Abstract
Oxytocin (Oxt) and vasopressin (Avp) are important for a wide variety of behaviors and the use of transgenic mice lacking the peptides or their receptors, particularly when their loss is spatially and temporally manipulated, offers an opportunity to closely examine their role in a particular behavior. We used a cued fear conditioning paradigm to examine associative learning in three lines of transgenic mice: mice that constitutively lack vasopressin 1a (Avpr1a−/−) or Oxt receptors (Oxtr−/−) and mice that have oxytocin receptor loss restricted to the forebrain that begins post-weaning (OxtrFB/FB). Oxtr−/− and Avpr1a−/− mice have normal conditioned freezing. OxtrFB/FB mice have a reduction in freezing behavior during acquisition, as well as during context and cue retention. In addition to reduction of Oxtr in the central nucleus of the amygdala, in vitro receptor autoradiography revealed that the OxtrFB/FB mice have significantly reduced levels of Avpr1a only in that structure. Our results show that post-weaning alteration of the distribution of Oxtr receptors is critically important for fear behavior, an effect mirrored in the neural structures that mediate it. While constitutive knockouts of Oxtr and Avpr1a are useful for identifying the neural underpinnings of some behaviors, compensatory mechanisms within some circuits may obscure other behavioral roles.
Introduction
Oxytocin (Oxt) and vasopressin (Avp) have effects on a wide variety of behaviors, most notably social behavior (Bielsky et al., 2004; Lee et al., 2008; Wersinger et al., 2002). While Avp and Oxt receptors have been found in the amygdala, hippocampus and other areas involved in fear behavior (Insel et al., 1991; Lee et al., 2008; Veinante and Freund-Mercier, 1997) little is known about their role in fear conditioning. Fear conditioning is a learning paradigm in which a neutral stimulus (conditioned stimulus; CS) such as a tone, and a mild aversive stimulus, such as a shock (unconditioned stimulus; US), are paired in a manner that results in an association between the two. The neural circuitry mediating the association between an aversive event and the stimulus that predicts it has been extensively characterized, and the amygdala plays a key role (Fanselow and LeDoux, 1999; LeDoux, 2007; Shi and Davis, 2001). Oxtr and Avpr1a have been found in several amygdalar areas, including the central nucleus (CeA) (Cassell et al., 1999; Tribollet et al., 1988; Veinante and Freund-Mercier, 1997). Recent evidence suggests that Avpr1a and Oxtr in the CeA may play a role in fear behavior (Huber et al., 2005).
The present study utilized an associative learning paradigm to examine fear behavior in two total receptor KOs (Oxtr−/− and Avpr1a−/−)(Hu et al., 2003; Lee et al., 2008) and one relatively forebrain-specific KO of Oxtr (OxtrFB/FB)(Lee et al., 2008). While the Oxtr and Avpr1a lines lack receptors from the time of conception, the OxtrFB/FB mice have an induced receptor loss after postnatal day (PND) 21 when expression of cre recombinase, under control of the Camk2a promoter, begins (Lee et al., 2008). This line has some phenotypic differences from the Oxtr line: in particular, there is a more pronounced deficit in social memory (Macbeth et al., 2009; Lee et al., 2008), an effect mediated in part by the medial nucleus of the amygdala (Ferguson et al, 2001). Some have suggested Oxt and Avp may control behavior as part of balanced, individually tuned circuits within the amygdala (Viviani and Stoop, 2008). Since Oxt has organizational effects during the pre- and neonatal periods (Takayanagi et al., 2005; Winslow et al., 2000), this balance may be uniquely affected by the post-weaning loss in the OxtrFB/FB line.
We predicted, based on the studies by Huber et al. (2005), that Avpr1a−/− mice would display a deficit in fear conditioning, while Oxtr−/− mice would show enhanced fear. Since OxtrFB/FB have a more profound deficit in social recognition and the post-weaning loss of Oxtr may have a more profound effect on the Oxtr/Avpr1a balance in the CeA, we thought it possible to see a potentiation of fear behavior, compared to the Oxtr−/− mice.
