Abstract
Alzheimer's disease (AD) is the 6th leading cause of death in United States afflicting >5 million Americans. This number is estimated to triple by the middle of the century if effective treatments are not discovered. Current therapy for AD is mainly symptomatic. Effective disease-modifying treatments are needed that would eliminate the cause rather than the symptoms of the disease. Polymerization of monomeric beta-amyloid peptide (Aß) into dimers, soluble oligomers and insoluble fibrils is considered the prime causative factor in triggering AD pathogenesis. Based on these facts, removal/reduction of Aß has gained importance as a primary therapeutic target in treating the cause of the disease. In that regard, passive immunotherapy with direct delivery of anti-Aß antibodies to the brain has shown great promise, but awaits the challenge of overcoming greater influx of anti-Aß antibody into the brain. This investigation was undertaken to maximize direct delivery of immunotherapeutics to the brain by using Wheat Germ Agglutinin (WGA) as a novel axonal transporter-carrier to be conjugated with anti-Aß antibody (6E10) raised against EFRHDS 3-8 amino acid (aa) epitopes of Aß known to react with 1-16 aa residues of mono-/di-/oligomeric Aß. This is the first report showing the use of WGA as an efficient axonal transporter carrier that not only enhanced the influx of anti-Aß antibody directly into the brain but also resulted in greater reduction of cerebral Aß compared to the unconjugated anti-Aß antibody delivered intranasally in Alzheimer's 5XFAD model.
Keywords: Alzheimer's disease, Intranasal passive immunization, Wheat germ agglutinin, Olfactory sensory neurons, Endocytic uptake, Anterograde axonal transport
Introduction
Alzheimer's disease (AD) is an age-dependent progressive neurodegenerative disorder functionally characterized by mild cognitive impairment (MCI) at its onset leading to subsequent cognitive decline; and pathologically characterized by the deposition of ß-amyloid (Aß) neuritic plaques (NP) derived from ß-amyloid precursor protein (APP), and deposition of neurofibrillary tangles (NFTs) resulting from abnormal phosphorylation of tau proteins within the brain parenchyma [1, 2]. Since formation of Aß is considered the key causative seeding event in Alzheimer's pathogenesis that produces neurotoxicity, synaptic degeneration, neuroinflammation, and tau phosphorylation, with concomitant cognitive deficits [3-7], removal/reduction of Aß has been explored as the prime therapeutic target in Alzheimer's pre-clinical research. In that regard, immunotherapeutic strategies have shown great progress and promise over the past few decades. Antibodies to Aß derived from active or passive immunization showed reduction of cerebral Aß and improvement in cognitive deficits [8-14]. Although partially successful, all immunization strategies explored this far are posed with various limitations. By and large, passive immunization using anti-Aß antibodies delivered directly to the brain have shown greater benefits. More specifically, selection of antibody and facilitation of greater influx of antibody into the brain are critical in advancing immunotherapy for Alzheimer's disease.
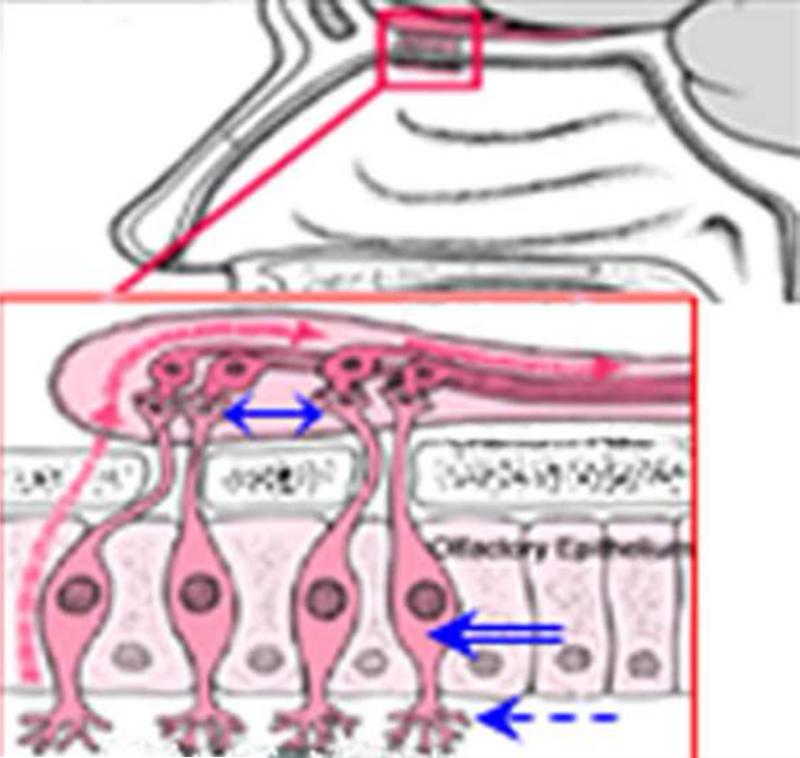
Intranasal route is largely considered as a non-invasive, simple and practical route for the delivery of therapeutics to the central nervous system (CNS) that can bypasses the blood brain barrier (BBB) and systemic adversities. The unique anatomic and physiologic characteristics of nasal mucosa such as the large surface area available for drug absorption and close proximity to CNS and CSF [15-18] facilitate drug uptake despite minor limitations posed by nasal milieu itself, i.e. exo-/endo-peptidase(s)-mediated degradation of drugs or mucociliary clearance [16, 18]. The olfactory epithelium is located just below the cribriform plate separating the nasal cavity from the cranial cavity (Fig. 1). Besides olfactory supporting cells and basal cells, the olfactory epithelium contains olfactory sensory bipolar neurons (OSNs) (Fig. 1, blue double-lined arrow) with a single dendritic process bearing non-motile cilia (Fig. 1, blue dotted arrow), and non-myelinated axons that connect with neighboring axons forming a bundle surrounded by glial cells penetrating into the cranial cavity through small holes in the cribriform plate (Fig. 1, blue two-sided arrow) [16] which merge with the afferent axons connected to the olfactory tracts of the olfactory bulb. Thus, OSNs congregate directly with the CNS.
Fig. 1.
