Abstract
A common method for modeling pathological and behavioral aspects of Alzheimer's disease (AD) is the transgenic mouse. While transgenic strains are often well characterized pathologically, behavioral studies of cognitive deficits often employ a limited set of aversively motivated, spatial learning and memory tests, under brief testing periods. Here we illustrate an alternative operant behavioral methodology to provide a comprehensive characterization under repetitive testing conditions, and with appetitive motivation. In this study, we employed the commonly used Tg2576 murine model of Alzheimer's disease amyloid pathology, since it has been the subject of many previous behavioral studies. In these mice, we compared the learning of simple and complex, as well as spatial and non-spatial rules. The mice were assessed on a progressively more complex and interlocking battery of operant tasks, ranging from simple rule learning to delayed recall, as well as tests of motor and sensory ability. In general, as compared to wild type control mice, within-group variability was high in the Tg2576 mice, and deficits were most apparent in more complex discrimination tasks. Furthermore, a consistent decrease in the rate at which Tg2576 mice completed testing trials was observed, pointing to a potential motivation difference or speed-accuracy tradeoffs as a defining characteristic of this strain under these test conditions. Using sensitive adjusting retention interval procedures, it was also possible to isolate a difference in retention interval and separate it from non-mnemonic processes. Overall, these experiments demonstrate the utility of this novel operant approach for characterizing the cognitive deficits of transgenic murine models of dementia.
Keywords: Learning, Memory, Operant, Tg2576, Transgenic
Introduction
Alzheimer's disease (AD) is a progressive, debilitating disease pathologically characterized by amyloid plaques, neurofibrillary tangles and neuronal loss. The disease clinically manifests with memory loss, disorientation, and other behavioral changes (Alzheimer, 1907; Davies & Maloney, 1976; Murrell et al., 1991; Price, et al., 1992; Selkoe, 2001, 2004). AD has been studied in spontaneous mammalian animal models, including primates and rodents by using lesions of various specificities by using pharmacological challenges such as anticholinergic drugs, and by using aged animals (Bartus, 2000; Sherman, et al., 1981). In the last two decades, transgenic murine lines have become one of the dominant methods for studying the etiology, neuropathology, and treatment strategies for AD (Iqbal et al., 2005; Götz et al., 2007). Most transgenic mouse lines were created from introducing gene mutations related to familial early-onset forms of AD, alone or in combination, into transgenes encoding human amyloid ß-protein precursor (AßPP) and characterized by the appearance over the lifespan of various histopathological and behavioral abnormalities, particularly learning and memory alterations.
One such well-studied transgenic model is the Tg2576 mouse line, introduced in 1996 by Hsiao et al., which contains the Swedish double mutation (K670N/M671L) under the control of a Hamster PrP promoter. As early as six months, but virtually universally by 12–14 months of age, these mice show substantial accumulations of human Aβ species, especially Aβ42, congophilic plaques, and inflammation in cortical and limbic structures. While this is one of the most thoroughly studied human AßPP transgenic mouse lines, one important limitation is in the behavioral characterization of this line. There is reliance on tasks such as passive/active avoidance and object recognition, or on spatially intensive learning and memory tests such as spontaneous alternation, the Morris water maze, and the Barnes maze (early studies reviewed in Ashe, 2001; Arendash & King, 2002; Barnes & Good, 2005; Corcoran, Lu, Turner & Maren, 2002; Deacon, et al., 2008; Good & Hale, 2007; Hale & Good, 2005; King & Arendash, 2002a, b; Middei, et al. 2006; Ognibene, et al., 2005; Westerman, et al., 2002; Zhuo, et al., 2007). These tasks are often aversively motivated (e.g. Morris water maze and passive/active avoidance most notably) or take place in exposed settings, such that they presumably evaluate subjects exclusively in a high state of vigilance and arousal. And while some incorporation of operant tasks exist in this literature (e.g. Adriani et al., 2006; Lagadec et al., 2010), relatively little work has evaluated the Tg2576 or other related lines along a progressive spectrum of tasks, where simple components are initially assessed and then more complex and demanding tests drawing on those simpler skills are introduced. Operant techniques, using appetitive reinforcers, are especially well suited to these progressive, moderate arousal analyses of cognitive functions, and to potentially sensitive operant tests likely to reveal deficits potentially missed in other less complex learning and memory tests.
In the present study, we sought to demonstrate the feasibility of the operant approach for making sophisticated dissociations of advanced cognitive skills in mice well trained in the underlying procedures. Our experimental model was based on an interlocking, progressive operant regimen that has been employed successfully in rats pathology (Kritzer et al, 2007), and altered it to be conducted using Tg2576 mice, during their early development of underlying Aβ–related pathology (Westerman et al., 2002). Much work has been done with several of the tasks employed in this study using rats as subjects, including signal detection, Delayed Nonmatch-to-Position, and progressive ratio. These tasks have been used to evaluate ascending cholinergic system deficiencies (e.g. Jensen et al., 1987; Sarter et al., 2005), hippocampal damage (Dunnett, et al., 1988, Heyser, et al., 1993, Winters & Dunnett, 2004), and mesolimbic dopamine alterations (Hodos & Kalman, 1963; Zhang et al., 2003), respectively. This paper represents a first step in investigating the utility of adding operant regimens to systematically evaluate transgenic models of Alzheimer's disease symptomology.
Methods
Subjects
Tg2576 (B6/129) mice were obtained from Jackson Laboratories. The study employed eight Tg2576 animals and eight wild type controls of the same age and genetic background, both groups were mixed-sex (four and four of each). All mice were singly housed in standard 27.5cm long × 16.5cm wide mouse cages throughout the duration of the experiments in a vivarium with controlled temperature and humidity on a 12-hour light/dark schedule. Operant training and testing procedures began at six months of age, and concluded at approximately twelve months. The subjects were then euthanized and the brain tissue harvested for histological and neurochemical analysis. The Stony Brook University Institutional Animal Care and Use Committee approved all procedures.