Methods
Animals
The development and genotyping of all lines has been described in detail previously (Hu et al., 2003; Lee et al., 2008). Oxtr−/− and OxtrFB/FB mice were developed using transgenic Flp and Cre recombinase lines on a C57BL/6J background, resulting in Oxtr−/− and OxtrFB/FB mice that have now been crossed further into C57BL/6J (from Jackson Labs, Bar Harbor, ME) for at least nine generations. All OxtrFB/FB mice are heterozygous for the Cre recombinase transgene. Avpr1a−/− subjects were originally an equal mix of the C57BL/6J and 129X1/SvJ strains [RW4 embryonic stem cell line (Shipley et al., 1996)] but have been repetitively backcrossed for greater than 10 generations with C57BL/6J mice. All subjects were littermates from crosses of non-sibling heterozygous mice. Subject males were group-housed (2–5 mice per cage) upon weaning at 21 days old.
All mice were maintained on a 12-hour light/dark cycle (lights on at 0400) with food and water available ad libitum. Behavioral tests were conducted during the light phase. All animal procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines on the care and use of animals.
Procedures
Fear Conditioning
Fear conditioning experiments used 49 male Avpr1a mice (25 WT, 24 KO) taken from 15 different litters, 56 male Oxtr mice (28 WT, 28 KO) taken from 16 different litters and 48 male FB Oxtr mice (24 WT, 24 KO) taken from 15 litters. All mice were between 60 and 130 days old at the time of testing. Subjects were age matched such that there was no difference in age at the time of testing for any of the lines (p>0.10 for all lines). Mice used in fear conditioning experiments were not re-used for shock reactivity or receptor autoradiography experiments so that these results would be unaffected by the prior experience of fear conditioning.
Mice were pair housed in a clean cage at least three days prior to the start of the experiment and their tails were marked for identification. During all phases of the experiment, mice were transported to and from the behavioral testing in their home cage. They were allowed to sit undisturbed in the behavioral suite for ten minutes prior to experimental procedures and for five minutes following.
The fear conditioning apparatus (Coulbourn Instruments Whitehall, PA) consisted of a chamber (17.8 × 17.8 × 30.5 cm) with a shock grid floor (1.1 cm, center to center) and two clear Plexiglas and two stainless steel walls. The chamber was placed inside an isolation cubicle (71.1 × 45.7 × 48.3 cm) with a fan (background noise level 68dB). A light inside the fear chamber produced illumination of 4.6 lux. 80dB, 2800Hz tones were delivered through a speaker near the top of the fear chamber. The shock grid was connected to a programmable shocker (Coulbourn Instruments). Tones and shocks were controlled by software on a PC (Freezeframe, Actimetrics, Wilmette, IL). A camera mounted in the ceiling of the cage recorded mouse behavior, which was later analyzed using the same software. The dependent variable (freezing) was defined as the cessation of movement except for breathing for a period at least 750ms long. Previous experiments analyzed with the Freezeframe software and scored by an observer resulted in concordance greater than 95%.
There were two between subject variables: Genotype (WT vs. KO) and Group (paired vs. unpaired). The paired group received tones and shocks that temporally overlapped and co-terminated. The unpaired group received the tones during the first day and shocks on the second. The unpaired control group receives the same number of tones and shocks as the paired group, but in a manner that does not support fear to the tone. The explicit unpairing of the CS and US is a control used in a wide array of associative learning paradigms to measure non-learning related changes in behavior due to pseudoconditioning, sensitization, handling stress or novelty. This control ensures that the behavioral changes seen following paired training are due to learning and not a result of exposure to tones and shocks or some other feature of the experiment. This method of unpaired training results in very low levels of freezing to the tone during retention (Smith et al., 2007), though levels may be higher than during baseline for the reasons just mentioned.
