Abstract
Mechanisms underlying chronic graft-versus-host disease (cGVHD) are numerous, including skewing of Th1/Th2 cytokine expression. cGVHD has biological differences between early and late onset cGVHD. To test whether different Th1/Th2 cytokines are associated with early or late onset cGVHD, peripheral blood was collected from 63 children enrolled on the Children’s Oncology Group phase III trial ASCT0031 evaluating hydroxychloroquine therapy for newly diagnosed extensive cGVHD. mRNA expression of interferon gamma (IFN-γ) and interleukins 2, 4 and 10(IL-2, IL-4 and IL-10) from stimulated peripheral blood mononuclear cells was evaluated by quantitative polymerase chain reaction (Q-PCR). We found that early onset cGVHD (n=33) was characterized by decreased expression of IFN-γ and IL-2 mRNA after non-specific PMA-Ionomycin stimulation. In contrast, late onset cGVHD (n=11) was characterized by decreased expression of IL-4 and IL-2 mRNA after anti-CD3 stimulation of T cells. Receiver Operator Characteristic (ROC) curve analysis revealed that IFN-γ production could determine the absence of early cGVHD (AUC=0.77) and IL-4 (AUC=0.89) and IL-2 (AUC=0.84) the absence of late cGVHD. We did not find any correlation between cytokine expression and a specific immune cell subset. We also showed an increased expression of Foxp3 mRNA in early onset cGVHD and late controls. The different time-dependent cytokine profiles in newly-diagnosed cGVHD suggests that mechanisms underlying cGVHD are temporally regulated. While larger validation studies are needed our data suggests cytokine profiles could potentially be used as biomarkers for the diagnosis of cGVHD.
Introduction
Myeloablative allogeneic blood and marrow transplantation (BMT) is the only successful cellular based immunotherapy for high-risk hematopoietic malignancies. It is also the only curative treatment for numerous marrow failure syndromes [1], non-malignant blood disorders [2], primary immunodeficiencies [3], autoimmune diseases [4], and inherited metabolic diseases [5]. However due to the increased usage of unrelated donors, more than half of patients who receive an allogeneic BMT will develop chronic graft-versus-host disease (cGVHD) [6] which has become the leading cause of transplantation-related morbidity and mortality [7]. In adults with cGVHD there is a 60% mortality after 8 years [8] and in children a 20% mortality after 15 years [9]. Numerous potential mechanisms have been investigated in cGVHD, but previous human clinical studies have been hindered by a number of factors: 1) the insidious onset and multiple organ involvement of cGVHD, 2) samples taken at different times in the course of the disease and taken from patients who are often on a variety of immunosuppressants, 3) failure to consider time of onset and 4) lack of proper controls to account for patterns of normal immune recovery post-BMT.
Our group has already previously shown evidence that the biology of cGVHD is temporally different and influenced by immune reconstitution after BMT. We have shown that there are different patterns of biomarkers in early onset (3–8 months post BMT) and late onset (≥ 9 months) cGVHD [10]. Soluble B-cell activation factor (sBAFF), anti-dsDNA antibody, soluble IL-2 receptor alpha (sIL-2Rα), and soluble CD13 (sCD13) were elevated in patients with early-onset cGVHD compared with controls. sBAFF and anti-dsDNA were elevated in patients with late-onset cGVHD. This previous finding suggests that the pathophysiology of cGVHD is heterogeneous with different mechanisms operative at different times after BMT. The results presented in this paper further try to characterize the differences between early and late onset of chronic GVHD.
There are a number of different effector cell populations thought to be important in the pathophysiology of cGVHD, including: 1) B cells, 2) regulatory T (Treg) cells and 3) effector and memory T cells. B cells have been increasingly recognized as playing an important role in the pathophysiology of cGVHD. These conclusions were initially identified in a murine model by our group [11]. Later human data confirmed the importance of B cells in cGVHD by establishing a role for autoantibodies, such as HY antibodies in male recipients with female donors correlating with cGVHD development [12–14], high levels of soluble B-cell activation factor (sBAFF) [10,15], increased plasma cell populations [16] and CD21-CD27+ B cells [17]. Their importance is also clinically supported by the successful treatment of steroid-refractory cGVHD with rituximab, an anti-CD20 (B-cell antigen) monoclonal antibody [18–20].
The role of Tregs in cGVHD is less clear. Mouse models show that Tregs play an important role in prevention of GVHD [21] and that adoptive transfer of freshly isolated or ex vivo expanded CD4+CD25+ T cells can prevent GVHD [22–23]. In humans, there is conflicting data as to the importance of Tregs in cGVHD, with studies showing decreased, unchanged, or increased numbers of these regulatory cells [24–27].
The role of other T cell populations appears to be equally varied and unclear. cGVHD has been associated with a preponderance of IFN-γ, IL-4, IL-5, and IL-2 producing CD4+ effector memory cells [28–29] and with infiltration of CD8+ T cells in skin [30] and intestine [31]. OX40, an activation marker on both CD4+ and CD8+ T cell populations may be associated with cGVHD onset [32]. Predominance of cytokine producing Th1/Th2 immune responses has also been postulated. A review of current literature offers contradictory results in regards to the synthesis of cytokines such as IFN-γ. In some studies, increased IFN-γ mRNA expression is associated with extensive cGVHD [33–34]. In contrast, other studies show that patients with microsatellite polymorphisms within the first intron of the IFN-γ gene associated with decreased production have higher rates of cGVHD [35]. In mouse models, high IFN-γ production by NK-T cells results in lower rates of cGVHD [36–37]. There is no data in children.
Based on our findings showing different biomarker profiles in early and late onset cGVHD, we hypothesized that there were distinctive Th1/Th2 cytokine profiles associated with early and late cGVHD. In order to verify our hypothesis, we made use of a well-controlled Children’s Oncology Group study, ASCT0031, evaluating hydroxychloroquine therapy for newly diagnosed extensive cGVHD from 2002 to 2005. As part of the biological studies associated with this clinical trial, we prospectively measured mRNA levels of IFN-γ, IL-2, IL-4 and IL-10 of peripheral blood mononuclear cells after either non-specific mitogen stimulation with PMA/Ionomycin or T-cell stimulation with anti-CD3 in newly diagnosed cGVHD patients and comparing these levels to time-matched BMT control subjects who did not develop cGVHD. We also collected data on percentage and absolute counts of immune cell subsets. Furthermore, we retrospectively analyzed mRNA levels of Foxp3 in resting or anti-CD3 stimulated peripheral blood mononuclear cells to investigate the temporal role of regulatory T cells in cGHVD.
Materials and Methods
Patients
Samples were obtained from patients enrolled on the Children’s Oncology Group trial, ASCT0031. All subjects enrolled in these studies signed informed consent agreements that were approved by individual center’s Institutional Review Board in accordance with the Declaration of Helsinki. The study was a Phase III randomized, placebo-controlled, double-blinded trial evaluating two treatment regimens for patients 1–30 years of age with newly diagnosed extensive chronic graft-versus-host disease (cGVHD). cGVHD patients received a standard regimen of cyclosporine and alternate-day prednisone with either hydroxychloroquine or placebo. Eligibility requirements allowed concurrent steroids for treatment and/or prophylaxis of acute GVHD if prednisone dose was ≤ 2 mg/kg/day (or equivalent) at study entry. Enrolment was entirely voluntary by each center and there was no attempt to establish matched controls for the cGVHD subjects enrolled. The decision to allow a center to participate in control enrolment was not based on the characteristics of the patients enrolled, but on the resources available to identify and follow such individuals. Newly diagnosed extensive cGVHD was documented with biopsy confirmation of at least one organ system (e.g. lip, skin, liver) and either 1) generalized skin involvement, or 2) localized skin involvement and/or liver dysfunction plus at least one of the following: liver histology showing chronic aggressive hepatitis, bridging necrosis, or cirrhosis; eye involvement (Schirmer’s test with < 5 mm wetting); involvement of minor salivary glands or oral mucosa on lip biopsy; or involvement of any other target organs, or (3) involvement of at least two target organs. Peripheral blood samples in patients with cGVHD were collected at study entry and at 2, 6 and 12 months post study entry. For controls, peripheral blood was collected from patients without documented cGVHD at 6 and 12 months after transplantation. There were a total of 82 patients enrolled in the original Phase III trial. There were a total of 44 patients with cGVHD and 19 control patients without cGVHD included in this part of the study for a total of 63 patients which was 77% of original sample size. Samples collected were used for a number of different studies and we were limited by the amount of sample received for some patients. Control samples were collected 6 months and 12 months post BMT on patients who did not have chronic GVHD to address the potential confounding factor of immune reconstitution after BMT. A time matched comparison was performed with days post BMT at the time of cGVHD diagnosis used to divide the patients into early onset (3–8 months) or late onset (≥9 months) group to allow comparison against samples from control patients drawn at 6 and 12 months post transplant, respectively. All blood samples were collected prior to initiation of protocol therapy.
Patient Characteristics
Patients with chronic GVHD and controls at each time-point were compared for differences in age, sex, donor source, donor type, acute GVHD, GVHD prophylaxis and concurrent steroid use (≤2 mg/kg/day) (Table 1). There appeared to be a clinical difference in unrelated donors, peripheral blood source and acute GVHD at the early time-point as expected, as all are associated with higher rates of cGVHD, but there was no apparent statistically significant difference between the groups at each time-point.
Table 1.
