.
Including sites with involvement by at least 2 patients.
Circulating cell isolation
CTCs were isolated and analyzed using a standard protocol for the Veridex CellSearch® System with this work performed in the Colorado Molecular Correlates Laboratory (CMOCO). Blood (7.5 ml) was collected from patients using CellSave Preservative tubes, specimens were analyzed within 96 hours of collection. CTC were separated using a monoclonal antibody to EpCAM conjugated to magnetic nanoparticles. After separation the remaining cells were stained for Cytokeratin (CK) 8, 18 and/or 19 (phycoerythrin; PE), CD45 (allophycocyanin; APC), and DAPI for nuclei. CTCs are known to stain positive for CK and DAPI. In contrast, leukocytes stain positive for CD45 and DAPI. Dual stained leukocytes (positive for both CK and CD45) were excluded from CTC counts. Enumeration was performed by a pathologist. The cell staining and separation was performed on the CellTracks® AutoPrep® instrument and image capture and analysis was performed on the CellTracks® Analyzer II imager.
Fluorescent in situ hybridization (FISH)
Cell suspensions were received in the Veridex cartridges after processing using the CellSearch® system. The cartridges were initially centrifuged upright for 20 minutes at 1000 rpm and the bottom of 100 μl of suspension was transferred to a microcentrifuge vial and mixed with 100μl of fresh fixative (3:1 methanol/glacial acetic acid). After centrifugation for 10 minutes at 3,400 rpm, the supernatant was completely discarded inverting the vial and the pellet allowed to air dry. Then, 15μl of fresh fixative was added to the vial, the pellet thoroughly resuspended, applied to an etched slide and allowed to dry at room temperature. For the FISH assays, specimens were put through ethanol dehydration series then digested in 0.008% pepsin/0.01M HCl at 37°C for 10–14 minutes followed by a 5 minute 2xPBS wash and 10 minute incubation in 1% formaldehyde solution at room temperature. After dehydration the probe mixture was applied, the area sealed and DNA denaturation was performed at 85°C for 7–10 minutes. Hybridization was allowed to occur at 37°C for 20 hours and post-hybridization washes were performed with 2xSSC/0.3% NP40 at 74°C for 2 min and room temperature 2xSSC for 2 min. Chromatin was counterstained with 7μl of DAPI/antifade. The probe used for this study was the Vysis® UroVysion® Bladder Cancer Recurrence™ (Abbott Molecular). This probe consists of a mixture of DNA sequences homologous to specific regions on chromosomes 3, 7, 9, and 17: Chromosome Enumeration Probe (CEP) 3 in SpectrumRed, CEP7 in SpectrumGreen, CEP 17 in SpectrumAqua and Locus Specific Identifier LSI p16 in SpectrumGold.
Statistics
We calculated descriptive statistics for age, metastatic status, and detectable CTCs. The Kaplan-Meier survival14 was estimated and used to describe the pattern of survival for patients with any CTC vs. no CTC separately for the 14 patients with metastatic disease and the 30 patients with localized disease. Median survival times with their 95% confidence intervals were calculated for each group. There was no formal hypothesis testing done, as these results are preliminary and the event rate low. Analyses were done in SAS 9.2 (SAS Institute, Inc, Cary, NC).
Results
Of the 44 evaluable subjects, detectable CTCs were observed in 12 (27%) at the time of the baseline CTC assessment. Among the 14 with metastatic disease, 7 (50%) subjects had detectable CTCs ranging from 1–177 CTC in 7.5 ml of blood. Five of the 14 (36%) metastatic patients had 5 or more CTC in the 7.5 ml sample. Of the 28 pre-operative subjects, all of whom had clinically localized disease, 5 (18%) had detectable CTC, ranging from 1–6 CTC in 7.5 ml of blood with only one subject having 5 or more CTCs in the sample. Neither of the post-operative subjects had detectable CTCs (Table 2). Among the 5 pre-operative patients found to have circulating tumor cells, the pathologic stages at the time of surgery were T3a, T3b, T4a, T4a and metastatic disease. Three of these 5 subjects had lymph node positive disease, one was negative and one’s lymph node status was not assessed. At the time of this analysis, 4 of these 5 patients was alive. Among the metastatic group, bone disease was more common in those with detectable CTCs (5 out of 7) compared to those without detectable CTCs (1 out of 7).
Table 2.