Schema showing the intranasal route of transfer of materials to the brain. Pink outlined inlet showing olfactory epithelium located just below the cribriform plate separating the nasal cavity from the cranial cavity. The olfactory epithelium contains olfactory supporting cells, basal cells, and olfactory sensory bipolar neurons (OSNs) (blue double-lined arrow) with a single dendritic process bearing non-motile cilia (blue dotted arrow), and non-myelinated axons that connect with neighboring axons forming a bundle surrounded by glial cells penetrating into the cranial cavity through small holes in the cribriform plate (blue two-sided arrow). A pink dotted arrow indicates receptor-mediated endocytosis into the OSNs followed by intracellular transport to olfactory bulb.
Intranasal administration conventionally utilizes 3 potential pathways to reach CNS [19]: (i) Adsorptive or receptor-mediated endocytosis into the OSNs followed by intracellular transport to the olfactory bulb (Fig. 1, pink dotted arrow); (ii) Non-specific fluid phase endocytosis into the OSNs followed by intracellular transport to olfactory bulb (Fig. 1, pink dotted arrow); (iii) Extracellular diffusion along the open inter-olfactory clefts directly to the olfactory bulb/subarachnoid space/CSF. The intranasal route has been used to deliver neurotrophic factors [19, 20], cytokines [21], neuropeptides [22], and antibodies [23, 24] to CNS. Enhancing intranasal delivery of therapeutics includes conjugation of candidate drugs with microspheres, liposomes, chitosan, cytodextrins, bile salts/surfactants and lectins [18]. Among all, the lectin Wheat Germ Agglutinin (WGA) is unique in that it contains N-acetyl glucosamine and sialic acid, both of which are abundant in nasal mucosa, reducing the chances for self-rejection/removal by nasal mucosa [22, 25]. Most importantly, WGA has a preferred selectivity for adsorptive endocytic uptake by OSN anterograde axonal transport to the olfactory bulb [19, 26]. Thus, conjugation of a candidate drug with WGA is expected to enhance intranasal delivery of therapeutics to the brain both by enhancing active endocytic uptake and passive diffusion.
Despite rigorous pre-clinical immunization approaches explored in the models of AD, there are additional challenges to be met in order to improve/advance immunotherapy. Considering the potential toxicity produced by mono/di/oligomeric Aß, the immunotherapy that would target monomers, dimers, and oligomers of Aß while simultaneously enhancing influx of immunotherapeutics into the brain, is expected to overcome most limitations of passive immunotherapy for AD. With this consideration, we chose anti-Aß antibody 6E10, that is raised against EFRHDS 3-8 amino acid (aa) epitopes of Aß, and is know to react with 1-16 aa residues of mono-/di-/oligomeric Aß. Furthermore, in order to increase the influx of anti-Aß antibody, we tested if conjugation of anti-Aß antibody (6E10) with the unique axonal transporter carrier plant lectin-WGA [19, 26] will enhance influx of intranasally delivered anti-Aß antibody (6E10) into the brain.
This study investigated if conjugation of anti-Aß antibody (6E10) with WGA will enhance cerebral uptake of anti-Aß antibody and will efficiently reduce cerebral Aß levels after intranasal delivery in 5XFAD mice, as evaluated by binding ability of WGA labeled anti-Aß antibody to native Aß plaques and by measuring of histological and biochemical profiles of cerebral Aß.
Materials and Methods
The 5XFAD mice harboring mutations APP K670N/M671L + I716V + V717I and PS1 M146L + L286V, were bred by crossing 5XFAD heterozygous male(s) (original founder breeder males obtained from Dr. Vassar, Northwestern University, Chicago, IL), with B6/SJL F1 females (Jackson labs, Bar harbor, ME) [27]. Presence of transgene was identified by PCR genotyping of tail genomic DNA with specific forward and reverse primers (Eurofins Operon, Fermentas Life Sciences). Since this study involved examination of binding of WGA-labeled anti-Aß antibody to the cerebral Aß plaques, only transgenic mice (Tgs) were included in the study.
Transgenic mice (Tgs) were divided into 4 groups (N=5/Group), each group intranasally administered with horse radish peroxidase (HRP)-labeled: (1) non-immune immunoglobulin (IgG) only (Covance) (Group 1: HRP+IgG); (2) WGA only (Group 2: HRP+WGA); (3) Anti-Aß antibody only (6E10, Covance) (Group 3: HRP+anti-Aß antibody); (4) and WGA labeled anti-Aß antibody (6E10, Covance) (Group 4: HRP+WGA+anti-Aß antibody). Tagging IgG or anti-Aß antibody either with WGA or HRP, separately or together, was performed commercially (NOVUS Biologicals, LLC). Mice were administered with a total dose of 40ug/mouse/week [(10μg/5μl/naris) = (20μg/mouse), 2x week =40μg/mouse, on Day1 & Day3/week]. All mice were euthanized at the end of the week on Day 7. Brains were harvested after decapitation under mild sedation and processed for frozen sectioning. The sections were subjected to standard diaminobenzidine chromogen development procedure to reveal HRP label [28]. No nuclear counter stain was used. Sections were subjectively observed by 3 independent investigators. Images were captured with the use of ImagePro and the densitometric or area measurements were performed with the use of NIH/ImageJ software. Total number and diameter of cortical Aß plaques within 10 high power fields (hpfs) (200μm2/each hpf) bilaterally per animal with a total of 50 hpfs per group (N=5/group) were analyzed.
Another set of experiment was conducted to measure cerebral levels of Aß40/42 with the use of ELISA. Transgenic mice (Tgs) were divided into 4 groups (N=5/Group), each group intranasally administered with: (1) non-immune immunoglobulin (IgG) (Covance) alone (Group 1: IgG); (2) WGA alone (Group 2: WGA); (3) Anti-Aß antibody (6E10, Covance) (Group 3: anti-Aß antibody); (4) WGA labeled anti-Aß antibody (6E10, Covance) (Group 4: WGA+anti-Aß antibody). Mice were administered with a total dose of 40μg/mouse/week [(10μg/5μl/naris) = (20μg/mouse), 2x week =40μg/mouse, on Day1 & Day3/week]. All mice were euthanized on Day 7at the end of the week.
All animal procedures were performed in accordance with the Jesse Brown VA Medical Center institutional Animal Care and Use Committee approval, National Institute of Health Guide for the Care and Use of Laboratory Animals, and policy and guidance of the Society for Neuroscience.