Comparison of Patients with Chronic GVHD and Controls for Differences in Age, Sex, Donor Type, Donor Source, Acute GVHD, GVHD Prophylaxis and Concurrent Steroids
| Early controls (N=18) |
Early cGVHD (N=33) |
Difference (95% CI) |
Late controls (N=11) |
Late cGVHD (N=11) |
|
|---|---|---|---|---|---|
| Age: | |||||
| Median (Range) | 9.0(1.3,20.7) | 11.3(2.0,21.0) | 7.3(1.2,15.4) | 13.5(3.2,21.8) | |
| Mean (SD) | 9.7(6.0) | 12.0(5.7) | 7.0(4.8) | 13.2(5.5) | |
| Sex: | |||||
| Male | 12 (67%) | 23 (70%) | 7 (64%) | 8 (73%) | |
| Female | 6 (33%) | 10 (30%) | 4 (36%) | 3 (27%) | |
| Donor Source: | |||||
| Bone marrow | 11 (61%) | 13 (39%) | 21.7%(-6,49) | 7 (64%) | 7 (64%) |
| Peripheral blood | 4 (22%) | 14 (42%) | 20.2%(-45,5) | 2 (18%) | 2 (18%) |
| Umbilical cord blood |
3 (17%) | 6 (18%) | 2 (18%) | 2 (18%) | |
| Donor type: | |||||
| Sibling | 13 (72%) | 15 (45%) | 27%(0,54) | 7 (64%) | 5 (45%) |
| Unrelated | 5 (28%) | 18 (55%) | 27%(-53,0) | 4 (36%) | 6 (55%) |
| Acute GVHD: | |||||
| Yes | 6 (33%) | 20 (61%) | 27%(-54,0) | 4 (36%) | 6 (55%) |
| No | 12 (66%) | 13 (39%) | 27%(0,54) | 7 (64%) | 5 (45%) |
|
GVHD prophylaxis: |
|||||
| CSA | 14 (78%) | 26 (79%) | 10 (91%) | 9 (82%) | |
| MTX | 13 (72%) | 22 (67%) | 8 (73%) | 6 (55%) | |
| Steroids | 6 (33%) | 8 (24%) | 4 (36%) | 3 (27%) | |
| Tacrolimus | 6 (33%) | 7 (21%) | 3 (27%) | 2 (18%) | |
| MMF | - | 2 (6%) | - | - | |
| ATG | - | 2 (6%) | - | - | |
|
On Prednisone or equivalent (≤ 2 mg/kg/day) at study entry: |
|||||
| Yes | 8 (44%) | 16 (48%) | 5 (45%) | 3 (27%) | |
| No | 10 (56%) | 17 (48%) | 6 (55%) | 8 (73%) | |
Quantitative RT-PCR
Th1 (IL-2 and IFN-γ) cytokine, Th2 (IL-4 and IL-10) cytokine and Foxp3 mRNA levels were determined by Q-PCR. Blood from cGVHD and control subjects were collected in heparinized tubes and then shipped overnight at room temperature to the reference laboratory at the University of Iowa. 0.5 – 1.0 × 106 peripheral blood mononuclear cells (PBMC), isolated by Ficoll-Hypaque separation, were maintained in complete RPMI media (10% fetal calf serum, penicillin 1000U/ml, streptomycin 1000U/ml and glutamine 20mM), then placed in Costar plates with medium alone or stimulated with Phorbol 12-Myristate 13 Acetate (75 ng/ml, Sigma, St. Louis MO) plus ionomycin (1 µM, Sigma) or with plate bound anti-CD3 (biotinylated anti-CD3, Pharmingen, San Diego, Ca). Following an 18-hour incubation, cells were lysed and RNA isolated with the Qiagen RNeasy mini kit (Qiagen, Valencia, CA). cDNA was generated using Gibco BRL Superscript First Strand Synthesis system (Invitrogen Corp., Carlsbad, CA). Q-PCR was performed according to manufacturer’s instructions as previously described [38]. TaqMan primers and probes for human cytokines were purchased from Applied Biosystems, and samples were analyzed utilizing the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). For each sample, mRNA expression level was normalized to the level of GAPDH housekeeping genes. The value of each sample was an average of three independent Q-PCR measurements. Data from Q-PCR experiments were analyzed using the comparative CT method for multiplex reactions, as previously described [39].
Flow Cytometric Analysis of Immune Cell Populations