Apparatus and Setting Procedures
The operant chamber (MED Associates) was located inside a sound-attenuating chamber, with an exhaust fan providing white noise. The chamber was 19×22cm at the floor with two front nose-poke ports that are 4.5cm directly right and left of the water dipper, and one nose-poke port in the rear, directly opposite of the dipper. Nose poke ports were 1.5 cm in diameter and contained a small light, and a single photo-beam that registered nose-poke responses. The chamber had a metal rung floor, and was lit by a small house light.
During the operant testing, all the mice had no access to water for 23 hours, followed by a 30 minute test session in which water was available as reinforcers, and then 30 minutes of free water consumption in their home cage following the test session.
Behavioral Testing
All mice received an identical program of behavioral testing, described as follows and summarized in Table 1. Trial initiation and reinforcement procedures were uniform across all behavior tests. Trials always began with a nose-poke into an illuminated front nose-poke port. Following a correct response, both nose-port well lights were extinguished, and the water reinforcer ladle or “dipper” was made available for 10 seconds. When the dipper was removed, the nose-port lights were illuminated, beginning the next trial. Following an incorrect response, both nose-port lights were extinguished for the ten-second intertrial interval, and then re-illuminated to indicate the start of the next trial. All sessions were 30 minutes in duration.
Table 1.
List of progression of operant behavioral paradigms employed, number of training sessions, and primary psychological construct assessed by each task. The numerator in Number Completing Training ratio indicates how many mice were unable to complete the full number of training sessions, and the denominator the number who began training in that task.
| Task | Main Construct(s) Measured | Sessions | Number Completing Training |
|---|---|---|---|
| Fixed Ratio | Simple operant contingency | ~3 | WT=8/8 Tg2576=7/8 |
| Alternation | Simple response rule learning | ~8 | WT=8/8 Tg2576=6/7 |
| Light-Dark Discrimination | Simple discrimination | 10 | WT=8/8 Tg2576=6/6 |
| Chained FR1-FR1-FR1 | Completion of a response sequence; behavioral flexibility | 5 | WT=8/8 Tg2576=6/6 |
| Non-Match to Position | Complex, conditional discrimination; behavioral flexibility | 40 | WT=8/8 Tg2576=5/6 |
| Delayed NMTP | Short-term (working memory) | 28 | WT=8/8 Tg2576=3/6 |
| Reaction Time | Motor initiation | 15 | WT=8/8 Tg2576=2/3 |
| Signal Detection | Sustained attention | 10 | WT=4/4* Tg2576=3/3# |
| Progressive Ratio | Reinforcer strength | 10 | WT=4/4 Tg2576=3/3 |
WT = wild type mice
Randomly discontinued to balance N's
one mouse returned after illness
In order to normalize performance on each task, no animal progressed to the next step/task until they had successfully acquired the previous one. In the early tasks a set number of responses per 30 min trial was required, in the later tasks, the requirement was a percentage of correct responses, out of all trials that were attempted. As a result, there was a short break between tasks for the better performing animals, while the animals who had not acquired the task continued until reaching criteria. The operant programs progressed in a set order, the same for all mice, as follows:
Magazine (dipper) training
In this procedure, both front nose-poke ports were illuminated at the start of all trials. The water dipper was activated non-contingently every thirty seconds and remained raised for ten seconds in the testing chamber. A nosepoke response in either port when lights were illuminated produced an additional reinforcer. The mice were exposed to this procedure for two days. This task helped shape the behavior of the mice to nose-poke, in preparation for later tasks.
Fixed Ratio
In this task, both front nose-poke ports were lit and mice were required to nose-poke in order to obtain a reward. The mice could poke in either port, at any time when the dipper was not already activated. This program continued until the mice reached a criterion of a total of fifteen or more responses in one session combined to either port, or at least ten more responses on one side than the other.
Alternation
During this task, only one of the front nose poke wells was lit at a time, the lit lamp alternating from right to left on subsequent correct response trials and resetting to the same side following error trials. This program forced mice to use both left and right response equally to maximize reinforcement rate. This alternation program continued until animals completed a criterion of thirty or more responses per session.
Light Dark Discrimination
During this task, only one front nose-poke well light was lit at a time, and alternated at random, separated by a 10 sec intertrial interval. A correct response was made in the illuminated port.
Chained FR1-FR1-FR1 Reinforcement Schedule
This task required a specific sequence of nose poke responses. First, one of the front nose-poke wells was lit randomly. Following a correct response, the rear port was illuminated and a response required there. Following this response, the front port not first cued was illuminated. Errors were defined by and incorrect response in the final FR1 component and there were no scheduled consequences to a response in a non-cued port during either the first or second FR1 component. Reinforcement followed only after the successful completion of all three FR1 segments. This task required both sustained attention and memory of the previously learned rule to respond only in wells that were illuminated.
Non-Match to Position (NMTP)
This program built on the same response sequence as the chained schedule task previously, except that the final FR1 response was not cued. Rather, after the rear port nose poke, both front wells were illuminated, and the mouse was required to recall which well they poked previously in the first components, and poke the opposite well in order to receive a reinforcer. This task requires generalization of the light dark discrimination to two novel positions as well as the orderly completion of a sequence of spatially discrete responses. Like the chained schedule, this task required the orderly completion of a sequence of spatially discrete responses, but placed the additional demand of remembering the initial front response (the “sample”). An animal's performance on this task required sustained attention and short-term memory.