Brains were harvested after decapitation under mild sedation, homogenized in the modified RIPA buffer (85mM HEPES, 250mM NaCl, 5 mM MgCl2, 10mM EDTA, 5mM NaF, 5mM Na Pyrophosphate, 1mM PMSF, 1%Triton X100, 10% Glycerol) containing 1x Protease & Phosphatase inhibitor cocktail (Sigma), and centrifuged at 14,000 RPM at 4°C. Supernatant was collected (P1 Fraction) and the resulting pellet was subjected to Formic acid extraction (P2 fraction). Total protein content of P1 and P2 fractions was determined using Bradford Protein assay. An aliquot of 100μg/100μl from each sample was used for ELISA measurement of soluble (s) Aß40/42 forms (P1 fraction) and fibrillar (f) Aß40/42 forms of cerebral amyloid using commercial kit (Covance). Briefly, the 96-well plates (Nunc) were pre-coated with the respective capture antibody (1:500) for 24 h at 4°C, followed by incubation with 100 μl of sample containing 100 μg of total protein or standards [100-1000 pg of purified synthetic peptides (BioSource) dissolved in 100 μl of PBS, pH 7.4], for 90 min at 37°C; followed by incubation with the respective reporter antibody for 90 min at 37°C. After 3 washes, 100 μl of anti-mouse IgG-HRP or anti-rabbit IgG-HRP conjugate (1:200) (Molecular Probes) was added to each well, incubated for 90 min followed by incubation with 3,3’,5,5’-tetramethyl benzidine dihydrochloride (TMB) and hydrogen peroxide as a substrate (1:1) for 30 min. The reaction was stopped by the addition of 100 μl of 0.5N H2SO4. The absorbance was read at 450 nm using ELISA reader (Molecular Devices). Values were normalized with the standard curve generated using custom synthetic peptides [Aß1-40 peptide (03-136); Aß1-42 peptide (03-111) (Covance)]. All samples were analyzed in triplicates and used to obtain individual average value(s).
Data were statistically analyzed with the use of GraphPad Prism (V 4.0). The individual average values were subjected to column statistics to obtain respective group means with standard deviation (SD). The data were further subjected to omnibus analysis of variance (ANOVA) to determine if there was a main effect of the treatment across the groups, followed by Tukey post hoc test for comparisons between control and experimental groups. A value of p<0.05 was considered statistically significant.
Results
Binding Pattern of intranasally delivered anti-Aß antibody (6E10) with or without WGA Conjugation
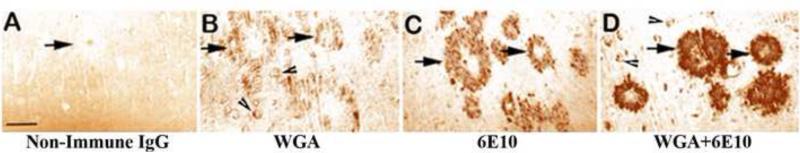
Results imply that the Tg mice administered with HRP+IgG showed non-specific diffuse background staining within the brain parenchyma, while no HRP label was revealed within the Aß plaques or neuronal perikarya per se. As seen in Fig. 2A, the Aß plaques remained unlabeled, but still identifiable (Fig. 2A, arrow). Tg mice administered with HRP-labeled WGA (HRP+WGA) showed diffuse binding of HRP label within the brain parenchyma including Aß plaques (Fig. 2B, arrows). Interestingly, this group showed very distinct labeling of neuronal perikarya (Fig. 2B, arrowheads). Tg mice administered with HRP-labeled anti-Aß antibody (HRP+anti-Aß antibody) indicated binding of HRP label to Aß plaques within the brain parenchyma (Fig. 2C, arrows). This group did not show neuronal perikaryal labeling. Among all the experimental groups, the group that received intranasal administration of HRP+WGA+anti-Aß antibody, exhibited very strong label of HRP bound to the native Aß plaques within the brain parenchyma (Fig. 2D, arrows). In addition, this group also showed distinct labeling of neuronal perikarya (Fig. 2D, arrowheads).
Fig. 2.
Representative photomicrographs showing the distribution of horse radish peroxidase (HRP) label in the transgenic mice intranasally administered with: (A) HRP+non-immune globulin (IgG), (B) HRP+Wheat germ agglutinin (WGA), (C) HRP+Anti-Aß antibody, (D) HRP+WGA+Anti-Aß antibody. Note diffuse non-specific binding of IgG within the brain parenchyma and Aß plaques without any label in (A). Note moderate labeling of WGA within the Aß plaques (arrows) but distinct labeling in neuronal perikarya (arrowheads) in (B). Note moderate labeling of Aß plaques but no perikaryal staining in (C). Note robust labeling of Aß plaques and distinct labeling of neuronal perikarya in (D). Scale bar = 100 μm.
These results clearly indicate that: (1) Within a week's time, WGA-antibody complex was able to reach cerebral cortex and maximally bind to amyloid plaques compared to the groups that received stand-alone HRP+WGA or HRP+anti-Aß antibody; (2) Conjugation of WGA drastically increased the ability of anti-Aß antibody influx into the brain and its binding to cerebral amyloid plaques, in addition to facilitating intraneuronal access.
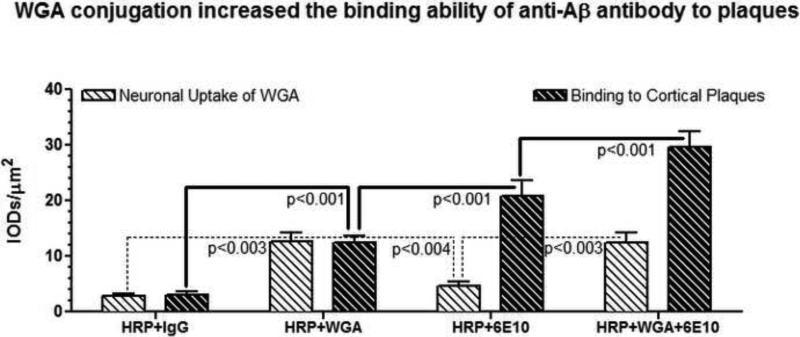
As shown in Fig. 3, densitometric quantitation of HRP binding among various experimental groups revealed that the control group which received HRP-labeled non-immune globulin (IgG) showed a diffuse reaction within the brain parenchyma indicating binding of non-immune globulin to non-specific IgG epitopes. This value served as the baseline background correction.
Fig. 3.