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque gradient centrifugation. Following isolation, cells were washed twice in FACS staining buffer (PBS, 0.1% sodium azide, 1% fetal calf serum) then stained with a panel of fluorescein-conjugated antibodies. Expression of cell surface markers was quantitated by FACScan (Becton Dickinson Immunocytometry Systems, San Jose, CA) and Cellquest V3.2 software (Becton Dickinson) as previously described [40]. Mouse anti-human monoclonal antibodies (mAb), conjugated with FITC, PE, or PerCP, and reacting with T cell (CD3, CD4, CD8), B cell (CD19), NK cell (CD16/56), or various other markers (CD25, CD57, CD69, HLA-DR) were purchased from Becton Dickinson (Mountain View, CA).
Statistical Analysis
Descriptive statistics were generated on all data using Prism version 4 for PC (GraphPad Software, San Diego, CA). The percentage change in cell surface molecule expression was defined as the difference in the percentage of positive cells after culture with stimulation and the percentage of positive cells after culture in otherwise identical conditions with no stimulation. The degree of dispersion from the mean was calculated as a standard deviation (σ). Significance of observed changes was determined using the Mann-Whitney U-test. The alpha (P) value was set at 0.05, making all p values <0.05 statistically significant. Even when a p value of <0.05 was seen, results were not considered biologically significant unless the difference was either 50% higher or lower in the GVHD value compared to the control value. The receiver operating characteristic (ROC) curve methodology was used to assess the ability of cytokine expression to predict likelihood of cGVHD. The ability to discriminate between the presence or absence of cGVHD was evaluated using the area under the receiver operating characteristic (ROC) curve. A value of 0.5 for the area under the curve indicates that the measurement is no better than chance in discriminating between the two conditions, whereas a value of 1 indicates perfect discriminatory power. ROC analysis allowed us to determine cut offs for cytokine levels to maximize accuracy.
Results
IFNγ, IL-4, IL-2 and IL-10 Concentrations in Early Chronic GVHD
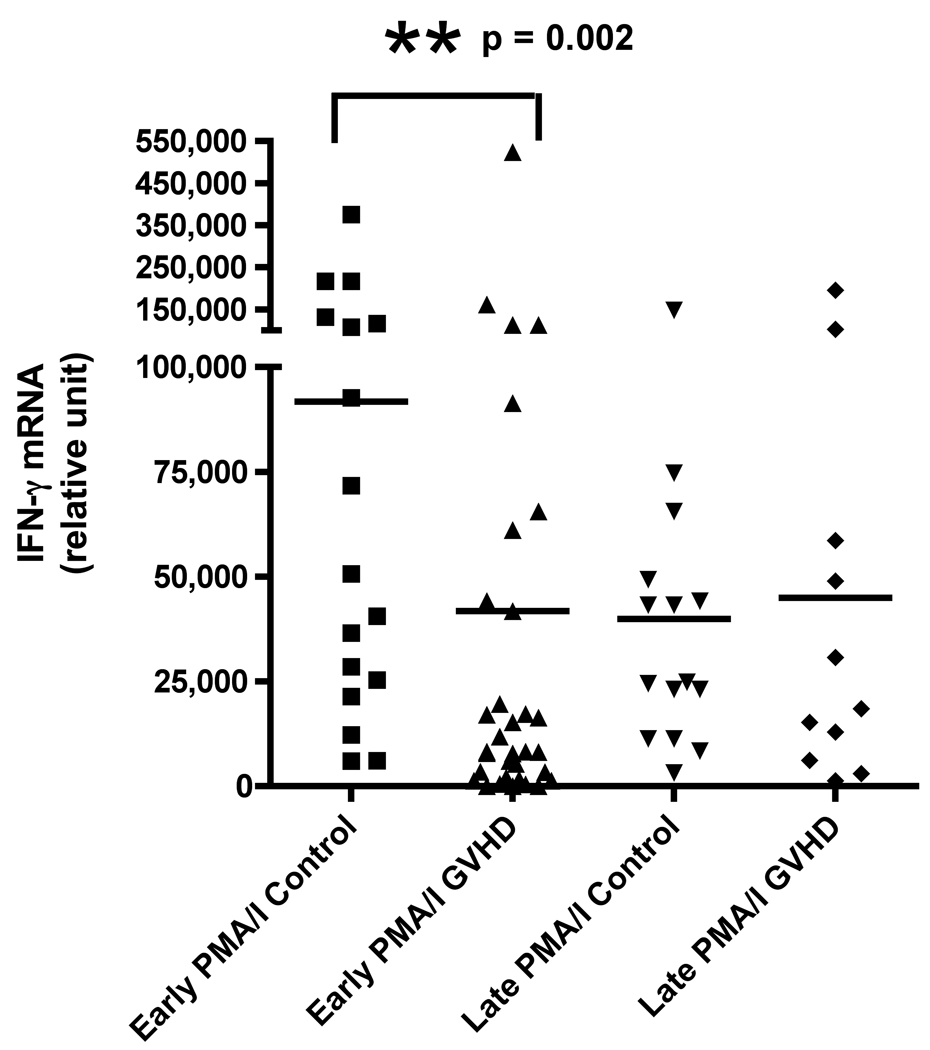
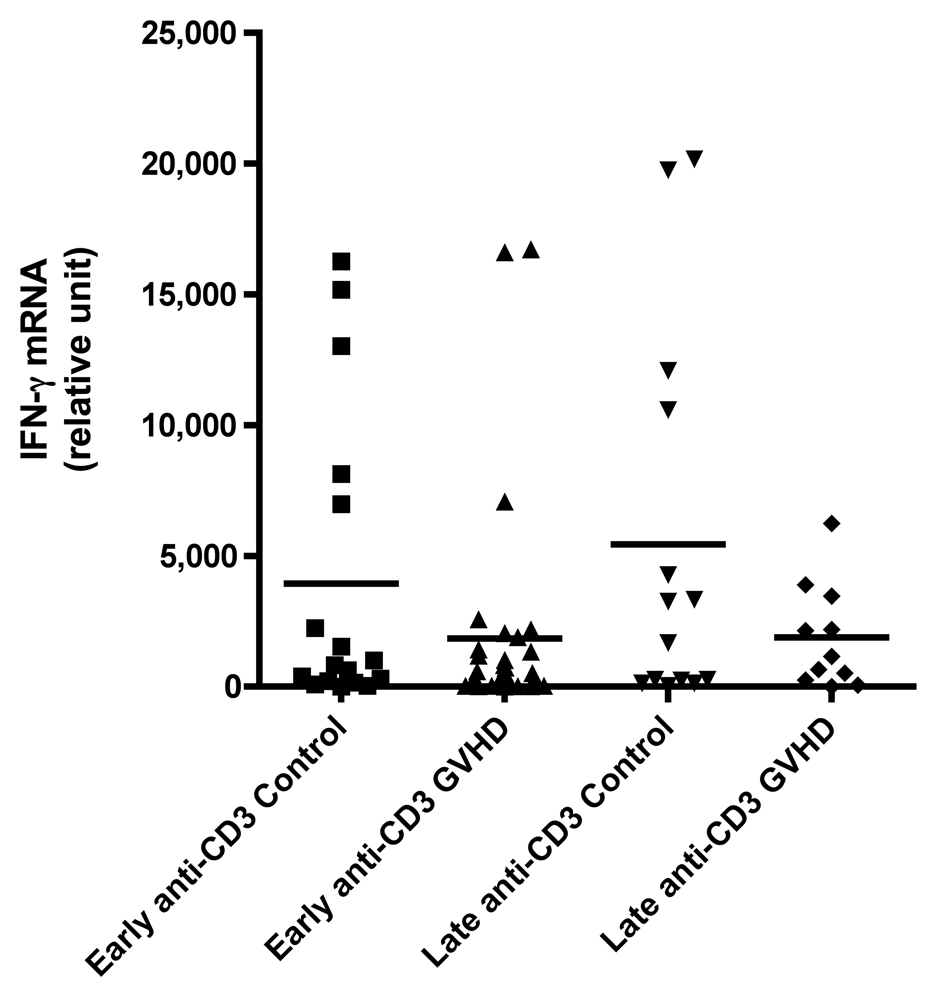
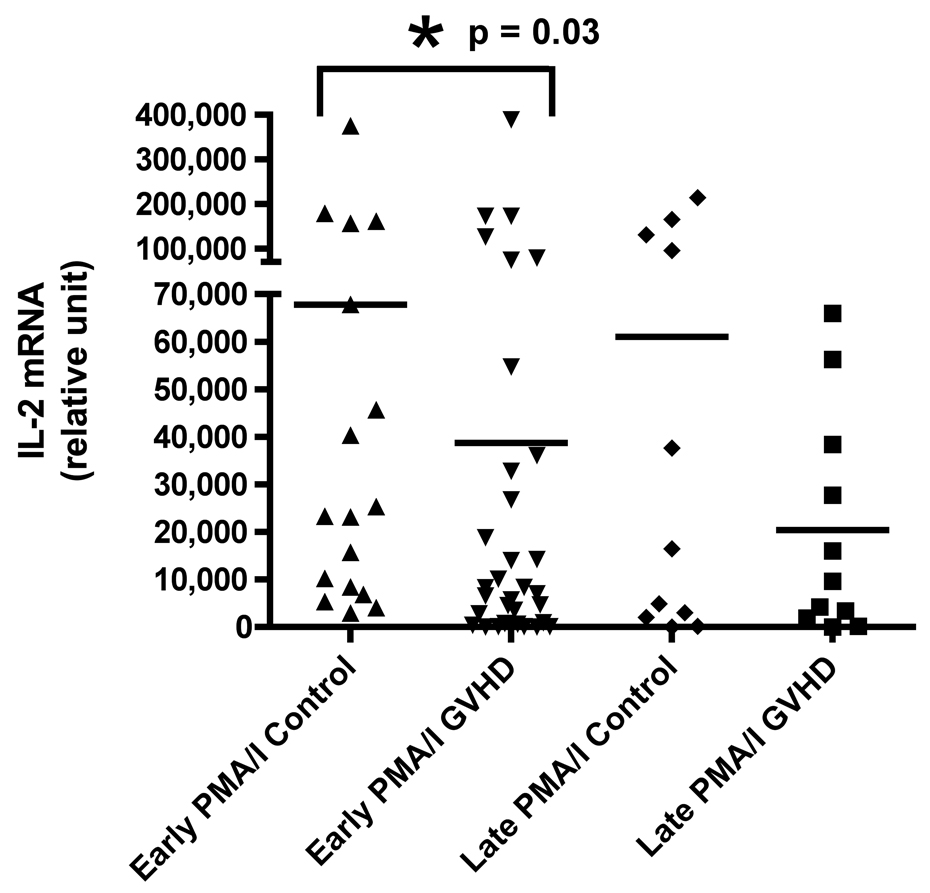
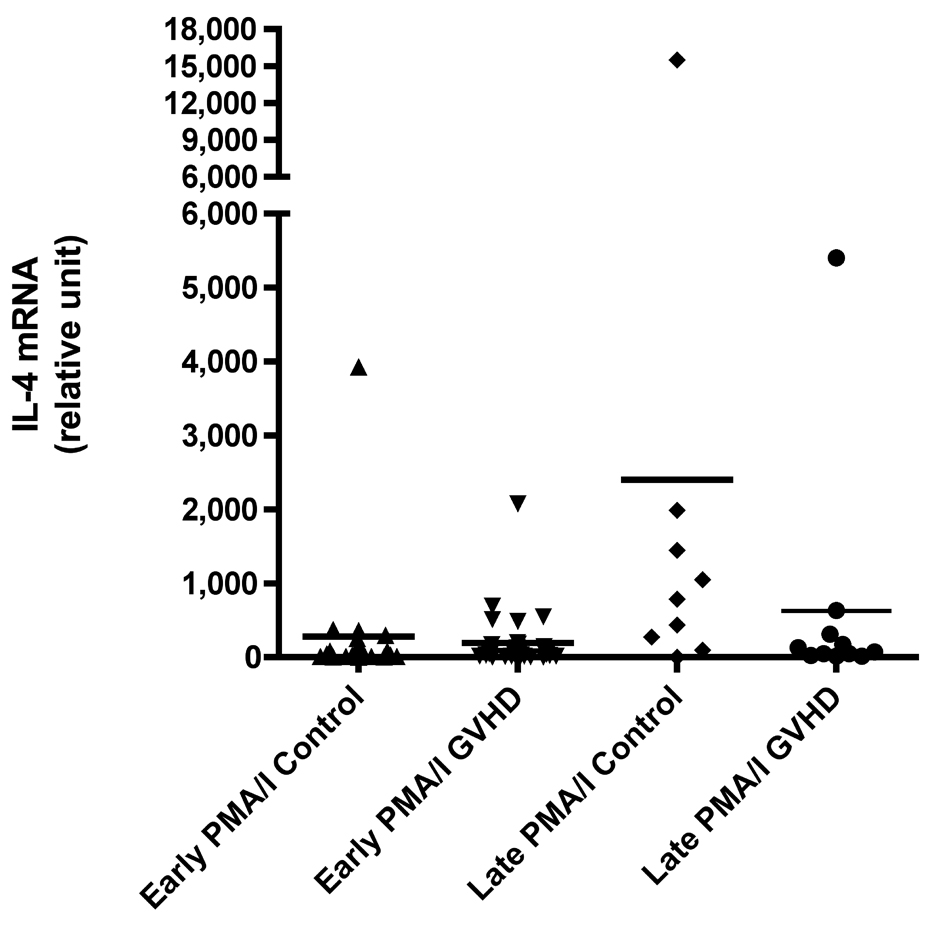
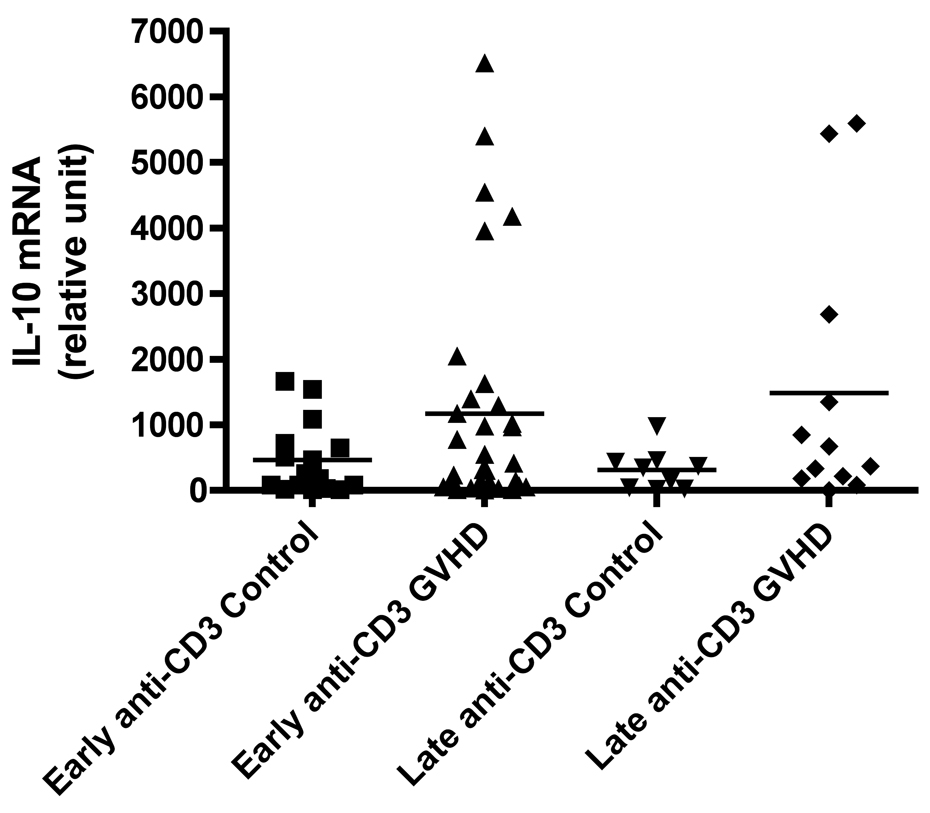
We evaluated cytokine mRNA expression from PBMCs after activation with a nonspecific mitogen, PMA/Ionomycin (PI), which stimulates all T cells, NK cells, monocytes, and B cells and compared these findings to cytokine expression after T cell-specific stimulation with anti-CD3 monoclonal antibody (mAb). We found a decreased production of IFN-γ in patients with early cGVHD versus early controls (Figure 1A, p = 0.002) after PI stimulation. There was no difference in IFN-γ production after anti-CD3 mAb stimulation (Figure 1B). Similarly, there was also a decreased IL-2 production in patients with early cGVHD versus early controls after PI stimulation (Figure 1C, p = 0.03) with no significant difference after anti-CD3 mAb stimulation. No differences were seen for either PI or anti-CD3 mAb stimulated IL-4 production in early cGVHD (Figure 1E and F). IL-10 production was only assessed after anti-CD3 mAb stimulation. There appeared to be a higher expression in patients with early cGVHD, but we could not show a statistical difference (Figure 1G, p = 0.12). A previous history of aGVHD did not have a significant effect on expression of all cytokines.