The experiment consisted of acclimation, acquisition and retention phases that occurred on consecutive days. One the first day, the acclimation phase was 720s of exposure to the fear chamber for the paired group. The unpaired group had six 20s, 80db (2800Hz) tones starting at 120s, with an inter-trial interval (ITI) of 80s. Freezing was measured during the 720s of acclimation for both groups.
On the second day, during acquisition, the paired group received 6 tone presentations and a 2s, 0.5mA shock that co-terminated with the tone. Tone-shock pairings (18s inter-stimulus interval) started after a 260s stimulus free baseline period and were presented with an 80s ITI. Six shocks were given to the unpaired group at the same interval used for the paired group. Freezing was measured during the stimulus free period (baseline) and the 18s preceding the shock for both paired and unpaired groups (tones 1–6).
On the third day, retention day, both groups were tested for fear to the context and, 2 minutes later, for fear to the cue. Mice were exposed to the testing environment to measure freezing to the training context for 240s and were then returned to their cages for approximately 2 min. Tone fear was tested in an opaque plastic cylinder with an open top (Container & Packaging Supply, Eagle, ID) scented with vanilla extract placed inside the fear chamber after the isolation chamber’s fan was disconnected. Four 20s tones were presented after a 200s stimulus-free period with an 80s ITI. Freezing was recorded during the 200s stimulus free period (baseline) and during the tone presentations. This arrangement produced little freezing during the stimulus-free period prior to the presentation of the first tone indicating no generalization of fear to the new context. The fear conditioning chambers were cleaned with a 70% ethanol solution between groups of mice. The chamber used for testing cue retention was cleaned with Clidox solution. The use of separate cleaners and the addition of vanilla scent to the cylinder were done to minimize similarities between the training context and cylinder used to test fear to the tone (Davis et al; 2005; Gould & Wehner, 1999).
The data were analyzed separately for each day of the experiment. Acquisition was a within subjects variable with 7 levels (baseline, tones 1–6), Context Retention had two levels (baseline and context retention), and Cue Retention had 2 levels (baseline and cue retention). Data from the 120s stimulus free period on the Acquisition day was used as the baseline for Acquisition and Context Retention. Since Cue Retention was run in a different context (plastic cylinder) the baseline was the 200 stimulus-free seconds prior to tone onset. Results were analyzed using mixed model ANOVA, with p-values adjusted using the Greenhouse-Geisser correction for the within subject variable on the Acquisition phase. Post hoc analyses were performed using Tukey’s honestly significant difference (HSD) test.
Shock Reactivity
A role for oxytocin in pain perception has been suggested (Breton et al., 2009; Rojas-Piloni et al., 2010), though this may in fact be mediated by oxytocin acting at the Avpr1a in mice (Schorscher-Petcu et al., 2010). To test for altered shock sensitivity, we examined reactivity to shock in a startle chamber.
The shock reactivity experiment used 14 FB Oxtr male mice (7 WT, 7 KO) taken from 5 litters. Mice were housed and transported to the behavioral suite in the manner mentioned above. Shock reactivity was measured in a startle chamber (San Diego Instruments, San Diego, CA) to allow for quantitative measurement of the response to shock. Mice were placed in a Plexiglas chamber (8.9 × 2.79 cm) mounted on a Plexiglas base inside of a Formica-laminated chamber and after one minute received eight, 1s footshocks that increased in intensity (.12mA, .24mA, .37mA, and .50mA) at one-minute intervals. Shocks were administered through a metal grid consisting of seven bars connected to a mechanical scrambler. Mice received each level of shock two times, in order of intensity. Chamber displacement following shock was measured with a piezoelectric strain gauge attached to the bottom of the Plexiglas chamber. SR-LAB software (San Diego Instruments) on a PC controlled the delivery of shocks and recorded cage displacement. Cages were cleaned with a 70% ethanol solution between mice. The average amplitude for the two shock presentations at a given level was averaged to yield a single measure of reactivity at each level of shock intensity. Results were analyzed using ANCOVA with weight as a covariate. The effect of shock intensity and its potential interaction with genotype were also assessed with a mixed model ANOVA, and followed up with Tukey’s HSD.