Adjusting Delayed Non-match to Position
This procedure was identical to the NMTP procedure, except there was an addition of an adjusting retention interval between the first front nose-poke and the rear nose poke. For session one, a three second delay separated the first front a rear responses. Thereafter, the retention interval that each animal was exposed to on subsequent trials was determined on a daily basis. If choice accuracy was equal to or greater than 75% in a single session, then the retention interval delay was increased two seconds on the subsequent trial. If choice accuracy for that animal was less than 75%, then the retention interval was shortened by two seconds on the subsequent trial, to a minimum of one second. This adjusting procedure was designed to isolate the retention capacity performance component by holding the overall rate of reinforcement relatively constant and avoiding floor and ceiling effects. This task allowed for isolation of the ability to perform accurately over a retention interval.
Reaction Time
This task was identical to the light-dark discrimination task tested earlier except that a response was required within 2.5 seconds of stimulus onset. This task measured response initiation in the mice.
Signal Detection
This task was identical to the light dark discrimination task except that the ITI was unpredictable and variable between 3–17 sec, and the duration of the stimulus was varied in the same adjusting manner as with the DNMTP task along a range of 3.0, 1.0, 0.5, 0.3, or 0.1sec. If mice received more than 25% error on a given day, the stimulus duration was lengthened the following day (from 1 to 3 sec). This task measured sustained attention and visual acuity in the mice.
Progressive ratio
At the start of this task, only the left nose-poke well was lit. On the first trial, mice were only required to make one response for a reward, and the number of responses per reinforcer increased by one on each trial. This task assessed reinforcer strength.
At the completion of operant training, brief testing in several non-operant tasks was also conducted (data not shown).
Histological Characterization
Perfusion and tissue treatment
At the completion of behavioral testing, all mice were deeply sedated with sodium pentobarbital before being transcardially perfused with 1°C saline. Brains were immediately removed and bisected in the mid-sagittal plane. One hemisphere was snap-frozen and used for the protein analyses. The other hemisphere was placed in 70% ethanol, followed by xylene treatment and embedding in paraffin for immunohistochemical and histological analyses.
ELISA for Aß peptides
The levels of soluble and insoluble Aß40 and Aß42 peptides in mouse brain lystates were determined using a highly specific sandwich ELISA assays as previously described (DeMattos et al., 2002).
Immunohistochemical analysis for Aß
Brain tissue sections from hippocampus and cortex were cut in the sagittal plane at 10μm thickness using a microtome, deparaffinated and rehydrated. Antigen retrieval was performed by treatment with proteinase K (0.2 mg/ml) for 10 min at 22°C for Aß and for collagen staining, and by 10 mM sodium citrate solution (pH 9.0) for 30 min at 90°C in a water-bath for activated microglia staining. For detection of Aß the mouse monoclonal antibody 66.1, which recognizes residues 1–5 of human Aß (1:200) was used, primary antibody were detected with horseradish peroxidase-conjugated secondary antibody and visualized with a stable diaminobenzidine solution (Invitrogen, CA) as substrate. Sections were counterstained with hematoxylin. Thioflavin-S staining for fibrillar amyloid was also conducted (Dickson & Vickers, 2001).
Data Analysis
Unless otherwise noted, the data were analyzed using the StatView program by SAS. Where parametric statistical analyses were performed (as described in Results), repeated measures ANOVAs were performed, with groups as a between-subjects variable and session as within. As indicated in the text, some data were analyzed at the level of individual subjects from the Tg2576 groups compared against 95% confidence intervals drawn around the WT control group. This technique offers the advantages of producing comparable inference to ANOVA for data from single subjects, while avoiding type II errors resulting from violation of assumptions of the ANOVA (Cohen, 1994).
Results
Behavioral Results
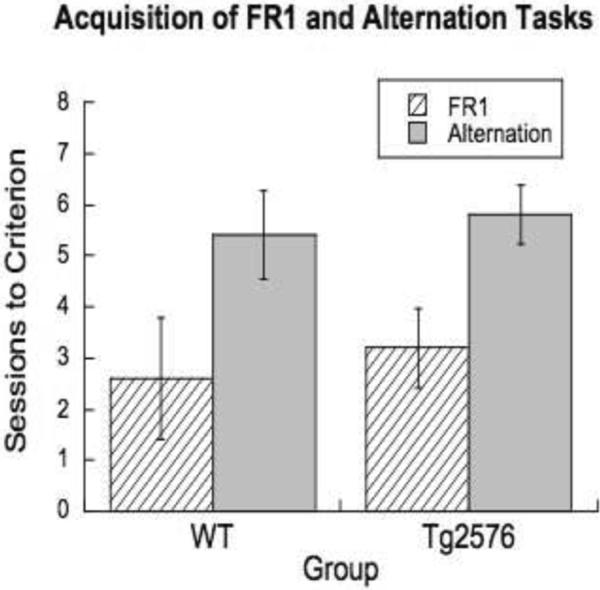
Before operant testing could begin, mice first had to learn the basic operation of the operant box: that is, they needed to learn that a nose poke into either of the wells at the front of the chamber resulted in the delivery of a water reward. Two of the eight Tg2576 animals did not reliably respond despite considerable training effort through the first two tasks, and could not be continued in the study. One never acquired a reliable FR response and the second never reached the required criterion of ten responses per session in alternation. Table 1 summarizes the attrition of subjects over the course of the study. The remaining members of the Tg2576 group at 6–7 months of age were able to acquire the fixed ratio (F1,12=0.23, n.s.) and alternation tasks (F1,12=0.16, n.s.). Including the outlier subject who did not reach alternation criterion into the analysis still did not produce a significant difference in FR between the groups (F1,13=1.6, p<.21). This indicates that, with the two exceptions, these animals had a spared ability to learn the contingency of the nose poke for water reward as indicated by FR success, and the ability to learn a simple win-shift (i.e. response alternation) rule, as indicated by successful alternation (see Figure 1).