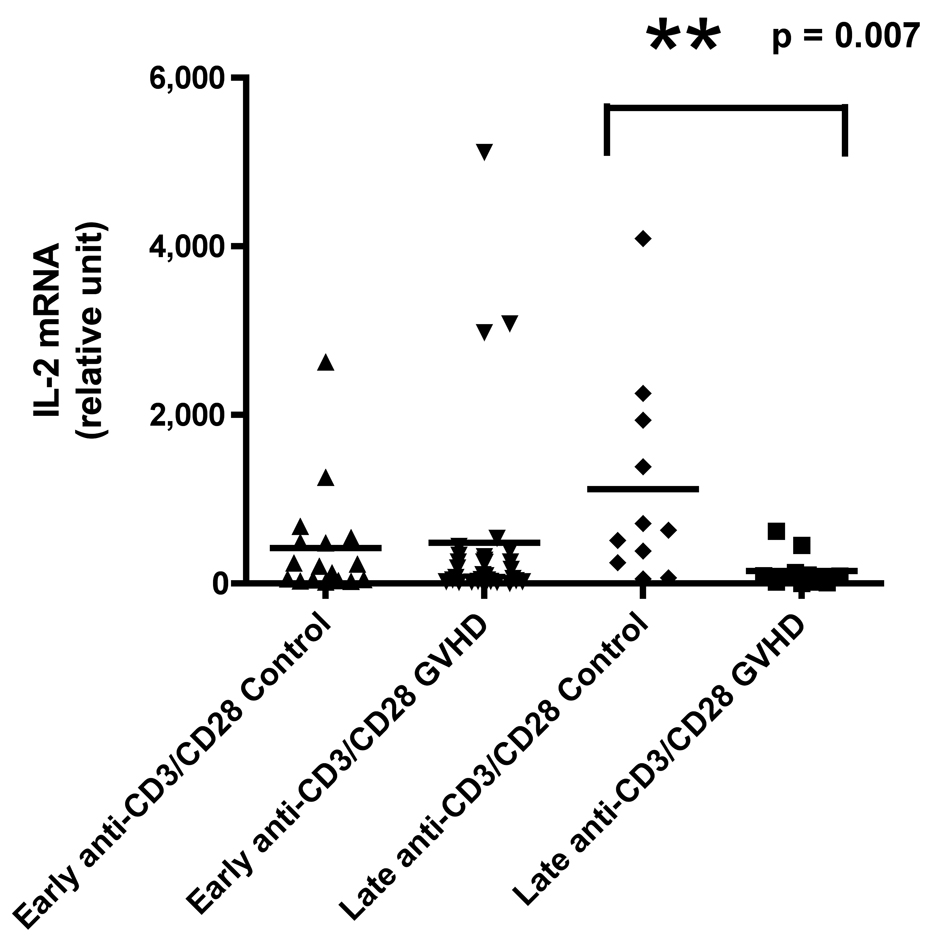
Figure 1.
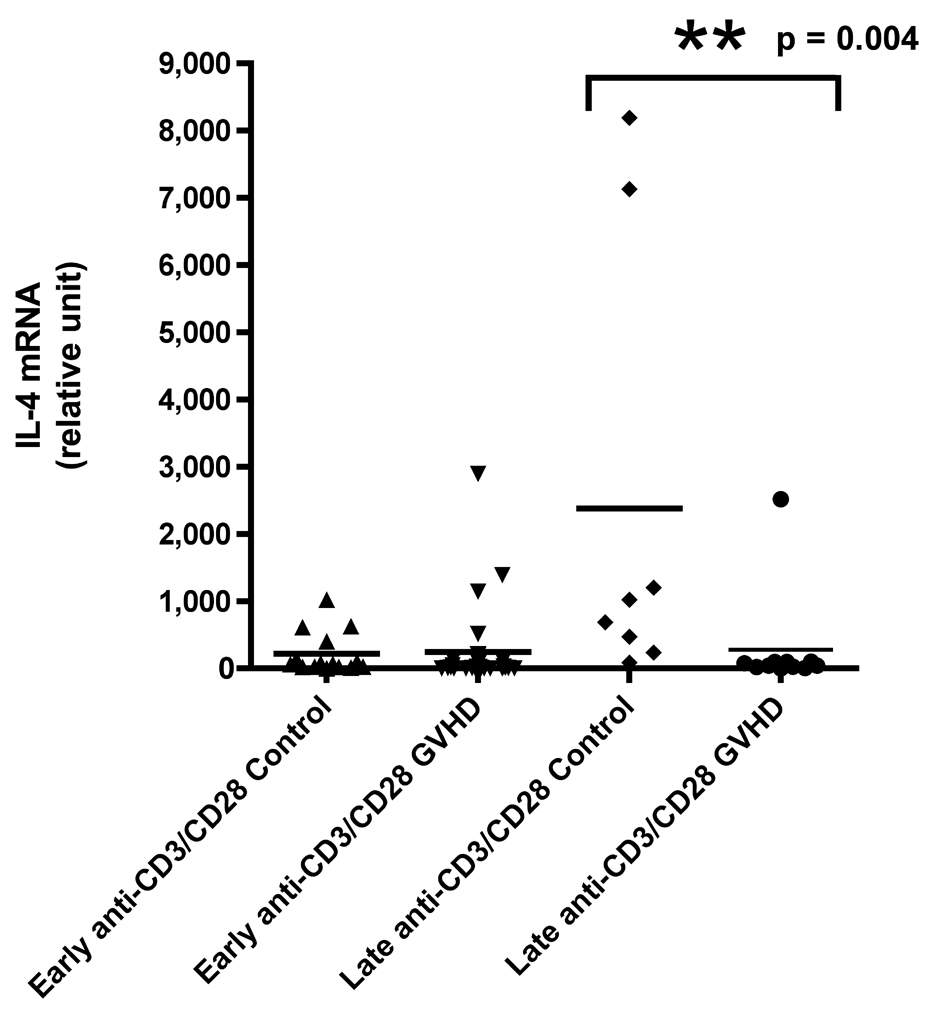
A) IFN-γ mRNA production at early and late timepoints in controls and patients with cGVHD after PI stimulation for 18 hours; B) IFN-γ mRNA production at early and late timepoints in controls and patients with cGVHD after anti-CD3 mAb stimulation for 18 hours; C) IL-2 mRNA production at early and late timepoints in controls and patients with cGVHD after PI stimulation for 18 hours; D) IL-2 mRNA production at early and late timepoints in controls and patients with cGVHD after anti-CD3 mAb stimulation for 18 hours; E) IL-4 mRNA production at early and late timepoints in controls and patients with cGVHD after PI stimulation for 18 hours; F) IL-4 mRNA production at early and late timepoints in controls and patients with cGVHD after anti-CD3 mAb stimulation for 18 hours; G) IL-10 mRNA production at early and late timepoints in controls and patients with cGVHD after anti-CD3 mAb stimulation for 18 hours.