Summary of CTC results by group
| Characteristics | Metastatic disease | Localized (pre-operative) | Localized disease (post-operative) |
|---|---|---|---|
| Number in group | 14 | 28 | 2 |
| Number with any CTCs | 7 (50%) | 5 (18%) | 0 |
| Range of CTC’s | 1–177 | 1–6 | 0 |
| Number with CTCs > 5 | 5 | 1 | 0 |
CTC is circulating tumor cells, measured in 7.5 mls of blood
The neoplastic nature of the cells identified as CTCs by immunomagnetic separation was assessed by FISH, using the 4 DNA target UroVysion probe set. Twenty samples from 18 unique subjects were analyzed by FISH, with 9 of these subjects having detectable CTCs. Cells for scoring were selected by large nuclear size and irregular nuclear boundaries and chromatin texture and included cells that were not included in the enumeration results (i.e. apoptotic cells, leukocytes, granulocytes, macrophages, etc.), since the entire cassette was provided for cytogenetic evaluation. On average, 50 cells were scored per specimen (range = 9 to 104). In those specimens without detectable CTCs, none of the scored cells were found to have abnormal copy number for the tested DNA targets, supporting the accurate identification of non-cancer cells using the CellSearch® System interpretation criteria. In contrast, of the 9 subjects with detectable CTCs analyzed by FISH, 5 displayed copy number gain for multiple targets, indicating occurrence of chromosomal aneusomy consistent with malignant transformation (FIGURE 1). Therefore, the molecular cytogenetic analysis confirmed the presence of neoplastic cells in circulation, with this finding limited to those subjects with CTCs detected via this immunomagnetic method.
Figure 1.
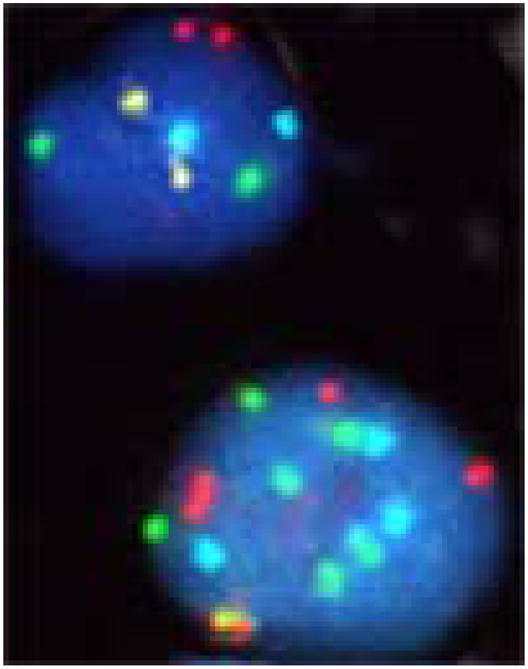
FISH assessment of cells isolated by immunomagnetic capture. A comparison between a normal cell (upper) and a cancer cell (lower) is shown, with a significant increase in copy number in the cancer cell from a patient in this series positive for CTCs. Probes: Chromosome Enumeration Probe (CEP) 3 in SpectrumRed, CEP7 in SpectrumGreen, CEP 17 in SpectrumAqua and Locus Specific Identifier in SpectrumGold.
The survival of subjects was assessed with regard to CTC status. All 7 of the metastatic patients with detectable CTCs had died at the time of this data assessment. In contrast, 3 of 7 (43%) metastatic patients without CTCs had died. Using the Kaplan-Meier estimator for censored data, the median survival time for these subjects was 156 versus 337 days for the detectable and undetectable groups, respectively, as shown in Figure 2. The survival of clinically localized subjects was also assessed with respect to CTC status, but only a small number of events have been recorded in this sub-population to date.
Figure 2.
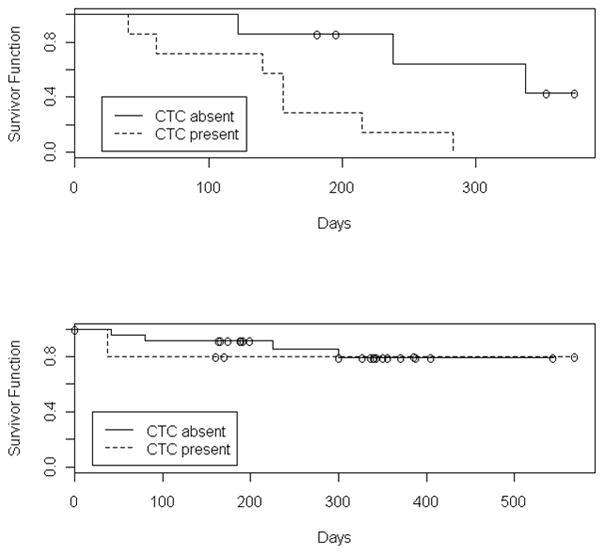
Kaplan-Meier estimate of the survival function in patients with metastatic disease (Upper panel) and clinically localized disease (Lower panel), both with and without detectable CTCs. ‘O’ indicates a censored observations.