Figure 1.
Sessions to criterion of >/= 10 responses on the FR1 or response alternation programs. No difference is evident on rate of acquisition for either task. Shown are mean±s.e.m.
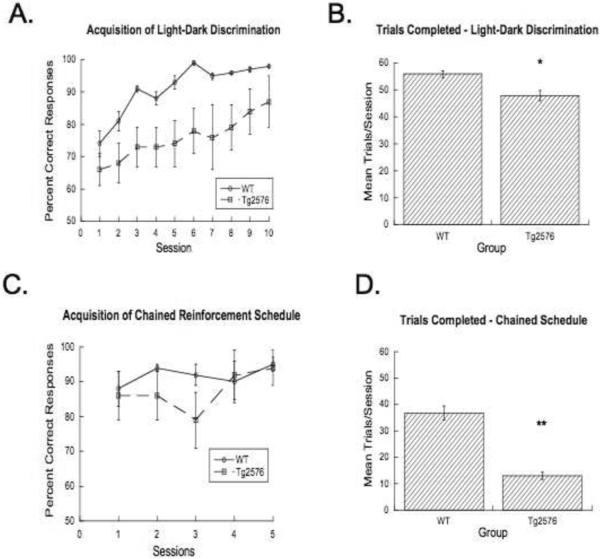
In contrast to these findings, striking between-group dissociations are apparent in the non-spatial light-dark discrimination learning at this early age (Figures 2a & 2b), clearly indicating that Tg2576 animals have deficits in learning a spatially irrelevant simple discrimination task. The Tg2576 mice acquired the light dark discrimination task more slowly than WT controls (main effect: F1,12=5.6, p<.03), though most were able to ultimately learn to a high level of performance. Although Tg2576 mice completed a high number of trials they completed significantly fewer than WT controls (main effect: F1,12=2.6, p<.05). An equality of variances test also confirmed the greater variability of choice accuracy in the Tg2576 group (p<.0001).
Figure 2.
Acquisition curve (A) and the average number trials completed per session (B) for the light dark discrimination (A, B) and chained FR1-FR1-FR1 reinforcement schedule (C, D). Shown are mean±s.e.m.
In the chained schedule task (Figures 2c & 2d), a marked drop-off in the rate of trials completed per session (main effect: F1,12=13.7, p<.003) is evident, though accuracy in the remaining animals was not substantially altered (e.g. p<.15 on session 3). This indicates that the sequence can be completed successfully by simply following the light cues (main effect: F1,12=0.57, n.s.).
In the learning of the NMTP conditional discrimination (Figure 3), the Tg2576 mice as a group were able to learn this demanding rule at a rate that was not significantly different in a repeated measures ANOVA (F1,10=1.1, n.s.). However, some difference in the ease and uniformity of learning of the WT controls and in a manner which suggested sluggishness or speed-accuracy tradeoff, as reflected in a visual inspection of the choice accuracy data and in the diminished but only borderline trial completion rate (F1,10=3.9, p<.07). The inherent variability on the Tg2576 responses in the NMTP along with the progressive subject dropout in the groups, prompted us to move to a more sensitive and revealing single subject analysis combined with means +/− 95% confidence intervals as the inference method. The value of this approach is illustrated by an examination of the individual performances. Tg254 and Tg247 show comparable or even superior performance to the WT group, but Tg248 and Tg263 show some impairment.
Figure 3.
Acquisition curves for individual Tg2576 mice (A–D) plotted against the mean and 95% confidence intervals for the WT group for the nonmatching-to-position (NMTP) conditional discrimination task. Panel E shows the group means ± s.e.m. of the same curves and panel F shows the average number trials completed per session (means ± s.e.m.).
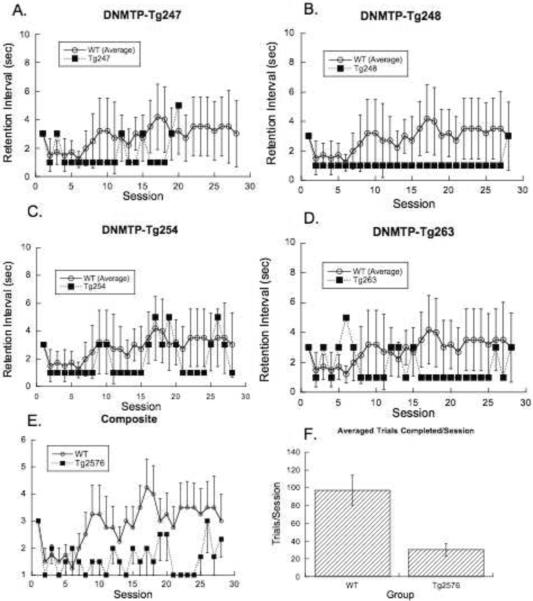
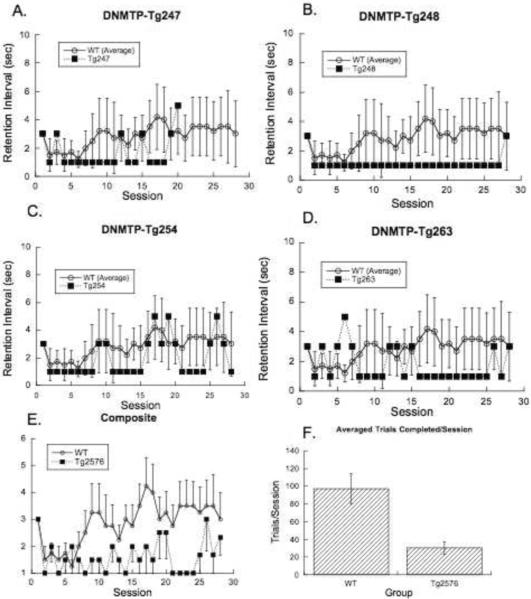
During the adjusting DNMTP task (Figure 4) the ideographic analysis isolated another subtle dissociation between the Tg2576 mice and the WT controls. These mice, who presumably were the best performing of the original cohort members, were unable to reliably maintain greater than 75% accurate performance at anything more than the minimal retention intervals, while the WT animals maintained a modest but longer than minimal retention interval. The trial completion rate (F1,9=5.1, p<.04) is also again reduced for the Tg2576 mice.