IFNγ, IL-4, IL-2 and IL-10 Concentrations in Late Chronic GVHD
Neither PI nor anti-CD3 mAb stimulations resulted in a significant difference for IFN-γ in patients with late onset cGVHD versus controls (Figure 1A and B). IL-2 production, lower in patients with late cGVHD versus late controls after PI stimulation, only became significant after stimulation with anti-CD3 mAb (Figure 1D, p = 0.007). Similarly, there was decreased IL-4 production after PI stimulation in patients with late cGVHD versus late controls (Figure 1E, p = 0.07) although it was not significant. This difference did become significant after T-cell specific anti-CD3 mAb stimulation (Figure 1F, p = 0.004). IL-10 production was only assessed after anti-CD3 mAb stimulation. There appeared to be a higher expression in patients with late cGVHD, but we could not show a statistical difference (Figure 1G, p = 0.1). A previous history of aGVHD did not have a significant effect on expression of all cytokines.
Receiver Operating Characteristic (ROC) Analysis of Cytokine Levels to Predict the Absence of Chronic GVHD
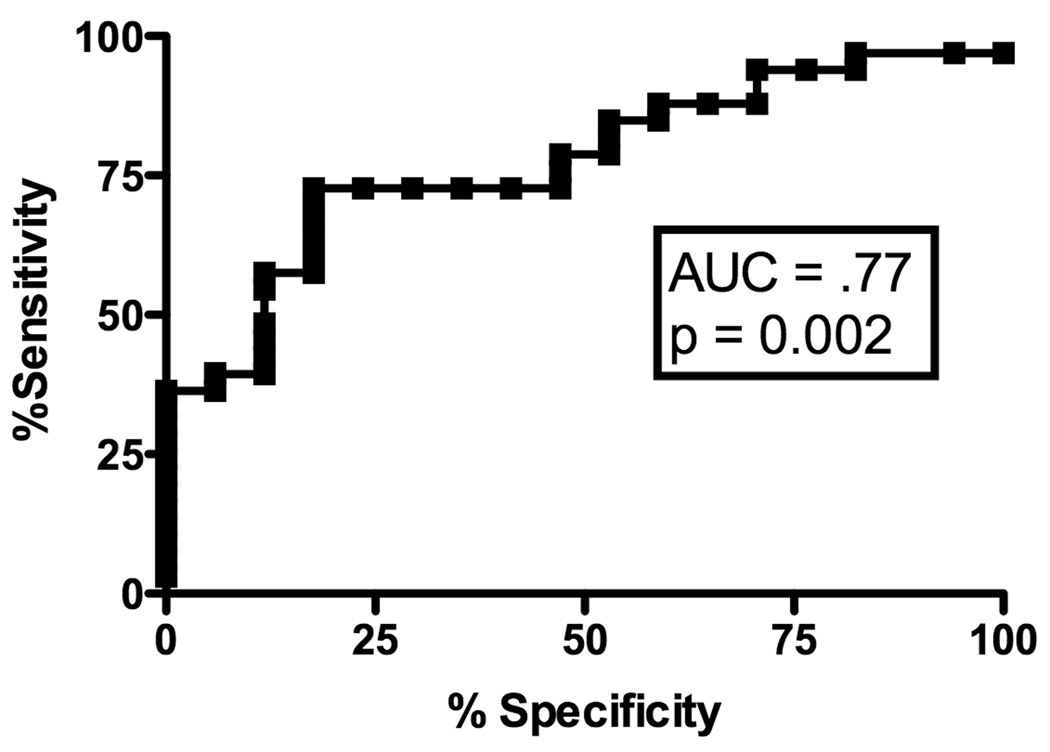
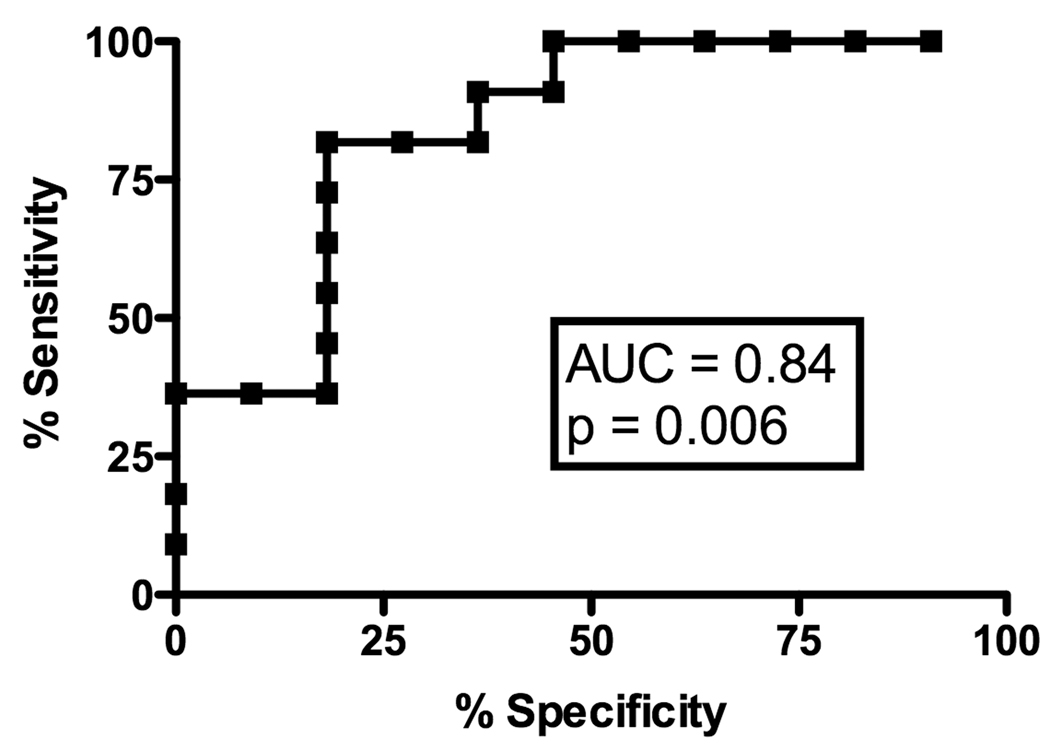
Receiver Operating Characteristic (ROC) curve analyses were used to explore if and at what level each of the cytokines mRNA might be useful in predicting the presence or absence of cGVHD. PI-induced IFN-γ production was potentially useful in predicting the absence of GVHD at the early time-point with an AUC of 0.77 (Figure 2A, p = 0.002), with a 80% specificity at a value of 20544 (sensitivity of 73%). Analysis of PI-induced IL-2 production at the early time-point did not yield a high enough AUC (AUC .69, p = 0.03) to be predictive. We found no significant association for either IFN-γ (AUC 0.62, p = 0.18) or IL-2 (AUC 0.57, p = 0.40) after T-cell specific stimulation with anti-CD3 mAb. IL-4 did not have any predictive value in the early time-point.
Figure 2.
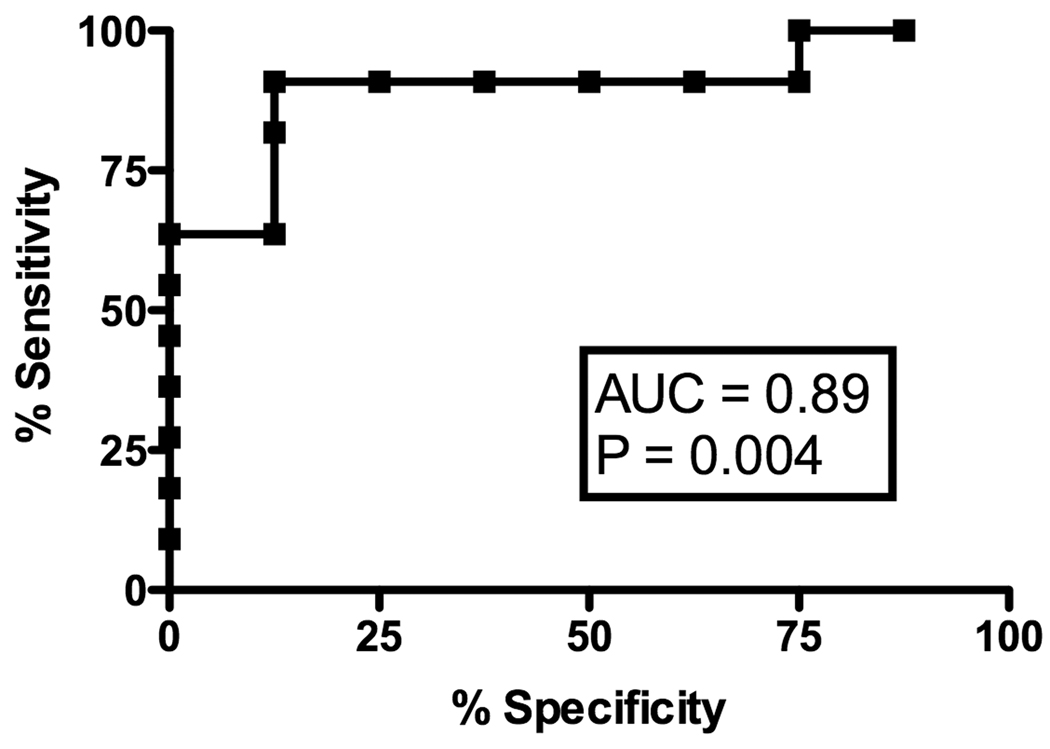
A) ROC curve analysis of IFN-γ mRNA production after PI stimulation for predicting the absence of early cGVHD; B) ROC curve analysis of IL-2 mRNA production after anti-CD3 stimulation for predicting the absence of late cGVHD; C) ROC curve analysis of IL-4 mRNA production after anti-CD3 stimulation for predicting the absence of late cGVHD.
