Abstract
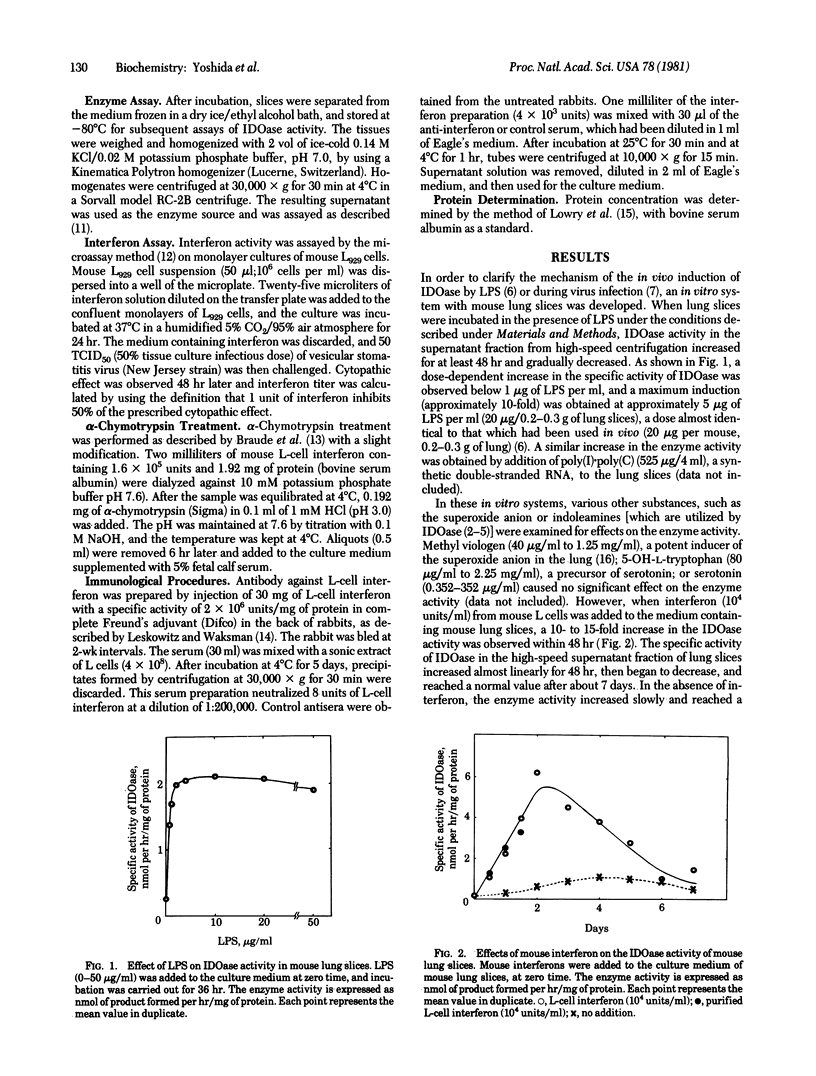
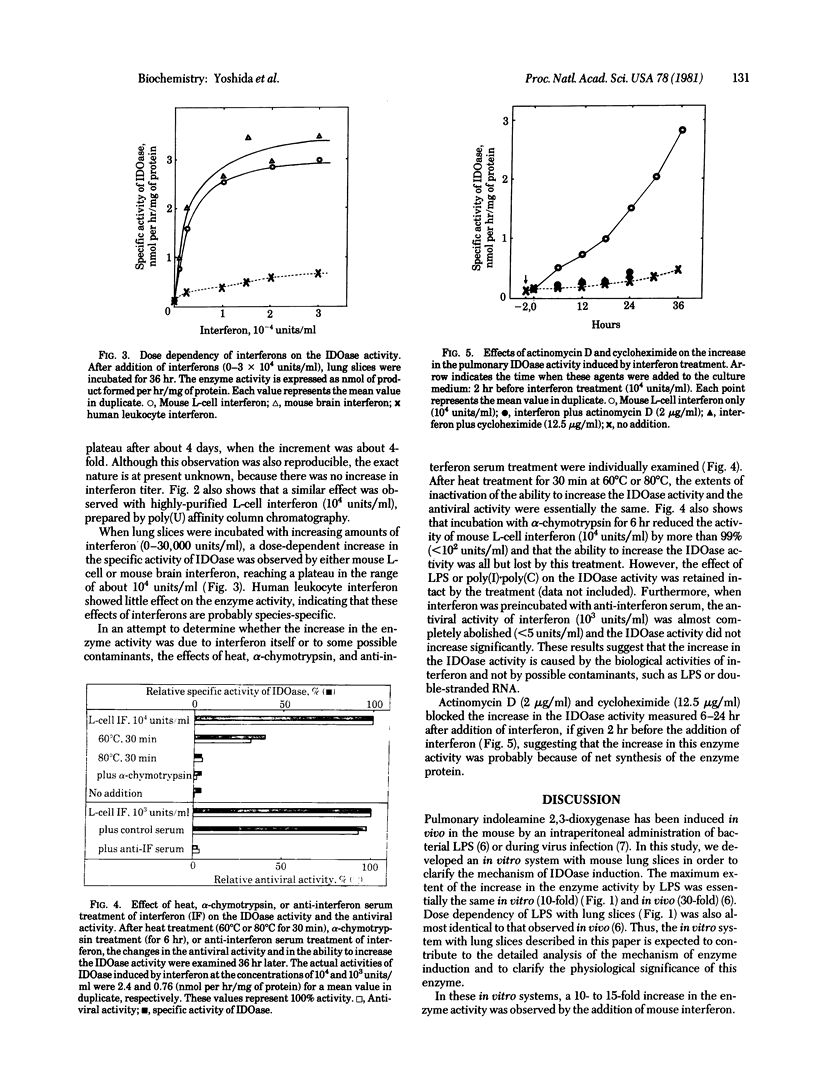
Pulmonary indoleamine 2,3-dioxygenase [indoleamine: oxygen 2,3-oxidoreductase(decyclizing)] has been found to be induced (30- to 100-fold) in the mouse after a single intraperitoneal administration of bacterial endotoxin [Yoshida, R. & Hayaishi, O. (1978) Proc. Natl. Acad. Sci. USA 75, 3998-4000] or during in vivo virus infection [Yoshida, R., Urade, Y., Tokuda M. & Hayaishi, O. (1979) Proc. Natl. Acad. Sci. USA 76, 4084-4086]. In the present study, an in vitro system with mouse lung slices was developed in which bacterial endotoxin (5 micrograms/ml)produced an induction (approximately 10-fold) of indoleamine 2,3-dioxygenase. The endotoxin was substituted by interferon from mouse L cells or mouse brain. The pulmonary enzyme activity increased almost linearly for 48 hr after addition of mouse interferon (10(4) units/ml) to lung slices. Interferon from mouse L cells or mouse brain produced a 10- to 15-fold increase in the enzyme activity, whereas that from human leukocytes was all but ineffective. The effect also was observed using highly purified L-cell interferon, prepared by poly(U) affinity column chromatography. When interferon was treated either by heat, alpha-chymotrypsin, or anti-interferon serum, such increase in the enzyme activity was diminished essentially to the same extent as seen in the antiviral activity. The increase in the enzyme activity was blocked when actinomycin D or cycloheximide was added to the slices before interferon treatment. These results suggest that the enzyme induction was produced by interferon and not by possible contaminants in the interferon preparations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A., White C. N. Oligonucleotide inhibitor of protein synthesis made in extracts of interferon-treated chick embryo cells: comparison with the mouse low molecular weight inhibitor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1167–1171. doi: 10.1073/pnas.75.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude I. A., Lin L. S., Stewart W. E., 2nd Differential inactivation and separation of homologous and heterologous antiviral activity of human leukocyte interferon by a proteolytic enzyme. Biochem Biophys Res Commun. 1979 Jul 27;89(2):612–619. doi: 10.1016/0006-291x(79)90674-0. [DOI] [PubMed] [Google Scholar]