Figure 4.
Steady state performance during the adjusting delay nonmatching-to-position (DNMTP) task for individual Tg2576 mice (A–D) plotted against the mean and 95% confidence intervals for the WT group. Panel E shows the group means ± s.e.m. of the same curves and panel F shows the average number trials completed per session (means ± s.e.m.).
The analysis of the final three tasks (data not shown) showed spared ability in reaction time. However, in contrast to this, on the Signal Detection task Tg248 and Tg263, but not Tg254, required generally longer stimulus durations than WT controls to achieve the same moderate detection accuracy level (75% correct). Tg254 again was best off of the three Tg2576 animals. Tg248 and Tg263 showed a substantially lower “break-point” (i.e. reinforcers received per session) on the progressive ratio task than the age matched WT controls, but Tg254 showed a break point within the 95% confidence interval of the WT group.
Analysis of Aß
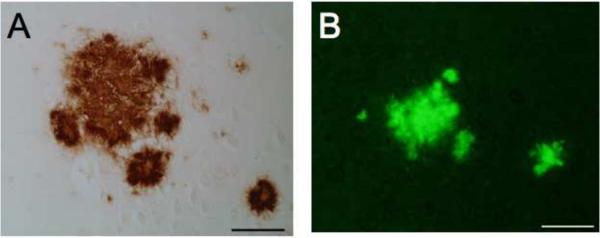
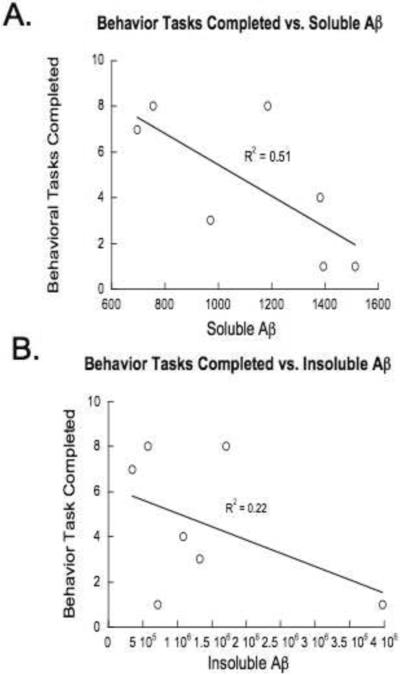
Immunoassaying for Aß identified abundant plaque deposits in the brain cortical and hippocampal parenchyma of all of the Tg2576 mice (Fig. 5A). Further, histological staining of the tissue sections with thioflavin-S confirmed that the plaque deposits were fibrillar in nature (Fig. 5B), characteristic of the amyloid pathology reported for these animals (Hsiao, 1996). ELISA analysis for Aß peptide levels in the brain confirmed that the Tg2576 animals had copious levels of Aβ by age ~14 months, mean = 618,860+/−279,040 pg/mg of total brain protein. This level is consistent with published reports (e.g. Hsiao, 1996) and the high degree of variance is also commonly observed in this line. Fig. 6 shows the relationship between the number of behavioral tasks completed by individual animals and the soluble versus insoluble Aβ fractions. The relationship was stronger between insoluble Aß and (P<.01) and the greater variance accounted for than the marginally significant soluble fraction (p<.06). However, even though this could be mediated by any number of factors, both support the general relationship between Aβ load at the individual subject level, more striking and overall relationship between the behavioral testing and a marker of underlying pathology is supported by this analysis.
Figure 5.
Representative immunostaining for Aß identifying abundant plaque deposits in the brain parenchyma common to all of Tg2576 mice (A). Histological staining of an adjacent tissue section with thioflavin-S confirmed that the plaque deposits were fibrillar in nature (B),
Figure 6.
Correlation plots of the number of behavioral tasks completed as a function of insoluble (A) or soluble (B) Aβ levels in individual subjects.
Discussion
The goal of this project was to explore the utility of a program of operant behavioral testing for understanding the behavioral impairments of transgenic mice models of AD amyloid pathology, using one frequently studied transgenic strain, Tg2576. The operant method could be a valuable addition to the field, which often tends to use a limited number of short term aversively motivated spatial memory tasks to test mnemonic deficits, such as Morris water maze (e.g. Holcomb et al., 1999, Hsiao, 1996) and fear conditioning (e.g. Wang et al., 2004, Billings et al., 2005). This experiment is part of a growing body of behavioral research in the AD field that uses non-aversively motivated, non-spatial tasks such as novel object recognition (e.g. Dodart, et al., 2002, Engel, et al., 2006) and transfer learning (Montgomery, et al., 2009).
Here, we sought to employ an approach reflective of the assumptions of a neuropsychological testing battery: a complex set of interlocking and progressively demanding cognitive operant tasks to test a variety of aspects of cognitive, sensory, and motor faculties (Wilner, 1991). This approach provides a comprehensive picture of the cognitive status of these animals and richly characterizes the progression and individual differences in the learning, memory, and executive functions lost in dementia than can be provided by employing spatial and aversively motivated maze tasks or simple avoidance learning tasks. This approach also allows for the examination of the animals under predictable, routine conditions unlikely to produce excess arousal which may also model the deficits of humans with moderate dementia functioning in the daily living setting (e.g. Zanetti et al., 2009). The ideographic analytical approach (i.e. examining single subjects data against 95% confidence intervals drawn around control groups) used to examine the data in the later experiments also is a particularly useful method for revealing individual differences in decline among animals while still retaining inferential statistical capability. Classic ANOVA approaches, especially when applied to relatively variable performance measures, can obscure rather than illuminate the variance in the behavior status between animals (Sidman, 1960; Cohen, 1997).