At the late time-point, ROC analysis revealed a strong predictive value of anti-CD3-induced IL-4 production for absence of cGVHD (Figure 2B, AUC .89, p = 0.004) with 80% specificity at a value of 170.5 (sensitivity of 91%) as well as IL-2 production (Figure 2C, AUC .84, p = 0.006) with 80% specificity at a value of 189.5 (sensitivity of 82%) in the late cGVHD population. PI-induced IL-4 production (AUC .74, p = 0.06) was not significantly predictive of the absence of late cGVHD. Contrary to early cGVHD, significant IL-2 results were only obtained with T-cell specific anti-CD3 mAb stimulation.
Evaluation of Other Factors That May Impact on Cytokine Production
The cytokine patterns in early and late cGVHD did not correlate with the donor type (related or unrelated), source (cord blood, peripheral blood or marrow) or organ involvement by cGVHD (data not shown).
Foxp3 mRNA Concentrations in Early and Late Chronic GVHD
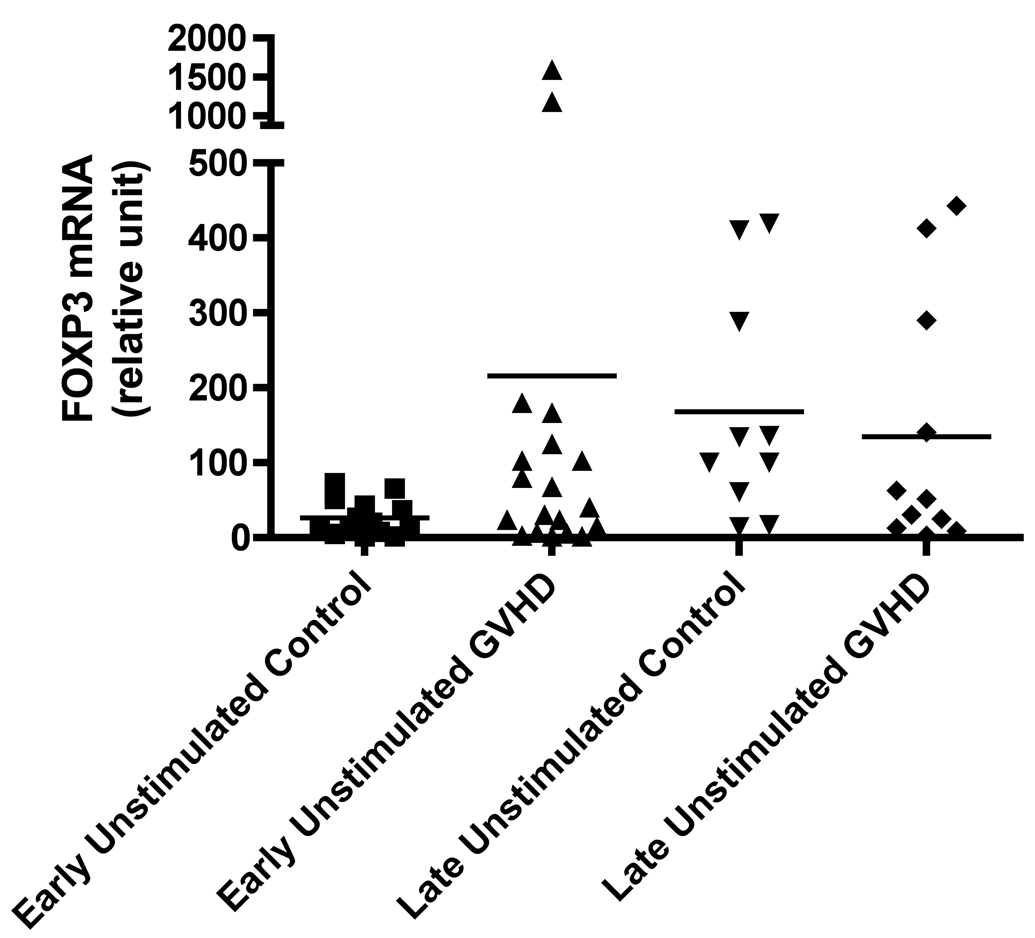
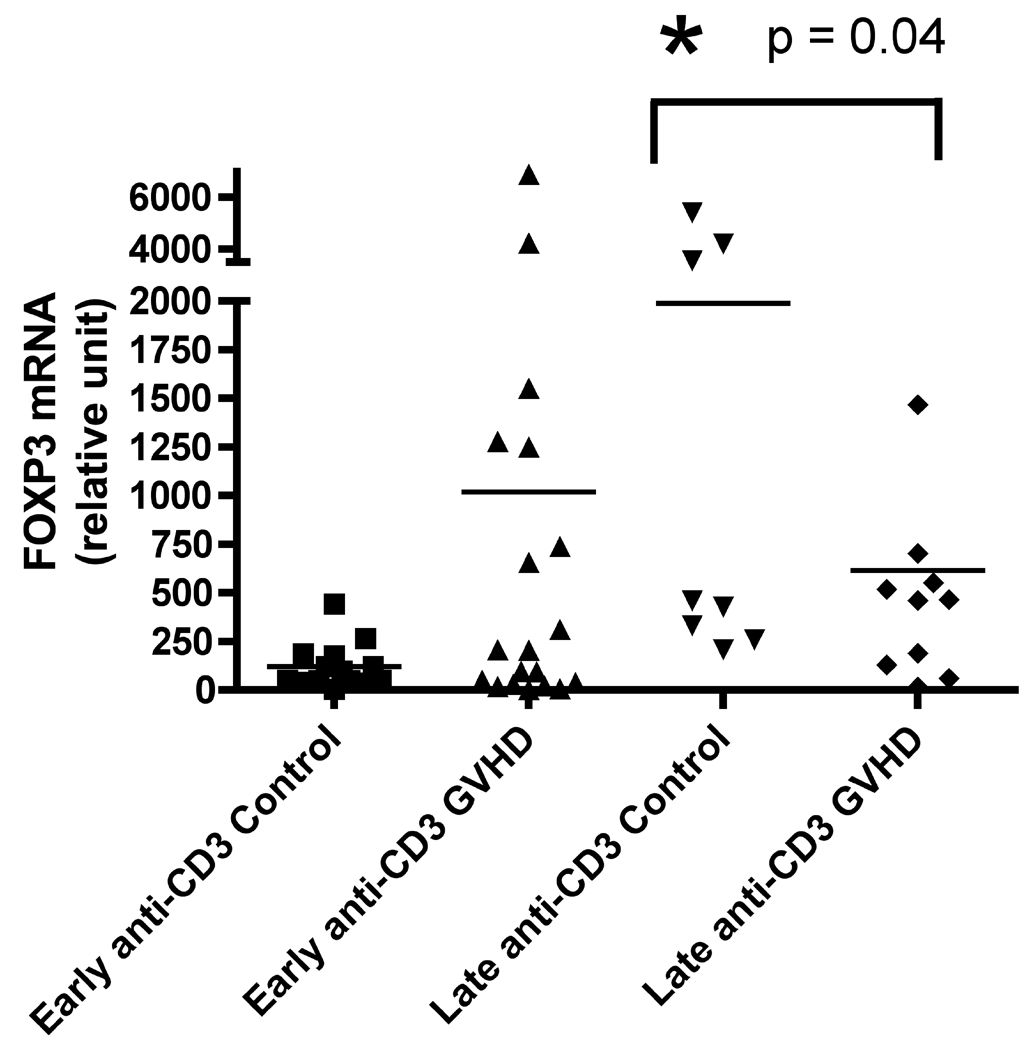
At the time of our retrospective analysis, there was no consistent Foxp3 antibody available for flow cytometry, therefore we measured mRNA expression in un-stimulated and anti-CD3 stimulated peripheral blood mononuclear cell samples. We found that Foxp3 mRNA expression was increased nearly 10-fold in patients with early onset cGVHD, although results did not reach significance in either the un-stimulated (Figure 3A, p = 0.08) or anti-CD3 stimulated (Figure 3B, p = 0.27) sets. Conversely, at the later time-point there was an increase in Foxp3 mRNA expression in control patients and this was significant in the anti-CD3 stimulated set (Figure 3B, p = 0.04).
Figure 3.
A) Foxp3 production at early and late timepoints in controls and patients with cGVHD in un-stimulated samples; B) Foxp3 production at early and late timepoints in controls and patients with cGVHD after anti-CD3 mAb stimulation for 18 hours.
Absolute Lymphocyte Count and Correlation of Immune Cell Subsets with Cytokine Production
We measured the absolute lymphocyte count (ALC) at the early and late time- point for all four combinations of acute (A) and chronic (C) GVHD: A-C-, A+C-, A-C+ and A+C+. At the early time-point, the highest ALC was in the A+C+ group with 2339 ± 3245/uL followed by A-C+ 2141 ± 1280/uL, A+C- 1586 ± 1122/uL and A-C- 1144 ± 477/uL. There was a statistically significant difference between the patients with or without cGVHD and no history of aGVHD (p=0.009). There were no significant differences between groups at the late time-point: A-C- 2013 ± 1669/uL, A+C- 2312 ± 1402/uL, A-C+ 2274 ± 3055/uL and A+C+ 2729 ± 2174/uL. There were no statistically significant differences in the percentages of cell phenotypes [T cells (CD3+, CD4+, CD4+CD57+, CD8+, CD8+CD57+), NK cells (CD3-CD16+CD56+), and B cells (CD19+)] evaluated between patients with cGVHD and controls at both time-points in un-stimulated samples. We also compared the percentages of activated T cell lymphocyte subsets (CD4+CD25+, CD8+CD25+, CD4+CD69+, CD8+CD69+, CD4+HLA-DR+, CD8+HLA-DR+) in un-stimulated versus anti-CD3 stimulated samples and found no significant differences. Furthermore, there was no correlation between mRNA expression of all cytokines and the percentage and absolute cell counts of anti-CD3 stimulated T cell lymphocytes evaluated at both time-points (data not shown).