- Cantell K., Hirvonen S. Large-scale production of human leukocyte interferon containing 10(8) units per ml. J Gen Virol. 1978 Jun;39(3):541–543. doi: 10.1099/0022-1317-39-3-541. [DOI] [PubMed] [Google Scholar]
- De Maeyer-Guignard J., Thang M. N., De Maeyer E. Binding of mouse interferon to polynucleotides. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3787–3790. doi: 10.1073/pnas.74.9.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O., Hirata F., Ohnishi T., Henry J. P., Rosenthal I., Katoh A. Indoleamine 2,3-dioxygenase: incorporation of 18O2-- and 18O2 into the reaction products. J Biol Chem. 1977 May 25;252(10):3548–3550. [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. New degradative routes of 5-hydroxytryptophan and serotonin by intestinal tryptophan 2,3-dioxygenase. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1112–1119. doi: 10.1016/0006-291x(72)90949-7. [DOI] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Studies on indoleamine 2,3-dioxygenase. I. Superoxide anion as substrate. J Biol Chem. 1975 Aug 10;250(15):5960–5966. [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Huang K. Y., Donahoe R. M., Gordon F. B., Dressler H. R. Enhancement of phagocytosis by interferon-containing preparations. Infect Immun. 1971 Nov;4(5):581–588. doi: 10.1128/iai.4.5.581-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. Y., Donahoe R. M., Gordon F. B., Dressler H. R. Enhancement of phagocytosis by interferon-containing preparations. Infect Immun. 1971 Nov;4(5):581–588. doi: 10.1128/iai.4.5.581-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACS A., HITCHCOCK G. Role of interferon in recovery from virus infections. Lancet. 1960 Jul 9;2(7141):69–71. doi: 10.1016/s0140-6736(60)91215-0. [DOI] [PubMed] [Google Scholar]
- Imanishi J., Yokota Y., Kishida T., Mukainaka T., Matsuo A. Phagocytosis-enhancing effect of human leukocyte interferon preparation of human peripheral monocytes in vitro. Acta Virol. 1975 Jan;19(1):52–58. [PubMed] [Google Scholar]