In vitro Receptor Autoradiography
Receptor autoradiography experiments used an additional 12 Avpr1a (6 WT, 6 KO) mice from 9 litters, 12 Oxtr (6 WT, 6 KO) mice from 10 litters and 12 FB Oxtr (6 WT, 6 KO) mice from 8 litters. In vitro receptor autoradiography was performed with modifications of previous methods (Barberis et al., 1995; Elands et al., 1988; Young and Kuhar, 1979). Briefly, mice were anesthetized using isoflurane and killed by cervical dislocation. The brains were quickly removed and frozen in powdered dry ice. Coronal sections were taken at 16 µM thickness, thaw-mounted onto slides (Fisherbrand Superfrost Plus, Fisher Scientific, Pittsburg, PA) and stored at −70°C. The slides with brain sections were warmed to room temperature, rinsed in 170mM Tris-HCl (pH 7.4), for 5 min, fixed in 0.5% formaldehyde in PBS for 5 min and rinsed in binding solution [160mM Tris-HCl (pH 7.4) containing 10mM MgCl2, 0.05% bacitracin, 0.1% BSA] for 5 min. They were then incubated for 1 hr in the same solution plus 50pM 125I-ornithine vasotocin analog (NEX 254, PerkinElmer, Waltham, MA) for Oxtr binding or 50pM 125I linear Avpr1a antagonist (NEX 310, PerkinElmer) for Avpr1a binding. The slides were then rinsed twice for 5 min and then for 35 min in 50mM Tris-HCl (pH 7.4) with 100mM MgCl2 at room temperature over a gently rotating stir bar. The slides were rapidly dipped in deionized water and then blown dry. Dried slides were apposed to phosphorimaging screens for 12 days and then scanned to create a digital image using a Cyclone Phosphor System (PerkinElmer) connected to a PC. Digital images were analyzed using OptiQuant software (PerkinElmer). Binding was measured in each structure of interest and in a same size section of tissue that was without apparent binding (background). The results are expressed as the ratio of binding within the structure to background minus 1. No specific binding (as is seen in the total KOs) equals 0. Results from the binding assay were analyzed using a one-way ANOVA, similar to (Insel et al., 1991).