Discussion
One of the most significant findings from the previously published COG ASCT0031 study is that there are immunological differences between cGVHD that occur early after BMT (3–8 months) compared to a later onset (≥9 months) [10] and that immune reconstitution post BMT can significantly affect the interpretation of cGVHD biomarkers. In the current analysis, the presence of early cGVHD was characterized by a decreased Th1 cytokine mRNA response while the presence of late cGVHD was characterized by a decreased Th2 cytokine mRNA profile. Despite the small number of patients, we were able to find significant patterns that can be subsequently validated in larger prospective clinical trials.
To better understand the immunopathogenesis of cGVHD, PBMCs isolated from patients with new onset cGVHD and time-matched controls were stimulated with a nonspecific lymphocyte mitogen, PI or T-cell specific anti-CD3 mAb. In early cGVHD patients, levels of IFN-γ and IL-2 mRNA expression were only significantly decreased after PI and not anti-CD3 mAb stimulation, suggesting that the initial effector cell populations may not be T cells. One possibility is that these effectors are NK cells, which are capable of producing inflammatory cytokines and mediate cytolytic activity [41]. The concept of an NK-mediated regulatory function is supported by the observation that a higher number of bone marrow NK cells is associated with a decreased incidence of cGVHD after HLA identical sibling bone marrow transplants in humans [42]. Recent findings indicate that this regulatory function can be mediated through dendritic cells (DCs). Through the production of IFNγ, NK cells can enhance the function of DCs, induce expression of costimulatory molecules and production of IL-12 and TNF-α, and skewthe T-cell stimulatory capacity of DCs toward Th1-type responses. It has been suggested that NK cells mediate reduction of cGVHD by direct lysis of T cells, inhibition of donor T cell proliferation, and induction of T cell apoptosis [43].
There is much less evidence suggesting a protective role for B cells in cGVHD. Effector B cells (‘Be1’ and ‘Be2’ cells) can secrete cytokines, such as IFN-γ, IL-12, IL-4 and IL-2, that reinforce and stabilize the cytokine profile of both effector Th1 and Th2 cells [44].
Our findings suggest that the ability to generate a Th1/Tc1 response (IFNγ and IL-2) early post-BMT protects against cGVHD. Unfortunately, we did not find any correlation between IFN-γ and IL-2 mRNA expression and the number of NK or B cells. Alimitation of this study was the lack of functional studies on immune cell subsets. It will be important in future trials to determine which immune cells are responsible for the production of our cytokines of interest.
At the later time point, the decrease in IL-4 mRNA expression in cGVHD patients supports the widely accepted hypothesis that a Th2 response is protective. In contrast to the earlier time point, there was significant elevation with both PI and anti-CD3-induced IL-4 production, suggesting the effector cell population may be a CD3+ T cell population. One promising candidate is the regulatory T cell, as previously described [21–23]. We also observed a significant decrease in IL-2 mRNA synthesis in early and late cGVHD patients. It is known that IL-2 plays a central role in Th2 differentiation [45]. IL-2 has also been shown to be necessary for the production and expansion of CD4+CD25+ T regulatory cells [46]. Mice deficient in either IL-2 or elements of its receptor develop a massive lymphoproliferative syndrome accompanied by severe utoimmunity [47–48]. IL-2 is necessary for B cell tolerance and its absence could contribute to the unchecked expansion of autoreactive B cells. A role for B cells in the pathophysiology of cGVHD has been suggested, and as part of this study we identified increased numbers of B cells, primed for a TLR9 response [49].. However, we did not find a difference in the absolute CD19+ B cell count between control and GVHD patients, though studies were limited by the lack of specific phenotype labelling (such as IgM memory B cells [50] or CD21 negative B cells [51]) and functional data.
In order to better address the potential role of regulatory T cells in cGVHD, we retrospectively measured Foxp3 mRNA expression from archived frozen cell lysates. During the initial design of the study, a proper Foxp3 antibody did not exist, and there was limited data on this cell type. At the early time-point, we were able to show a trend towards increased Foxp3 mRNA expression in anti-CD3 stimulated cell lysates from patients with cGVHD. In contrast, at the late time-point, an increase in Foxp3 mRNA expression in anti-CD3 stimulated cell lysates from patients without cGVHD was noted. While these results were not statistically significant, they could suggest the presence of different activated and/or regulatory T cell populations that are pathogenic at the early time-point and protective at the late time-point. We now know that Foxp3 expression is not uniquely associated with regulatory T cells [52, 53]. We can hypothesize that at the early time-point there is a pathological population of activated cytotoxic CD8+ T lymphocytes. Supporting this hypothesis, D’Asaro et al showed a correlation between the increase of the effector memory subset (TEMRA, CCR7-, CD45RA+) in CD8+ T cells and the occurrence of cGVHD in patients who underwent allogeneic peripheral blood stem cell transplantation [54]. Additionally, multiparous females have measurable levels of circulating HY, the male-specific minor H antigen, and specific CD8+ T-cytotoxic lymphocytes can be readily expanded in vitro. It is noteworthy that the use of multiparous females as donors significantly increases the risk for graft-versus-host disease [55].
The discovery of temporally regulated cytokine profiles in cGVHD has two potential applications that need further investigation. Firstly, it is possible that certain cytokine expression levels could serve as biomarkers in cGVHD. It is important that levels of expression of all the cytokines discovered in this study be applied prospectively to a new cohort of patients with cGVHD in order to be validated as biomarkers. This is currently underway. Cytokine expression can serve as a biomarker by: 1) providing another test to help confirm a diagnosis of cGVHD when there is uncertainty, 2) directing the choice of therapy, i.e. choosing a therapy that targets pathogenic immune subsets that are involved in the production of the cytokines and 3) determining whether a cGVHD patient is responding to therapy if cytokine levels change. Secondly, determining the cells of origin for these cytokines in future prospective studies will help us better understand the pathophysiology of cGVHD.
Combining our previous findings with the present results has led us to generate a tentative model in which T cell activation with sIL2Rα and sBAFF upregulation is part of early cGVHD and that IFNγ and IL-2 production is required to inhibit CD8+ T cell and B cell expansion and antibody production. Late onset cGVHD is characterized by a lack of Th2 shift and development of activated TLR9 high expressing B cells responding to upregulated BAFF expression from monocyte lineages. TLR9 activation in combination with sBAFF results in increased autoantibody production and makes these B cells potent antigen-presenting cells for T cells in the periphery. IL-4 down-regulates TLR9 transcripts and proteins in naïve B cells [56].
Data from this study is different than previous reports. The current study involved children and adolescents and it is unclear whether there are any immunological differences in cGVHD between these populations. Children have a lower incidence of cGVHD compared with adults. Zecca et al. [57] observed a cumulative probability of cGVHD of 27%, which is nearly half of the estimated probability in adults. This was even true for bone marrow stem cell sources, contesting the theory that the overall incidence of cGVHD in children is lower due to the increased usage of cord blood stem cells. It has so far been assumed mechanisms underlying cGVHD are the same in children and adults as their clinical manifestations and responses to therapy are similar. Similarly, laboratory features are comparable in studies involving both populations, including soluble BAFF [10,15].
One of the possible limitations of this study is that both cyclosporin A and tacrolimus can have a potent dose-dependent inhibitory effect on cytokine expression from lymphocytes after stimulation with PMA/I [58]. In the study by Puzik et al, the effect of calcium calmodulin inhibitors was dependent on donor age. In cord blood lymphocytes, the inhibitory effect was dose-dependent, while the function of adult lymphocytes was only impaired at high doses of both compounds. In our study, the lack of any statistical difference among the experimental groups in terms of GVHD prophylaxis and cord blood donors limits this confounding factor.
The cytokine expression results presented in this paper provide novel evidence that there are potentially different immune mechanisms underlying the timing of onset of cGVHD. Furthermore, the different cytokine profiles may explain the numerous contradictions in the literature regarding cytokine expression and immune dysregulation during cGVHD, particularly where the timing of disease onset is not taken into account.
Acknowledgments
We would like to thank Dr. Ruth Milner for her help with statistical analysis.
Financial Support: FDG, KRS and ALG R01CA84137 and KRS CIHR/Wyeth Clinical Research Chair in Transplantation. Research is also supported by the Chair’s Grant - U10 CA98543-08 and Statistics and Data Center Grant – U10 CA98413-08 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the NIH.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: All authors wish to disclose they do not have any existing or potential conflicts of interest.