Using this approach, the Tg2576 animals showed strikingly more overall within-group variability in their responses. This is reflected in the task-produced study attrition effect, shown in Table 1. This attrition was the result of some Tg2576 mice not being able to progress or sustain their responding in the next level of task complexity. In contrast, the WT animals generally showed quite uniform performances and seamless transitions between tasks throughout the entire testing regimen. This variability persisted in the Tg2576 group, in spite of the fact that the animals that learned tasks more slowly were given extra training at each of the earlier steps, until they reached the criteria to move to the next task, or completely ceased responding. Additionally, it was observed that Tg2576 animals, even at times when their accuracy was comparable to controls, showed consistent evidence of decreased speed/accuracy trade-off, reduced sensitivity to reinforcement, or both, as reflected in decreased trial completion rates. This speed-accuracy tradeoff is uniquely revealed by this kind of operant analysis, where multiple measures and sessions with high trial numbers can allow higher-order patterns among the measures to be revealed. Finally, relative sparing of simple operant abilities in the FR1 and Alternation tasks were retained in a majority of the mice, but deficits in the rate of learning emerged in several more complex tasks, which we show extend into non-spatial domains.
The deficits observed presently are generally consistent with those reported in the literature for Tg2576 mice (Eriksen & Janus, 2007). Tg2576 mice show relatively robust and reliable deficits in working memory measures of the Morris water maze and other learning and memory tasks (e.g. Arendish & King, 2002; Barnes, Hale & Good, 2004; Chapman, et al., 1999; Deacon et al., 2009; Hale & Godd, 2005; King & Arendash, 2002a; King et al., 1999; Middei et al., 2006; Ognibene et al, 2005; Ohno et al, 2006; Quinn et al., 2007; Ribes, et al., 2011; Rustay et al., 2009; Stackman et al., 2003; c.f. Bizon, Prescott & Nicolle, 2007) though the age of onset of these deficits shows considerable within and between study variance, consistent with our present data (Reed, et al., 2010). However, a particularly notable apparent discrepancy is Barnes, Hale & Good (2005), who reported that Tg2576 mice were impaired relative to controls in the acquisition of a T-maze force alternation task, but similar to controls during later working memory load manipulations. This finding contrasts with the present finding of impairment in the DNMTP task as the retention interval was lengthened, though may indicate a higher sensitivity to selective retention interval manipulations in the operant DNMTP task.
The robust accumulation of Aβ in these animals in hippocampal and cortical areas, along with the extensive activation of microglia in these areas corresponding to published reports, and variable deposition rates are likely to account for the within-group variations in behavioral of the Tg2576 mice (Hsiao, et al., 1996, Frautschy, et al., 1998). In general, Aβ levels were negatively correlated with completion of the behavioral test battery. However, clearly one must be cautious in interpreting these findings as the result of Aβ load and increasing task demand since task demand is confounded with age of the animals, and important variable in linking Aβ to behavior (Westerman et al., 2002), and some behavioral impairments have been reported to be evident in Tg2576 mice prior to major Aβ deposition (Arendash et al., 2004; King et al., 1999). Furthermore, it is also possible that it was not task demand per se, but carryover from previous tasks, in the form of negative transfer due to Tg2576 mice learning a response strategy different than that of the WT controls for the same task.
While this set of experiments demonstrates the value of this approach behavioral testing, working on certain design issues can improve upon the information revealed. First, the set of operant tasks employed clearly can be pruned, supplemented and repositioned in productive ways. For example, starting at earlier time points would allow a small set of tasks to be revisited repeatedly at different time points. If mice were fully trained by 6 months, the DNMTP task could be performed when still relatively unimpaired at 6 months, and then repeated as impairment increases over time. This would help to avoid one of the main limitations in this set of experiments; the inability to discern whether dropouts were due to increased task complexity or increased pathology with age. In addition, the order of the experiments could be changed, for example, following the learning of light-dark discrimination with the reaction time and signal detection tasks would allow experimenters to make sure that all animals are capable and motivated before moving on to test in the more long term complex memory-dependent tasks such as DNMTP. Also, with increased numbers of subjects in the studies, some animals can be sacrificed at different time points and quantitative measures of histopathology can be compared to behavioral performance. Establishing a relationship between histology and behavioral impairments at different time points is likely to produce important insights into the relationship between degree and quality of neuropathology, and the increasing difficulty animals show with learning, memory and executive functions when there is an increasing cognitive load. By repeating the same sequence of tasks or expanding the group sizes, different transgenic strains can be studied and compared. If test conditions are held uniform, this can be accomplished even at different time points as new strains emerge, as new testing data are compared to archived data. It is also clear that this approach is not high-throughput, so has limited application to rapid screening. Finally, the relationship, both temporal and anatomical, between the measures in the operant tasks and those in spatial maze tasks certainly should also be established. Clearly many behavioral tasks have been used together with histopathological analyses in the past that has provided useful information about transgenic models of AD (e.g. Ashe, 2009; Eriksen & Janus, 2007; Kokjohn & Roher, 2009). However, our findings demonstrate the value of this approach as a supplement to those, and which will be broadened by the study of other transgenic lines, and as treatment studies are performed using this approach. This testing strategy encourages researchers to prioritize and rigorously explore the behavioral symptoms, particularly in regard to variability of response in transgenic animals.