Densitometric quantitation of HRP label density within the cortical pyramidal neurons and cortical plaques represented as Mean and standard deviation (SD) derived from a total of 200 cerebral plaques and a total of 200 cortical pyramidal neurons averaged from (N=5/group). Note that WGA label is prominent within the neurons but not the plaques, and that the WGA+Anti-Aß Antibody combination revealed maximal labeling both within the cortical plaques and neurons.
WGA uptake in cortical pyramidal neurons of WGA-administered group (HRP+WGA) was 4.3-fold greater than the HRP+IgG group (p<0.003), and 2.8-fold greater than the HRP+ anti-Aß antibody (6E10) group (p<0.004) that did not receive WGA at all (Figs. 2 & 3). On the other hand, there was no significant difference with regard to WGA uptake in cortical pyramidal neurons of both WGA-administered groups i.e. HRP+WGA vs HRP+WGA+ anti-Aß antibody (6E10) (NS, p>0.05) (Figs. 2 & 3). Interestingly, WGA also diffusely labeled cortical plaques.
With regard to the binding of HRP label to cortical plaques, it was observed that the group administered with non-specific IgG (HRP+IgG) did not show any label in the plaques. The group that received HRP+WGA showed 4.0-fold increased plaque labeling compared to HRP+IgG group (p<0.001) (Figs. 2 & 3). Compared to HRP+WGA group, the group that received HRP-labeled anti-Aß antibody (6E10), exhibited 1.7-fold increased punctuate labeling of cortical neuronal plaques (p<0.001) (Figs. 2 & 3). Furthermore, the group that received WGA conjugated anti-Aß antibody i.e. (HRP+WGA+anti-Aß antibody (6E10) showed ~6-fold increase in the HRP density compared to (HRP+IgG) group, which translated to a 1.4-fold of additional increase in the HRP density of plaque label compared to HRP+ anti-Aß antibody (6E10) group (p<0.001), indicating maximally labeled cortical plaques in this group (Figs. 2 & 3).
These results indicate that: (1) The group that received WGA+HRP+ anti-Aß antibody exhibited maximum ability to reach cerebral cortex and showed maximal binding of WGA-antibody complex to Aß plaques compared to the groups that received stand-alone HRP+WGA or HRP+anti-Aß antibody; (2) WGA conjugation not only increased the binding of anti-Aß antibody to cerebral Aß plaques, but also facilitated intraneuronal access of anti-Aß antibody.
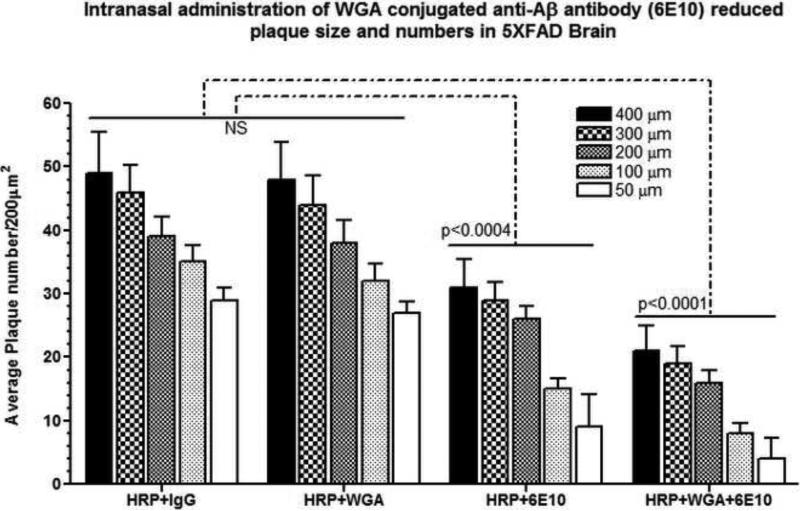
Neuropathological Evidence of Cerebral Aß Reduction after Intranasal Delivery of WGA Conjugated 6E10
As shown in Fig. 4, quantitation of total number and diameter of cortical Aß plaques within 10 high power fields (hpfs) (200μm2/each hpf) bilaterally per animal with a total of 50 hpfs per group (N=5/group) revealed that there was no significant difference between the HRP+IgG and HRP+WGA groups (NS, p>0.05). Compared to HRP+IgG or HRP+WGA groups, Intranasal treatment with anti-Aß antibody (6E10) significantly reduced Aß plaque numbers of different sizes. Aß plaques of ~200μm-400μm diameter were reduced by 1.5-fold, while those of ~100μm diameter were reduced by 2.3-fold, and of 50μm diameter were reduced by 3.2-fold (All values, p<0.0004). Conjugation of anti-Aß antibody (6E10) with WGA resulted in further reduction of Aß plaque numbers of different sizes by additional decrease(s) compared to HRP+anti-Aß antibody (6E10) group. In particular, Aß plaques of ~200μm-400μm diameter were reduced by 2.4-fold (additional decrease of 0.9-fold compared to the HRP+anti-Aß antibody group), those of ~100μm diameter were reduced by 4.3-fold (additional decrease of 2.0-fold compared to the HRP+anti-Aß antibody group), and of 50μm diameter were reduced by 7.2-fold (additional decrease of 4.0-fold compared to the HRP+anti-Aß antibody group) (All values, p<0.0001). These results indicate that conjugation of anti-Aß antibody with WGA had an additive effect in reducing Aß plaques, with more prominent reduction of smaller size (~50μm-100μm) plaques, in 5XFAD brain after intranasal delivery of WGA conjugated anti-Aß antibody (6E10).
Fig. 4.
Measurement of number and diameter of HRP labeled cortical plaques quantitated within 10 high power fields (hpfs) (200μm2/each hpf) bilaterally per animal with a total of 50 hpfs per group (N=5/group). Data are represented as integrated optical densities (IODs)/μm2) {Mean ± standard deviation (SD)}. Note that the WGA+Anti-Aß Antibody combination more prominently reduced smaller (~50μm-100μm), but not “core” (>200μm) plaques in 5XFAD brain after intranasal delivery of WGA conjugated anti-Aß antibody.