Results
Fear Conditioning
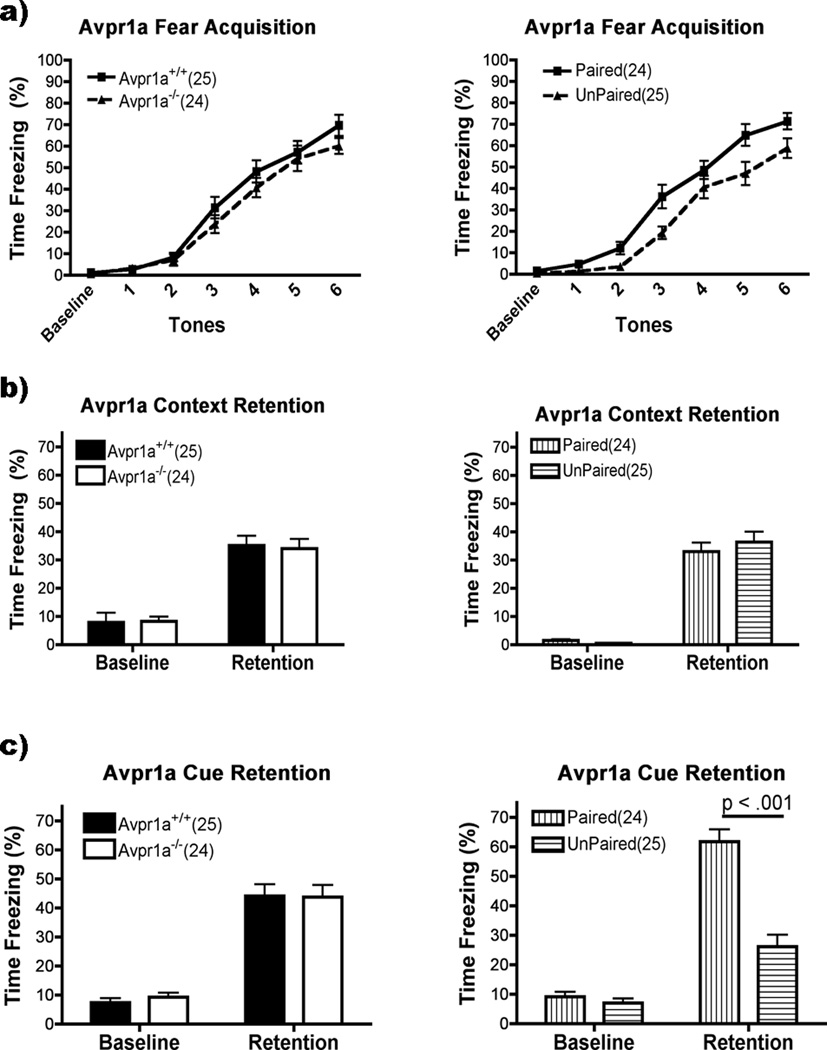
Avpr1a−/− mice
Avpr1a−/− mice had the same level of freezing as Avpr1a+/+ during fear acquisition (Fig. 1a). There was no main effect or interactions with Genotype (p >0.05), but there were significant main effects for Group (F(1,45)=8.63, p<0.01), with the paired group freezing more than the unpaired group overall. There was also a main effect for Acquisition (F(6,270)=151.77, p<0.001). Freezing during baseline was equivalent to the first two tones but freezing increased during the tone period for each successive tone thereafter (p<0.05). The Avpr1a−/− and Avpr1a+/+ mice also froze the same to the context twenty-four hours after training (Fig. 1b). There was no main effect or interactions of Genotype or Group (p>0.05), but significantly more freezing to chamber exposure during retention than prior to training (F(1,45)=116.82, p<0.001). Cue retention was unaffected by Genotype (p<0.05), but mice receiving paired tone/shock froze more than those that received unpaired tones and shocks (Group: F(1,45)=30.97, p<0.001) (Fig. 1c). There was also a significant main effect for Cue Retention (F(1,45)=182.10, p<0.001) and a Group × Cue Retention interaction (F(1,45)=39.72, p<0.001). There were no interactions with Genotype (p>0.05). Post hoc analysis of the Group × Cue Retention interaction showed that while both the paired and unpaired groups froze more during tone presentation than during the baseline period (p<0.001 for both), overall the paired group froze more than the unpaired (p<0.001).
Figure 1.
Avpr1a performance on fear conditioning (mean ± SEM). There were no effects of genotype on Acquisition, Context or Cue Retention (left panels, a,b,c; p>0.05). Group had an effect on acquisition but not context retention (right panels, a and b; P<0.01, P>0.05, respectively), but the paired group had higher levels of freezing during cue retention (P < 0.0001).