- Kimchi A., Shulman L., Schmidt A., Chernajovsky Y., Fradin A., Revel M. Kinetics of the induction of three translation-regulatory enzymes by interferon. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3208–3212. doi: 10.1073/pnas.76.7.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESKOWITZ S., WAKSMAN B. H. Studies on immunization. 1. The effect of route of injection of bovine serum albumin in Freund adjuvant on production of circulating antibody and delayed hypersensitivity. J Immunol. 1960 Jan;84:58–72. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Field A. K., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci U S A. 1967 Aug;58(2):782–789. doi: 10.1073/pnas.58.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Hirata F., Hayaish O. Indoleamine 2,3-dioxygenase. Potassium superoxide as substrate. J Biol Chem. 1977 Jul 10;252(13):4643–4647. [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Papamatheakis J. D., Chirigos M. A. Interferon: an inducer of macrophage activation by polyanions. Science. 1977 Aug 12;197(4304):674–676. doi: 10.1126/science.877584. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Nomiyama S., Hirata F., Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978 Jul 10;253(13):4700–4706. [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971 Nov;22(5):797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Hirata F., Hayaishi O. Intracellular utilization of superoxide anion by indoleamine 2,3-dioxygenase of rabbit enterocytes. J Biol Chem. 1977 Apr 25;252(8):2774–2776. [PubMed] [Google Scholar]
- Yamamoto S., Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967 Nov 25;242(22):5260–5266. [PubMed] [Google Scholar]
- Yoshida R., Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3998–4000. doi: 10.1073/pnas.75.8.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Urade Y., Tokuda M., Hayaishi O. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4084–4086. doi: 10.1073/pnas.76.8.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maeyer-Guignard J., de Maeyer E. Effect of antilymphocytic serum on circulating interferon in mice as a function of the inducer. Nat New Biol. 1971 Feb 17;229(7):212–214. doi: 10.1038/newbio229212a0. [DOI] [PubMed] [Google Scholar]