Biochemical Evidence of Cerebral Aß Reduction after Intranasal Delivery of WGA Conjugated 6E10
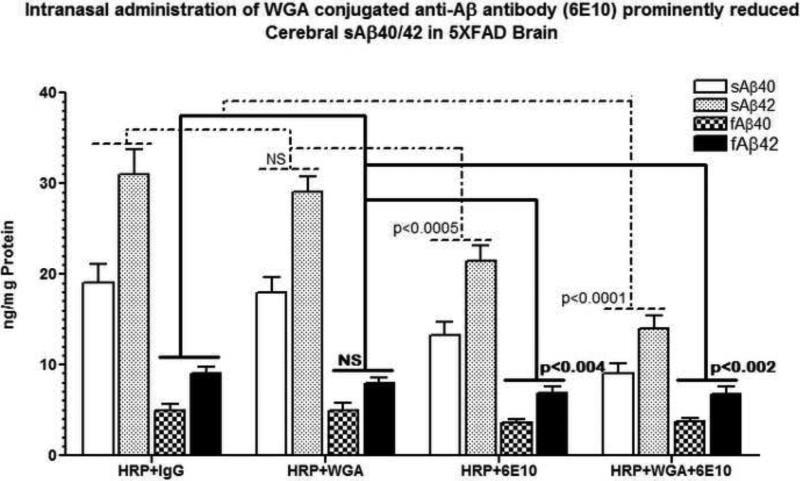
ELISA analysis of sAß40/42 from brain extracts of 5XFAD mice after intranasal administration of anti-Aß antibody (6E10) with and without WGA conjugation showed decreased levels of both soluble (sAß40/42) and fibrillar (fAß40/42) forms of cerebral amyloid. It is interesting to note that more pronounced decreases were observed in soluble (sAß40/42) than the decreases in fibrillar (fAß40/42) forms of cerebral amyloid. Results show that there was no significant difference between the HRP+IgG and HRP+WGA groups (NS, p>0.05). Compared to HRP+IgG or HRP+WGA groups, Intranasal treatment with anti-Aß antibody (6E10) significantly reduced cerebral sAß40/42 by 1.5-fold (p<0.0005) while the reductions in fAß40/42 were observed to be 1.3-fold (p<0.004). These amyloid reducing effects of intranasal anti-Aß antibody (6E10) were further enhanced after intranasal administration of WGA conjugated of anti-Aß antibody (6E10). As evidenced in Fig. 5, compared to HRP+IgG or HRP+WGA groups, WGA conjugation reduced cerebral sAß40/42 by 2.1-/2.2-fold respectively (p<0.0001) (additional decrease of 0.6-/0.7-fold compared to the HRP+anti-Aß antibody group), and levels of fAß40/42 were reduced by 1.3-fold (p<0.002). There was no additional decrease in the levels of fAß40/42 compared to the HRP+anti-Aß antibody group. These results indicate that conjugation of anti-Aß antibody with WGA had an additive effect in reducing soluble, but not fibrillar, Aß species in 5XFAD brain after intranasal delivery of WGA conjugated anti-Aß antibody (6E10).
Fig. 5.
ELISA quantitation of soluble (s) and fibrillar (f) cerebral amyloid (Aß40/42) in 5XFAD brain after intranasal delivery of anti-Aß antibody with and without WGA conjugation. Data are represented as (ng)/mg Protein) {Mean ± standard deviation (SD)}. Note that anti-Aß antibody with WGA conjugation showed prominent reduction of soluble, but not fibrillar, Aß species in 5XFAD brain.
Summary of Results
Conjugation with WGA enhanced binding ability of anti-Aß antibody to cerebral amyloid plaques after intranasal delivery.
Conjugation with WGA not only increased the binding ability of anti-Aß antibody to the plaques, but also facilitated intraneuronal access of anti-Aß antibody.
Conjugation of anti-Aß antibody with WGA had an additive effect in reducing Aß plaques, with more prominent reduction of smaller (~50μm-100μm), but not “core” (>200μm) Aß plaques in 5XFAD brain after intranasal delivery of WGA conjugated anti-Aß antibody.
Conjugation of anti-Aß antibody with WGA had an additive effect in reducing soluble, but not fibrillar, Aß species in 5XFAD brain after intranasal delivery of WGA conjugated anti-Aß antibody.
Discussion
All converging lines of evidence indicate that progressive production and accumulation of Aß plays a cardinal role in the pathogenesis of Alzheimer's disease [29]. Based on the premises of Aß being the primary causative trigger in the development of Alzheimer's disease, Aß-reducing strategies have gained much interest with regard to AD-disease modifying therapeutics. So far investigated Aß-reducing strategies include reduction of Aß production by ß- and/or Ɣ -secretase inhibition [30-32], α-secretase stimulation [33, 34], Aß degrading pathways [35-38], metal chelators [39-41], and immunization [42].
Among all, immunization strategy to eliminate excessive cerebral Aß has attained greater therapeutic importance [43] showing plaque reduction in various experimental models after active immunization with Aß peptide [44-48] or with Aß fragment [49], intranasal administration of phage-peptide [50], and systemic passive immunization with monoclonal antibodies against Aß epitopes injected intraperitoneally [51-56]. Clinical trials with active immunization against Aß peptides have been mounted with some optimistic results [57], but serious limiting problems with both active immunization and with passive systemic immunization have become apparent.
The potential for prolonged high concentrations of circulating anti-amyloid antibody to produce cerebrovascular pathology in conjunction with vascular amyloid has been demonstrated in animals [58]. Humans immunized with Aß showed reduced cerebral Aß and tau, but increased soluble oligomeric Aß, synaptic damage and signs of neuroinflammation [59], and lead to the development of meningoencephalitis [43, 60]. The international multicenter phase II clinical trial with active immunotherapy was interrupted because it resulted in lymphocytic inflammatory infiltrate, white matter lesions and meningoencephalitis [61]. Since active immunization was found to be associated with an autoimmune meningoencephalitis in a subset of humans, passive transfer of anti-Aß immunoglobulin was pursued. However, peripheral passive immunization of Tg2576 mice resulted in vasocentric mononuclear infiltration and meningoencephalitis [62]. In a time course study of peripheral passive immunization in APP transgenic mice, it was found that some markers of microglial activation increased transiently once the immunotherapy was initiated and continued to rise up to 3 months [63]. Another study in 3XTg-AD showed that peripheral passive immunization with monoclonal Aß protofibril antibody, although reduced cerebral Aß burden, but produced vasogenic edema and encephalitis with advancing stage of cerebral Aß burden [64]. These findings warrant the use of peripheral passive immunotherapy in humans.