Highlights
Behavioral studies of cognitive deficits in transgenic mouse models of AD often employ a limited set of aversively motivated, spatial learning and memory tests, under brief testing periods.
This study presents an alternative operant behavioral methodology to provide a comprehensive characterization under low arousal testing conditions, and with appetitive motivation.
The mice were assessed on a progressively more complex and interlocking battery of operant tasks, ranging from simple rule learning to delayed recall, as well as tests of motor and sensory ability.
Attrition from the study correlated with increasing task demands and amyloid load were seen.
These experiments demonstrate the utility of this novel operant approach for characterizing the cognitive deficits of transgenic murine models of dementia.
Acknowledgements
This work was supported in part by NIH grant NS55118. Antibody reagents for the Aß ELISAs were generously provided by Lilly Research Laboratories, Indianapolis, IN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Ognibene E, Heuland E, Ghirardi O, Caprioli A, Laviola G. Motor impulsivity in APP-SWE mice: a model of Alzheimer's disease. Behavioral Pharmacology. 2006;17:525–533. doi: 10.1097/00008877-200609000-00019. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Ueber eine eigenartige Erkrankung der Hirnrinde [On a peculiar disease of the cerebral cortex] Zeitschrift fuer Psychiatrie. 1907;64:146. [Google Scholar]
- Arendash GW, King DL. Intra- and intertask relationships in a behavioral test battery given to Tg2576 transgenic mice and controls. Physiology & Behavior. 2002;75:643–652. doi: 10.1016/s0031-9384(02)00640-6. [DOI] [PubMed] [Google Scholar]
- Arendash. GW, Lewis J, Leighty RE, McGowan E, Cracchiolo JR, Hutton M, Garcia MF. Multi-metric behavioral comparison of APPsw and P301L models for Alzheimer's disease: linkage of poorer cognitive performance to tau pathology in forebrain. Brain Research. 2004;1012:29–41. doi: 10.1016/j.brainres.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Ashe KH. Learning and memory in transgenic mice modeling Alzheimer's disease. Learning & Memory. 2001;8:301–308. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- Barnes P, Good M. Impaired Pavlovian cued fear conditioning in Tg2576 mice expressing a human mutant amyloid precursor protein gene. Behavioral Brain Research. 2005;157:107–117. doi: 10.1016/j.bbr.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Barnes P, Hale G, Good M. Intramaze and extramaze cue processing in adult APPSWE Tg2576 transgenic mice. Behavioral Neuroscience. 2004;118:1184–1195. doi: 10.1037/0735-7044.118.6.1184. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and other treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Experimental Neurology. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal AB causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Bizon J, Prescott S, Nicolle MM. Intact spatial learning in adult Tg2576 mice. Neurobiology of Aging. 2007;28:440–446. doi: 10.1016/j.neurobiolaging.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. The earth is round (p<.05) American Psychologist. 1994;47:997–1003. [Google Scholar]
- Corcoran KA, Lu Y, Turner RS, Maren S. Overexpression of hAPPswe impair srewarded alternation and contextual fear conditioning in a transgenic mouse model of Alzheimer's disease. Learning & Memory. 2002;9:243–252. doi: 10.1101/lm.51002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neuroscience. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Koros E, Bornemann KD, Rawlins JN. Aged Tg2576 mice are impaired on social memory and open field habituation tests. Behavioral Brain Research. 2009;197:466–468. doi: 10.1016/j.bbr.2008.09.042. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, O'dell MA, Parsadanian M, Taylor JW, Harmony JA, Bales KR, Paul SM, Aronow BJ, Holtzman DM. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer's disease. Proceedings of the National Academies of Science, USA. 2002;99:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer's disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunizaton reverses memory deficits without reducing brain AB burden in Alzheimer's disease model. Nature Neuroscience. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Evenden JL, Iverson SD. Delay-dependent short-term memory deficits in aged rats. Psychopharmacology. 1988;96:174–180. doi: 10.1007/BF00177557. [DOI] [PubMed] [Google Scholar]
- Engel T, Hernandez F, Avila J, Lucas JL. Full reversal of Alzheimer's disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. Neurobiology of Disease. 2006;26:5083–5090. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen JL, Janus CG. Plaques, tangles and memory loss in mouse models of neurodegeneration. Behavior Genetics. 2007;37:79–100. doi: 10.1007/s10519-006-9118-z. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizary M, Hyman B, Saido TC, Hsiao K, Cole GM. Microgial response to amyloid plaques in APPswe transgenic mice. American Journal of Pathology. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Good MA, Hale G. The “Swedish” mutation of the amyloid precursor protein (APPswe) dissociates components of object-location memory in aged Tg2576 mice. Behavioral Neuroscience. 2007;121:1180–1191. doi: 10.1037/0735-7044.121.6.1180. [DOI] [PubMed] [Google Scholar]
- Götz J, Deters N, Doldissen A, Bokhari L, Ke Y, Wiesner A, Schonrock N, Ittner LM. A decade of tau transgenic animals and beyond. International Society of Neuropathology. 2007;17:91–103. doi: 10.1111/j.1750-3639.2007.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G, Good M. Impaired visuospatial recognition memory but normal object novelty detection and relative familiarity judgments in adult mice expressing the APPswe Alzheimer's disease mutation. Behavioral Neuroscience. 2005;119:884–891. doi: 10.1037/0735-7044.119.4.884. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task-specific firing of hippocampal cells. Journal of Pharmacology and Experimental Therapeutics. 1993;264:294–307. [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforce volume on progressive ratio performance. Journal of Experimental Analysis of Behavior. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precurser protein and Presenilin-1 mutations: lack of association with amyloid deposits. Behavior Genetics. 1999;29:177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits Aβ elevation and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer's disease and other tauopathies. Biochimica et Biophysica Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Jensen LH, Stephens DN, Sarter M, Petersen EN. Bidirectional effects of beta-carbolines and benzodiazepines on cognitive processes. Brain Research Bulletin. 1987;19:359–364. doi: 10.1016/0361-9230(87)90104-3. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer's disease through 19 months. Physiology & Behavior. 2002a;75:627–642. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW. Maintained synaptophysin immunoreactivity in Tg2576 transgenic mice during aging: correlations with cognitive impairment. Brain Research. 2002b;926:58–68. doi: 10.1016/s0006-8993(01)03294-2. [DOI] [PubMed] [Google Scholar]