Five metastatic patients had 5 or more CTCs and the CTC level was followed every 2 months during chemotherapy until they fell to less than 5. Of note, the 2 patients with the highest CTC levels (99 and 177) both had rapidly progressive disease and died before their first reassessment point. Two other patients with baseline CTC levels of 27 and 34 did not have any detectable CTCs at the 2 month reassessment point. Of note, one had equivocal soft tissue changes with significant progression of bone disease at this time point and the other had reduction in the size of the soft tissue (lymph node) disease accompanied by clear progression of bone metastases at the 2 month point in their treatment with chemotherapy. The loss of CTCs on serial measurement did not correlate with the worsening bone disease in either of these patients. The final patient’s CTC counts were followed at 2 month intervals and found to be 11, 9, and 73 serially. At the initial reassessment point, with the CTC level falling to 9, the subject had clinically stable disease; in contrast, at the last time point with CTC count rising to 73, the subject had widespread and clear progression of disease.
Discussion
This trial demonstrates that CTCs are frequently detected in UC patients with metastatic disease using a commercially available assay. The survival analysis for the metastatic patients suggests a prognostic role of CTCs in this setting and is hypothesis generating for the design of future bladder cancer studies. For the first time, the malignant nature of the cells isolated by an immunomagnetic method was validated by FISH analysis in UC patients, demonstrating chromosomal aneusomy potentially associated with copy number gain of relevant genes, consistent with neoplastic transformation.
The role of CTCs as a pre-operative marker has been assessed in a limited manner in bladder cancer. One report from Germany describes the detection of CTCs in 1 of 5 pre-operative bladder cancer patients tested with the CellSearch® Technology using 25 ml of blood.13 More recently, 2 larger studies investigating CTCs in bladder cancer have been published. Guzzo et al assessed the peripheral blood of 43 with clinically-localized disease prior to a planned cystectomy.15 CTCs were identified in 9 (21%) of the subjects, ranging from 1–9 with a median of 1. In a second study, Rink et al describe the assessment of CTCs in 50 clinically-localized, preoperative bladder cancer subjects, with detectable CTCs in 15 (30%). 16 In this quickly changing field, our study demonstrates CTCs in 5 of 28 (18%) preoperative UC patients. Taken together, these findings suggest that CTCs may be observed frequently enough to merit further study of CTCs as a risk stratifying factor in the pre-operative setting for UC patients planning for radical cystectomy and future neoadjuvant treatment studies should incorporate CTC into their design.
Our study reports the first successful use of FISH to analyze CTCs in UC, demonstrating the malignant nature of the circulating cells found in our subjects. Molecularly, urothelial cancers overexpress many biologic targets of therapy, including vascular endothelial growth factor receptor,17 insulin-like growth factor,18 the epidermal growth factor receptor 1,19 and 2 (HER2/neu).19 Beyond confirming cancer, FISH assessment in CTCs may be able to investigate the genomic status of specific targets of therapy via the minimally-invasive interrogation of peripheral blood to aid in the future selection of targeted therapy. Considering the significant “targetable” markers in bladder cancer and the need to assess new biologic therapies in UC, assessment of CTCs in UC is a promising method to increase the speed of therapeutic development in this area. One of the challenges of FISH assessment in CTCs is the practical processing of cells in the capture cassette within the CellSerach system; our successful method is described here for future studies in UC.
The optimal threshold of CTCs for “positive finding” in UC is not known. In other metastatic cancer types, the threshold of 3 (colon cancer) or 5 (prostate, breast cancer) CTCs per 7.5 ml of blood has been utilized to define a positive test. The range of CTCs in metastatic patients studied in the present trial had a maximum of 177 per sample. The survival analysis divided patients into groups of no detectable CTCs or any CTCs, so that even 1 CTC per 7.5 ml of blood was considered positive. Larger cohorts of UC subjects are needed to better define a meaningful level of CTC in terms of clinical outcome, but our data suggest even the identification of a single CTC may be meaningful.
The very recent study by Rink et al reported that the presence of CTCs predicted worse overall and progression-free survival in the preoperative patients. The present study notes the survival time of metastatic UC patients on the basis of the presence or absence of CTCs, as shown in figure 2. While our study was not designed or powered to formally assess CTCs as a prognostic factor, the data presented here are hypothesis generating and future studies metastatic bladder cancer studies should be designed to incorporate CTC enumeration as both a prognostic and predictive marker.