Intracerebral passive immunotherapy tested in the transgenic mouse models of AD in our lab demonstrated potential efficacy of direct delivery of anti-Aß antibody to the brain with the advantage of bypassing the blood-brain barrier and systemic adversities. Administration of anti-Aß antibody directly into the 3rd ventricle was found to reduce cerebral amyloid and toxicity without producing inflammatory or perivascular hemorrhage in the transgenic mouse models of AD [65-67], consistent with the study showing removal of local amyloid plaques within 3 days after direct application of anti-Aß antibody on to the cerebral cortex [68]. Epitope mapping experiments have demonstrated that anti-Aß antibodies recognize residues between 1-15 at the N-terminal end of full-length Aß, and that the N-terminal 3-6 residues (EFRH) of Aß are the most critical [69-71]. Moreover, it is also suggested that the maximum clearance of Aß is achieved in the order of IgG1>IgG2a>IgG2b isotype(s), consistent with the findings from our laboratory showing that the (IgG1) isotype of anti-Aß antibody is the most efficient than those of IgG2a and IgG2b isotypes in reducing cerebral plaque burden in Swedish (K670M/N671L) plus Indiana (V717F) double mutant transgenic mouse model of AD (TgCRND8) [72]. Additional findings from our lab showed that a single injection of anti-Aß antibody lasted for 4 weeks post-injection for plaques-reappearance and for 2 weeks post-injection for microglial activation [67], and that the ameliorative effect of anti-oligomeric Aß antibody lasted longer than anti-Aß antibodies used this far [73].
Current studies from our laboratory in this line of investigation evaluated if direct intranasal delivery of anti-Aß antibody conjugated with the unique axonal transporter carrier plant lectin-WGA [19, 26] will enhance cerebral uptake of anti-Aß antibody (6E10) into the brain. Conjugation of immunotherapeutics with WGA has two distinct advantages to enhance cerebral uptake of candidate drugs both via active and passive mechanisms of transfer to the brain. Active mechanism of WGA conjugation involves its preferred selectivity of WGA for its active adsorptive endocytic uptake by OSN anterograde axonal transport to the olfactory bulb [19, 26]. While the passive mechanism of WGA is due to its nasal mucosa-like chemical properties that not only facilitate passive diffusion of immunotherapeutics but also reduce the chances of removal/self-rejection of administered immunotherapeutics by nasal mucosa [22, 25]. This is the first report showing enhanced uptake of anti-Aß antibody into the brain with the use of WGA as an efficient carrier. Observed comparison between only HRP+anti-Aß group and HRP+WGA+anti-Aß group substantiates preferred transport of WGA via intranasal route leading to enhanced delivery of anti-Aß antibody to the brain.
Results show very interesting pattern of the uptake of WGA by itself and of anti-Aß antibody with or without WGA tag. Uptake of WGA was observed to distinctly label neuronal perikarya in addition to binding with Aß plaques. This type of perikaryal labeling was not observed in the groups that received test materials with no WGA label. These observations indicate that anti-Aß antibody with WGA tag can enter the neuron via trans-axonal transfer, which may facilitate neutralization of intraneuronal Aß, in addition to its ability to bind and neutralize to extra neuronal Aß plaques. WGA also was found to diffusely label cortical plaques indicating its possible uptake even by damaged neuronal cytoskeleton entangled within the fabric of plaques. These findings provide direct proof of enhanced delivery of immunotherapeutics by WGA carrier utilizing active endocytic uptake by OSN anterograde axonal transport to the brain.
In addition, Conjugation of anti-Aß antibody with WGA had an additive effect in reducing Aß plaques, with more prominent reduction of smaller (~50μm-100μm), but not “core” (>200μm) plaques in 5XFAD brain after intranasal delivery of WGA conjugated anti-Aß antibody; and in reducing soluble, but not fibrillar, Aß species in 5XFAD brain after intranasal delivery of WGA conjugated anti-Aß antibody, indicating that WGA conjugation has not abated the immune-binding function of the antibody.
Conclusions
Given the lack of availability of disease modifying treatment(s) for Alzheimer's disease, current investigation validating the use of WGA as a novel vehicle for enhancing intranasal passive immunization in Alzheimer's disease has a great potential of clinical translation for treating Alzheimer's disease.
Research Highlights.
This is the first report demonstrating the use of WGA as a novel vehicle for enhancing intranasal passive immunization in Alzheimer's disease.
Conjugation with WGA enhanced binding ability of anti-Aß antibody to cerebral amyloid plaques after intranasal delivery.
Conjugation with WGA not only increased the binding ability of anti-Aß antibody to the plaques, but also facilitated intraneuronal access of anti-Aß antibody.
Given the lack of availability of disease modifying treatment(s) for Alzheimer's disease, current investigation validating the use of WGA as a novel vehicle for enhancing intranasal passive immunization in Alzheimer's disease has a great potential of clinical translation for treating Alzheimer's disease.