- King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ. Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. Behavioral Brain Research. 1999;103:145–162. doi: 10.1016/s0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Kokjohn TA, Roher AE. Amyloid precursor protein transgenic mouse models and Alzhiemer's disease: understanding the paradigms, limitations and contributions. Alzheimer's & Dementia. 2009;5:340–347. doi: 10.1016/j.jalz.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmont F, Davenport M, Robinson JK. Effects of gonadectomy on performance of operant tasks measuring prefrontal cortical function in adult male rats. Hormones & Behavior. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Lagadec S, Rotureau L, Hémar A, Macrez N, Delcasso S, Jeantet Y, Cho YH. Neurobiology of Aging. Sep 1, 2010. Early temporal short-term memory deficits in double transgenic APP/PS1 mice. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Montgomery KS, Simmons RK, Edwards G, III, Nicolle MM, Gluck MA, Myers CE, Bizon JL. Novel age-dependent learning deficits in a mouse model of Alzheimer's disease: implications for translational research. Neurobiology of Aging. 2009;32:1273–1285. doi: 10.1016/j.neurobiolaging.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middei S, Daniele S, Caprioli A, Ghirardi O, Ammassari-Teule M. Progressive cognitive decline in a transgenic mouse model of Alzheimer's disease overexpressing mutant hAPPswe. Genes, Brain & Behavior. 2006;5:249–256. doi: 10.1111/j.1601-183X.2005.00160.x. [DOI] [PubMed] [Google Scholar]
- Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with heredity Alzheimer's disease. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Middei S, Daniele S, Adriani W, Ghirardi O, Caprioli A, Laviola G. Aspects of spatial memory and behavioral disinhibition in Tg2576 transgenic mice as a model of Alzheimer's disease. Behavioral Brain Research. 2005;156:225–232. doi: 10.1016/j.bbr.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, Vassar R, Disterhoft JF. Temporal memory deficits in Alzheimer's mouse models: rescue by geneticdeletion of BACE1. European Journal of Neuroscience. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- Price DL, Walker LC, Martin LJ, Sisodie SS. Amyloidosis in aging and Alzheimer's disease. American Journal of Pathology. 1992;141:767–772. [PMC free article] [PubMed] [Google Scholar]
- Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, Stackman RW., Jr. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiology of Aging. 2007;28:213–225. doi: 10.1016/j.neurobiolaging.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Reed MN, Liu P, Kotilinek LA, Ashe KH. Effect size of reference memory deficits in the Morris water maze in Tg2576 mice. Behavioral Brain Research. 2011;212:115–120. doi: 10.1016/j.bbr.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes D, Torrente M, Vicens P, Colomina MT, Gómez M, Domingo JL. Alzheimer's Disease and AssociatedDisorders. 2011. Recognition memory and β-amyloid plaques in adult Tg2576 mice are not modified after oral exposure to aluminum. Epublished ahead of print. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Cronin EA, Curzon P, Markosyan S, Bitner RS, Ellis TA, Waring JF, Decker MW, Rueter LE, Browman KE. Mice expressing the Swedish APP mutation on a 129 genetic background demonstrate consistent behavioral deficits and pathological markers of Alzheimer's disease. Brain Research. 2010;1311:136–147. doi: 10.1016/j.brainres.2009.11.040. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Research Reviews. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Genes Proteins and Therapy. Physiological Reviews. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: Mechanistic understanding predicts novel therapies. Annals of Internal Medicine. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- Sherman K, Kuster JE, Dean RL, Bartus RT, Freidman E. Presynaptic cholinergic mechanisms in brain of aged rats with memory impairments. Neurobiology of Aging. 1981;2:99–104. doi: 10.1016/0197-4580(81)90006-3. [DOI] [PubMed] [Google Scholar]
- Sidman M. Tactics of scientific research. Basic Books; New York: 1960. [Google Scholar]
- Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer's disease by chronic Ginkgo biloba treatment. Experimental Neurology. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126:305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. Journal of Neuroscience. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilner P. Behavioral models in psychopharmacology. In: Willner P, editor. Behavioral models in psychopharmacology: Theoretical, industrial & clinical Perspectives. Cambridge University Press; Cambridge, U.K.: 1991. [Google Scholar]
- Winters BD, Dunnett SB. Selective lesioning of the Cholinergic Septo-Hippocampal Pathway does not disrupt spatial short term memory: A comparison with the effects of Fimbria-Fornix lesions. Behavioral Neuroscience. 2004;118:546–562. doi: 10.1037/0735-7044.118.3.546. [DOI] [PubMed] [Google Scholar]
- Zanetti O, Binetti G, Magni E, Rozzini L, Bianchetti A, Trabucchi M. Procedural memory stimulation in Alzheimer's disease: Impact of a training programme. Acta Neurologica Scandinavica. 1997;95:152–157. doi: 10.1111/j.1600-0404.1997.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Zhuo JM, Prescott SL, Murray ME, Zhang HY, Baxter MG, Nicolle MM. Early discrimination reversal learning impairment and preserved spatial learning in alongitudinal study of Tg2576 APPsw mice. Neurobiology of Aging. 2007;28:1248–1257. doi: 10.1016/j.neurobiolaging.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opoid, GABAergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behavioral Neuroscience. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]