Conclusions
CTCs are frequently detected in metastatic UC and are also found in a small number of patients with clinically localized UC. An analysis of the survival of metastatic UC patients suggests that CTCs may have prognostic significance in advanced disease. The detection of CTCs in pre-operative patients confirms the feasibility of this measurement, and future neoadjuvant trials in patients with localized bladder cancer may be designed and modeled with the data presented here.
Acknowledgments
The authors wish to acknowledge the invaluable research support provided by University of Colorado Cytogenetic core and Jennifer Gurshtein, BS, and also by the University of Colorado Cancer Center’s Clinical Investigations Core and Mary Anduha. This work was supported by a Paul Calabresi K12 clinical scholar grant awarded to the University of Colorado Denver (K12CA086913) (TWF). Veridex LLC provided the study supplies for the circulating cell assessments.
Footnotes
ClinicalTrials.Gov identifier: NCT00829920
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 3.Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–30. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, Gu B, Anand A, Smith K, Maslak P, Doyle GV, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–9. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 7.Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ, Bell DW, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 9.Osman I, Kang M, Lee A, Deng FM, Polsky D, Mikhail M, Chang C, David DA, Mitra N, Wu XR, et al. Detection of circulating cancer cells expressing uroplakins and epidermal growth factor receptor in bladder cancer patients. Int J Cancer. 2004;111:934–9. doi: 10.1002/ijc.20366. [DOI] [PubMed] [Google Scholar]
- 10.Gudemann CJ, Weitz J, Kienle P, Lacroix J, Wiesel MJ, Soder M, Benner A, Staehler G, Doeberitz MV. Detection of hematogenous micrometastasis in patients with transitional cell carcinoma. J Urol. 2000;164:532–6. [PubMed] [Google Scholar]
- 11.Naoe M, Ogawa Y, Morita J, Omori K, Takeshita K, Shichijyo T, Okumura T, Igarashi A, Yanaihara A, Iwamoto S, et al. Detection of circulating urothelial cancer cells in the blood using the CellSearch System. Cancer. 2007;109:1439–45. doi: 10.1002/cncr.22543. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher DJ, Milowsky MI, Ishill N, Trout A, Boyle MG, Riches J, Fleisher M, Bajorin DF. Detection of circulating tumor cells in patients with urothelial cancer. Ann Oncol. 2009;20:305–8. doi: 10.1093/annonc/mdn627. [DOI] [PubMed] [Google Scholar]
- 13.Karl A, Tritschler S, Hofmann S, Stief CG, Schindlbeck C. Perioperative search for circulating tumor cells in patients undergoing radical cystectomy for bladder cancer. Eur J Med Res. 2009;14:487–90. doi: 10.1186/2047-783X-14-11-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collett D. Modelling Survival Data in Medical Research. London: Chapman & Hall; 1996. p. 408. [Google Scholar]
- 15.Guzzo TJ, McNeil BK, Bivalacqua TJ, Elliott DJ, Sokoll LJ, Schoenberg MP. The presence of circulating tumor cells does not predict extravesical disease in bladder cancer patients prior to radical cystectomy. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Rink M, Chun FK, Minner S, Friedrich M, Mauermann O, Heinzer H, Huland H, Fisch M, Pantel K, Riethdorf S. Detection of circulating tumour cells in peripheral blood of patients with advanced non-metastatic bladder cancer. BJU Int. doi: 10.1111/j.1464-410X.2010.09562.x. [DOI] [PubMed] [Google Scholar]
- 17.Xia G, Kumar SR, Hawes D, Cai J, Hassanieh L, Groshen S, Zhu S, Masood R, Quinn DI, Broek D, et al. Expression and significance of vascular endothelial growth factor receptor 2 in bladder cancer. J Urol. 2006;175:1245–52. doi: 10.1016/S0022-5347(05)00736-6. [DOI] [PubMed] [Google Scholar]
- 18.Xie QX, Lin XC, Zhang MF, Han CX, Guo YH. Expression of IGF-I and IGF-IR in bladder cancer. Ai Zheng. 2004;23:707–9. [PubMed] [Google Scholar]
- 19.Rotterud R, Nesland JM, Berner A, Fossa SD. Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int. 2005;95:1344–50. doi: 10.1111/j.1464-410X.2005.05497.x. [DOI] [PubMed] [Google Scholar]