Acknowledgements
This work was supported by National Institute of Health (AG039625, NBC), and the Department of Veterans Affairs (VA MERIT Grant #B6285R, NBC). The support provided by the Westside Institute for Science and Education Chicago; and by the Departments of Pediatrics, and Anatomy & Cell Biology, University of Illinois at Chicago is duly acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finder VH. Alzheimer's disease: a general introduction and pathomechanism. J Alzheimers Dis. 2010;22(Suppl 3):5–19. doi: 10.3233/JAD-2010-100975. [DOI] [PubMed] [Google Scholar]
- 2.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009 Jul;118(1):5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 3.Eckert A, Schulz KL, Rhein V, Gotz J. Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Mol Neurobiol. 2010 Jun;41(2-3):107–14. doi: 10.1007/s12035-010-8109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang HC, Jiang ZF. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer's disease. J Alzheimers Dis. 2009;16(1):15–27. doi: 10.3233/JAD-2009-0960. [DOI] [PubMed] [Google Scholar]
- 5.Imahori K. The biochemical study on the etiology of Alzheimer's disease. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(1):54–61. doi: 10.2183/pjab.86.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maccioni RB, Farias G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer's disease. Arch Med Res. 2010 Apr;41(3):226–31. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008 Nov 26;60(4):534–42. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–93. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan NB, Siegel GJ. Intracerebroventricular passive immunization in transgenic mouse models of Alzheimer's disease. Expert Rev Vaccines. 2004 Dec;3(6):717–25. doi: 10.1586/14760584.3.6.717. [DOI] [PubMed] [Google Scholar]
- 10.Holtzman JL. Amyloid-beta vaccination for Alzheimer's dementia. Lancet. 2008 Oct 18;372(9647):1381. doi: 10.1016/S0140-6736(08)61578-0. author reply -2. [DOI] [PubMed] [Google Scholar]
- 11.Lemere CA, Maier M, Peng Y, Jiang L, Seabrook TJ. Novel Abeta immunogens: is shorter better? Curr Alzheimer Res. 2007 Sep;4(4):427–36. doi: 10.2174/156720507781788800. [DOI] [PubMed] [Google Scholar]
- 12.McGeer PL. Amyloid-beta vaccination for Alzheimer's dementia. Lancet. 2008 Oct 18;372(9647):1381. doi: 10.1016/S0140-6736(08)61579-2. author reply -2. [DOI] [PubMed] [Google Scholar]
- 13.Schenk DB, Seubert P, Grundman M, Black R. A beta immunotherapy: Lessons learned for potential treatment of Alzheimer's disease. Neurodegener Dis. 2005;2(5):255–60. doi: 10.1159/000090365. [DOI] [PubMed] [Google Scholar]
- 14.Wisniewski T, Konietzko U. Amyloid-beta immunisation for Alzheimer's disease. Lancet Neurol. 2008 Sep;7(9):805–11. doi: 10.1016/S1474-4422(08)70170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9(Suppl 3):S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illum L. Nasal drug delivery--possibilities, problems and solutions. J Control Release. 2003 Feb 21;87(1-3):187–98. doi: 10.1016/s0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- 17.Minn A, Leclerc S, Heydel JM, Minn AL, Denizcot C, Cattarelli M, et al. Drug transport into the mammalian brain: the nasal pathway and its specific metabolic barrier. J Drug Target. 2002 Jun;10(4):285–96. doi: 10.1080/713714452. [DOI] [PubMed] [Google Scholar]
- 18.Turker S, Onur E, Ozer Y. Nasal route and drug delivery systems. Pharm World Sci. 2004 Jun;26(3):137–42. doi: 10.1023/b:phar.0000026823.82950.ff. [DOI] [PubMed] [Google Scholar]
- 19.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–96. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Thorne RG, Frey WH., 2nd Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40(12):907–46. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 21.Hanson LR, Frey WH., 2nd Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J Neuroimmune Pharmacol. 2007 Mar;2(1):81–6. doi: 10.1007/s11481-006-9039-x. [DOI] [PubMed] [Google Scholar]
- 22.Gao X, Wu B, Zhang Q, Chen J, Zhu J, Zhang W, et al. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Release. 2007 Aug 28;121(3):156–67. doi: 10.1016/j.jconrel.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Bourgeois C, Bour JB, Aho LS, Pothier P. Prophylactic administration of a complementarity-determining region derived from a neutralizing monoclonal antibody is effective against respiratory syncytial virus infection in BALB/c mice. J Virol. 1998 Jan;72(1):807–10. doi: 10.1128/jvi.72.1.807-810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gigliotti F, Haidaris CG, Wright TW, Harmsen AG. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infect Immun. 2002 Mar;70(3):1069–74. doi: 10.1128/IAI.70.3.1069-1074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao X, Tao W, Lu W, Zhang Q, Zhang Y, Jiang X, et al. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006 Jun;27(18):3482–90. doi: 10.1016/j.biomaterials.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Thorne RG, Emory CR, Ala TA, Frey WH., 2nd Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995 Sep 18;692(1-2):278–82. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- 27.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006 Oct 4;26(40):10129–40. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chauhan NB, Siegel GJ, Lichtor T. Distribution of intraventricularly administered antiamyloid-beta peptide (Abeta) antibody in the mouse brain. J Neurosci Res. 2001 Oct 15;66(2):231–5. doi: 10.1002/jnr.1215. [DOI] [PubMed] [Google Scholar]
- 29.Cerpa W, Dinamarca MC, Inestrosa NC. Structure-function implications in Alzheimer's disease: effect of Abeta oligomers at central synapses. Curr Alzheimer Res. 2008 Jun;5(3):233–43. doi: 10.2174/156720508784533321. [DOI] [PubMed] [Google Scholar]
- 30.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999 Oct 22;286(5440):735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 31.Doerfler P, Shearman MS, Perlmutter RM. Presenilin-dependent gamma-secretase activity modulates thymocyte development. Proc Natl Acad Sci U S A. 2001 Jul 31;98(16):9312–7. doi: 10.1073/pnas.161102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001 Nov 8;414(6860):212–6. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 33.Pakaski M, Kasa P. Role of acetylcholinesterase inhibitors in the metabolism of amyloid precursor protein. Curr Drug Targets CNS Neurol Disord. 2003 Jun;2(3):163–71. doi: 10.2174/1568007033482869. [DOI] [PubMed] [Google Scholar]
- 34.Hock C, Maddalena A, Raschig A, Muller-Spahn F, Eschweiler G, Hager K, et al. Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer's disease. Amyloid. 2003 Mar;10(1):1–6. doi: 10.3109/13506120308995249. [DOI] [PubMed] [Google Scholar]
- 35.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4162–7. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckman EA, Watson M, Marlow L, Sambamurti K, Eckman CB. Alzheimer's disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J Biol Chem. 2003 Jan 24;278(4):2081–4. doi: 10.1074/jbc.C200642200. [DOI] [PubMed] [Google Scholar]