References
- 1.Mehta P, Locatelli F, Stary J, et al. Bone marrow transplantation for inherited bone marrow failure syndromes. Pediatr Clin North Am. 2010;57(1):147–170. doi: 10.1016/j.pcl.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Gaziev J, Sodani P, Lucarelli G. Hematopoietic stem cell transplantation in thalassemia. Bone Marrow Transplant. 2008;42 Suppl 1:S41. doi: 10.1038/bmt.2008.112. [DOI] [PubMed] [Google Scholar]
- 3.Filipovich A. Hematopoietic cell transplantation for correction of primary immunodeficiencies. Bone Marrow Transplant. 2008;42 Suppl 1:S49–S52. doi: 10.1038/bmt.2008.121. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan KM, Muraro P, Tyndall A. Hematopoietic cell transplantation for autoimmune disease: updates from Europe and the United States. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S48–S56. doi: 10.1016/j.bbmt.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad VK, Kurtzberg J. Cord blood and bone marrow transplantation in inherited metabolic diseases: scientific basis, current status and future directions. Br J Haematol. 2010;148(3):356–372. doi: 10.1111/j.1365-2141.2009.07974.x. [DOI] [PubMed] [Google Scholar]
- 6.Przepiorka D, Anderlini P, Saliba R, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98(6):1695–1700. doi: 10.1182/blood.v98.6.1695. [DOI] [PubMed] [Google Scholar]
- 7.Fraser C, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108(8):2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora M, Burns LJ, Davies SM, et al. Chronic graft-versus-host disease: a prospective cohort study. Biol Blood Marrow Transplant. 2003;9(1):38–45. doi: 10.1053/bbmt.2003.50003. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii H, Cuvelier G, She K, et al. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children’s Oncology Group. Blood. 2008;111(6):3276–3285. doi: 10.1182/blood-2007-08-106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz KR, Paquet J, Bader S, et al. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16(2):289–295. [PubMed] [Google Scholar]
- 12.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wechalekar A, Cranfield T, Sinclair D, et al. Occurrence of autoantibodies in chronic graft vs. host disease after allogeneic stem cell transplantation. Clin Lab Haematol. 2005;27(4):247–249. doi: 10.1111/j.1365-2257.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 14.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34(3):389–396. doi: 10.1016/j.exphem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cousineau S, Belanger R, Perreault C. Characterization of plasma cell populations at autopsy after human allogeneic bone marrow transplantation. Am J Pathol. 1986;124(1):74–81. [PMC free article] [PubMed] [Google Scholar]
- 17.Greinix HT, Pohlreich D, Kouba M, et al. Elevated numbers of immature/transitional CD21- B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(2):208–219. doi: 10.1016/j.bbmt.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease wih anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9(8):505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 19.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host diease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40(30):273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann P, Ermann J, Edinger M, et al. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young KJ, DuTemple B, Phillips MJ, et al. Inhibition of graft-versus-host disease by double-negative regulatory T cells. J Immunol. 2003;171(1):134–141. doi: 10.4049/jimmunol.171.1.134. [DOI] [PubMed] [Google Scholar]
- 24.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104(7):2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 25.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark FJ, Gregg R, Piper K, et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood. 2004;103(6):2410–2416. doi: 10.1182/blood-2003-06-2073. [DOI] [PubMed] [Google Scholar]
- 27.Meignin V, Peffault de Latour R, Zuber J, et al. Numbers of Foxp3-expressing CD4+CD25high T cells do not correlate with the establishment of long-term tolerance after allogeneic stem cell transplantation. Exp Hematol. 2005;33(8):894–900. doi: 10.1016/j.exphem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita K, Choi U, Woltz PC, et al. Severe chronic graft-versus-host disease is characterized by a preponderance of CD4+ effector memory cells relative to central memory cells. Blood. 2004;103(10):3986–3988. doi: 10.1182/blood-2003-09-3286. [DOI] [PubMed] [Google Scholar]
- 29.Xystrakis E, Bernard I, Dejean AS, et al. Alloreactive CD4 T lymphocytes responsible for acute and chronic graft-versus-host disease are contained within the CD45RChigh but not the CD45RClow subset. Eur J Immunol. 2004;34(2):408–417. doi: 10.1002/eji.200324528. [DOI] [PubMed] [Google Scholar]
- 30.Biedermann BC, Sahner S, Gregor M, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359(9323) doi: 10.1016/S0140-6736(02)08907-9. 2078-8. [DOI] [PubMed] [Google Scholar]
- 31.Patey-Mariaud de Serre N, Reijasse D, Verkarre V, et al. Chronic intestinal graft-versus-host disease: clinical, histological and immunohistochemical analysis of 17 children. Bone Marrow Transplant. 2002;29(3):223–230. doi: 10.1038/sj.bmt.1703329. [DOI] [PubMed] [Google Scholar]
- 32.Kotani A, Ishikawa T, Matsumura Y, et al. Correlation of peripheral blood OX40+(CD134+) T cells with chronic graft-versus-host disease in patients who underwent allogeneic hematopoietic stem cell transplantation. Blood. 2001;98(10):3162–3164. doi: 10.1182/blood.v98.10.3162. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie D, Seconi J, Wood C, et al. Prospective monitoring of tumor necrosis factor alpha and interferon gamma to predict the onset of acute and chronic graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(9):706–712. doi: 10.1016/j.bbmt.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Ochs LA, Blazar BR, Roy J, et al. Cytokine expression in human cutaneous chronic graft-versus-host disease. Bone Marrow Transplant. 1996;17(6):1085–1092. [PubMed] [Google Scholar]
- 35.Bogunia-Kubik K, Mlynarczewska A, Wysoczanska B, et al. Recipient interferon-gamma 3/3 genotype contributes to the development of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Haematologica. 2005;90(3):425–426. [PubMed] [Google Scholar]
- 36.Baker J, Verneris MR, Ito M, et al. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97(10):2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 37.Haraguchi K, Takahashi T, Matsumoto A, et al. Host-residual invariant NK T cells attenuate graft-versus-host immunity. J Immunol. 2005;175(2):1320–1328. doi: 10.4049/jimmunol.175.2.1320. [DOI] [PubMed] [Google Scholar]
- 38.Pashankar F, Singhal V, Akabogu I, et al. Intact T cell responses in ataxia telangiectasia. Clin Immunol. 2006;120(2):156–162. doi: 10.1016/j.clim.2006.04.568. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Kinet S, Bernard F, Mongellaz C, et al. gp120-mediated induction of the MAPK cascade is dependent on the activation state of CD4(+) lymphocytes. Blood. 2002;100(7):2546–2553. doi: 10.1182/blood-2002-03-0819. [DOI] [PubMed] [Google Scholar]
- 41.Rivas MN, Hazzan M, Weatherly K, et al. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol. 2010;184(12):6790–6798. doi: 10.4049/jimmunol.0902598. [DOI] [PubMed] [Google Scholar]
- 42.Larghero J, Rocha V, Porcher A, et al. Association of bone marrow natural killer cell dose with neutrophil recovery and chronic graft-versus-host disease after HLA identical sibling bone marrow transplants. Br J Haematol. 2007;138(1):101–109. doi: 10.1111/j.1365-2141.2007.06623.x. [DOI] [PubMed] [Google Scholar]
- 43.Olson JA, Leveson-Gower DB, Gill S, et al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effect. Blood. 2010;115(21):4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10(4):236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cote-Sierra J, Foucras G, Guo L, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci USA. 2004;101(11):3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74(6):961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 47.Wrenshall LE, Smith DR, Stevens ET, et al. Influence of interleukin-2 deficiency on the generation of autoimmune B cells. J Autoimmun. 2007;29(2–3):125–133. doi: 10.1016/j.jaut.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willerford DM, Chen J, Ferry J, et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 2005;3(4):521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 49.She K, Gilman AL, Aslanian S, et al. Altered Toll-like receptor 9 responses in circulating B cells at the onset of extensive chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(4):386–397. doi: 10.1016/j.bbmt.2006.12.441. [DOI] [PubMed] [Google Scholar]
- 50.D’Orsogna LJ, Wright MP, Krueger RG, et al. Allogeneic hematopoietic stem cell transplantation recipients have defects of both switched and IgM memory B cells. Biol Blood Marrow Transplant. 2009;15(7):795–803. doi: 10.1016/j.bbmt.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 51.Greinix HT, Pohlreich D, Kouba M, et al. Elevated numbers of immature/transitional CD21- B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(2):208–219. doi: 10.1016/j.bbmt.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Ioan-Facsinay A, van der Voort El, et al. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37(1):129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 53.Broady R, Yu J, Levings MK. ATG-induced expression of FOXP3 in human CD4(+)T cells in vitro is associated with T-cell activation and not the induction of FOXP3(+) T regulatory cells. Blood. 2009;114(24):5003–5006. doi: 10.1182/blood-2009-04-214437. [DOI] [PubMed] [Google Scholar]
- 54.D’Asaro M, Dieli F, Caccamo N, et al. Increase of CCR7- CD45RA+ CD8 T cells (T(EMRA)) in chronic graft-versus-host-disease. Leukemia. 2006;20(3):545–547. doi: 10.1038/sj.leu.2404079. [DOI] [PubMed] [Google Scholar]
- 55.James E, Chai JG, Dewchand H, et al. Multiparity induces priming to male-specific minor histocompatiblity antigen, HY, in mice and humans. Blood. 2003;102(1):388–393. doi: 10.1182/blood-2002-10-3170. [DOI] [PubMed] [Google Scholar]
- 56.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173(7):4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 57.Zecca M, Prete A, Rondelli R, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100(4):1192–1200. doi: 10.1182/blood-2001-11-0059. [DOI] [PubMed] [Google Scholar]
- 58.Puzik A, Schultz C, Iblher P, et al. Effects of ciclosporin A, tacrolimus and sirolimus on cytokine production in neonatal immune cells. Acta Paediatr. 2007;96(10):1483–1489. doi: 10.1111/j.1651-2227.2007.00484.x. [DOI] [PubMed] [Google Scholar]