- 37.Tucker HM, Kihiko M, Caldwell JN, Wright S, Kawarabayashi T, Price D, et al. The plasmin system is induced by and degrades amyloid-beta aggregates. J Neurosci. 2000 Jun 1;20(11):3937–46. doi: 10.1523/JNEUROSCI.20-11-03937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, et al. Metabolic regulation of brain Abeta by neprilysin. Science. 2001 May 25;292(5521):1550–2. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 39.Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994 Sep 2;265(5177):1464–7. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 40.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001 Jun;30(3):665–76. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 41.Ritchie CW, Bush AI, Mackinnon A, Macfarlane S, Mastwyk M, MacGregor L, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003 Dec;60(12):1685–91. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 42.Hamaguchi T, Ono K, Yamada M. Anti-amyloidogenic therapies: strategies for prevention and treatment of Alzheimer's disease. Cell Mol Life Sci. 2006 Jul;63(13):1538–52. doi: 10.1007/s00018-005-5599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenk D. Amyloid-beta immunotherapy for Alzheimer's disease: the end of the beginning. Nat Rev Neurosci. 2002 Oct;3(10):824–8. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 44.Hara H. [Abeta vaccine therapy for Alzheimer's disease]. Rinsho Shinkeigaku. 2005 Nov;45(11):867–9. [PubMed] [Google Scholar]
- 45.Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, et al. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann Neurol. 2000 Oct;48(4):567–79. [PubMed] [Google Scholar]
- 46.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000 Dec 21-28;408(6815):979–82. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 47.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000 Dec 21-28;408(6815):982–5. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 48.Das P, Murphy MP, Younkin LH, Younkin SG, Golde TE. Reduced effectiveness of Abeta1-42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging. 2001 Sep-Oct;22(5):721–7. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- 49.Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a nontoxic/nonfibrillar amyloid-beta homologous peptide reduces Alzheimer's disease-associated pathology in transgenic mice. Am J Pathol. 2001 Aug;159(2):439–47. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frenkel D, Katz O, Solomon B. Immunization against Alzheimer's beta -amyloid plaques via EFRH phage administration. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11455–9. doi: 10.1073/pnas.97.21.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000 Aug;6(8):916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 52.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8850–5. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solomon B. Immunological approaches as therapy for Alzheimer's disease. Expert Opin Biol Ther. 2002 Dec;2(8):907–17. doi: 10.1517/14712598.2.8.907. [DOI] [PubMed] [Google Scholar]
- 54.Solomon B. Immunotherapeutic strategies for prevention and treatment of Alzheimer's disease. DNA Cell Biol. 2001 Nov;20(11):697–703. doi: 10.1089/10445490152717550. [DOI] [PubMed] [Google Scholar]
- 55.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002 May;5(5):452–7. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 56.Mohajeri MH, Saini K, Schultz JG, Wollmer MA, Hock C, Nitsch RM. Passive immunization against beta-amyloid peptide protects central nervous system (CNS) neurons from increased vulnerability associated with an Alzheimer's disease-causing mutation. J Biol Chem. 2002 Sep 6;277(36):33012–7. doi: 10.1074/jbc.M203193200. [DOI] [PubMed] [Google Scholar]
- 57.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003 May 22;38(4):547–54. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 58.Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, et al. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002 Nov 15;298(5597):1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 59.Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer's disease pathogenesis. Acta Neuropathol. 2010 Sep;120(3):369–84. doi: 10.1007/s00401-010-0719-5. [DOI] [PubMed] [Google Scholar]
- 60.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003 Apr;9(4):448–52. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 61.Uro-Coste E, Russano de Paiva G, Guilbeau-Frugier C, Sastre N, Ousset PJ, da Silva NA, et al. Cerebral amyloid angiopathy and microhemorrhages after amyloid beta vaccination: case report and brief review. Clin Neuropathol. 2010 Jul-Aug;29(4):209–16. doi: 10.5414/npp29209. [DOI] [PubMed] [Google Scholar]
- 62.Lee EB, Leng LZ, Lee VM, Trojanowski JQ. Meningoencephalitis associated with passive immunization of a transgenic murine model of Alzheimer's amyloidosis. FEBS Lett. 2005 May 9;579(12):2564–8. doi: 10.1016/j.febslet.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 63.Morgan D. Modulation of microglial activation state following passive immunization in amyloid depositing transgenic mice. Neurochem Int. 2006 Jul;49(2):190–4. doi: 10.1016/j.neuint.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Minami SS, Sidahmed E, Aid S, Shimoji M, Niikura T, Mocchetti I, et al. Therapeutic versus neuroinflammatory effects of passive immunization is dependent on Abeta/amyloid burden in a transgenic mouse model of Alzheimer's disease. J Neuroinflammation. 2010;7:57. doi: 10.1186/1742-2094-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chauhan NB, Siegel GJ. Reversal of amyloid beta toxicity in Alzheimer's disease model Tg2576 by intraventricular antiamyloid beta antibody. J Neurosci Res. 2002 Jul 1;69(1):10–23. doi: 10.1002/jnr.10286. [DOI] [PubMed] [Google Scholar]
- 66.Chauhan NB, Siegel GJ. Intracerebroventricular passive immunization with anti-Abeta antibody in Tg2576. J Neurosci Res. 2003 Oct 1;74(1):142–7. doi: 10.1002/jnr.10721. [DOI] [PubMed] [Google Scholar]
- 67.Chauhan NB, Siegel GJ, Lichtor T. Effect of age on the duration and extent of amyloid plaque reduction and microglial activation after injection of anti-Abeta antibody into the third ventricle of TgCRND8 mice. J Neurosci Res. 2004 Dec 1;78(5):732–41. doi: 10.1002/jnr.20298. [DOI] [PubMed] [Google Scholar]
- 68.Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, et al. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001 Mar;7(3):369–72. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- 69.Solomon B. Towards Alzheimer's disease vaccination. Mini Rev Med Chem. 2002 Feb;2(1):85–92. doi: 10.2174/1389557023406575. [DOI] [PubMed] [Google Scholar]
- 70.Solomon B. Generation of anti-beta-amyloid antibodies via phage display technology towards Alzheimer's disease vaccination. Vaccine. 2005 Mar 18;23(17-18):2327–30. doi: 10.1016/j.vaccine.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 71.Solomon B. Alzheimer's disease immunotherapy: from in vitro amyloid immunomodulation to in vivo vaccination. J Alzheimers Dis. 2006;9(3 Suppl):433–8. doi: 10.3233/jad-2006-9s349. [DOI] [PubMed] [Google Scholar]
- 72.Chauhan NB, Siegel GJ. Efficacy of anti-Abeta antibody isotypes used for intracerebroventricular immunization in TgCRND8. Neurosci Lett. 2005 Mar 3;375(3):143–7. doi: 10.1016/j.neulet.2004.10.090. [DOI] [PubMed] [Google Scholar]
- 73.Chauhan NB. Intracerebroventricular passive immunization with anti-oligoAbeta antibody in TgCRND8. J Neurosci Res. 2007 Feb 1;85(2):451–63. doi: 10.1002/jnr.21110. [DOI] [PubMed] [Google Scholar]