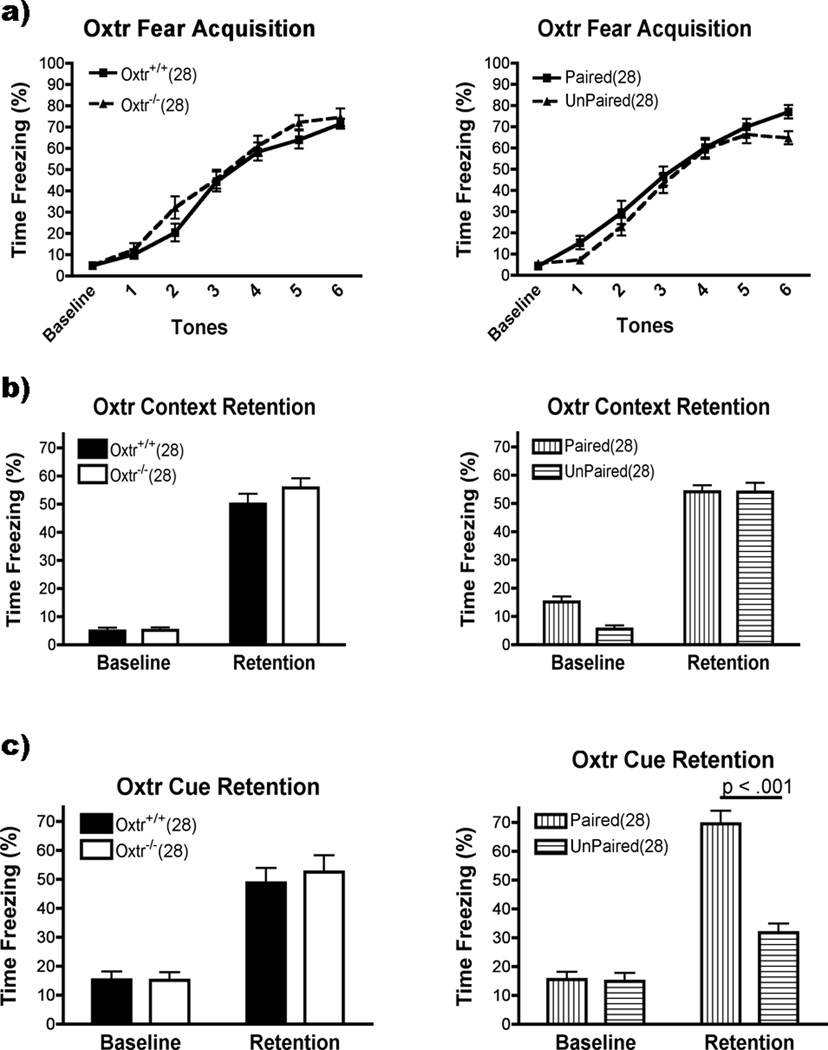
Oxtr−/− mice
Oxtr−/− and Oxtr+/+ had the same levels of freezing during fear acquisition (Fig. 2a). There was no main effect for Genotype or Group (p>0.05) and no interactions between them or with Acquisition. There was a main effect for Acquisition (F(6,312)=216.7,p<0.001). The baseline and first tone had equal levels of freezing, with more freezing in each successive tone thereafter (p<0.001 except for 4<5 which was p<0.05). Freezing to the context was also unaffected in the Oxtr−/− mice (Fig. 2b). There was no effect for Genotype or Group (p>0.05) and no interactions. There was a main effect for Context Retention, however (F(1,52)=253.3, p<0.001): mice froze more to the context after training than during the baseline period. Cue Retention was similarly unaffected in the Oxtr−/−, with no effects or interactions with Genotype (p<0.05)(Fig. 2c). There was a main effect for Group (F(1,52)=26.80, p<0.001) and Cue Retention (F(1,52)=211.60, p<0.001), and an Group × Cue Retention interaction (F(1,52)=58.40, p<0.001). The paired and unpaired groups did not differ on baseline freezing levels (p>0.05). Both groups froze more during tone presentation than during baseline (p<0.001) but the paired group froze more than unpaired (p<0.001).
Figure 2.
Oxtr performance on fear conditioning (mean ± SEM). There were no effects of genotype on Acquisition, Context or Cue Retention (left panels, a,b,c; p>0.05). Group had no effect on Acquisition or Context Retention (right panels, a,b; p>0.05), but the paired group had higher levels of freezing during Cue Retention (p<0.001).
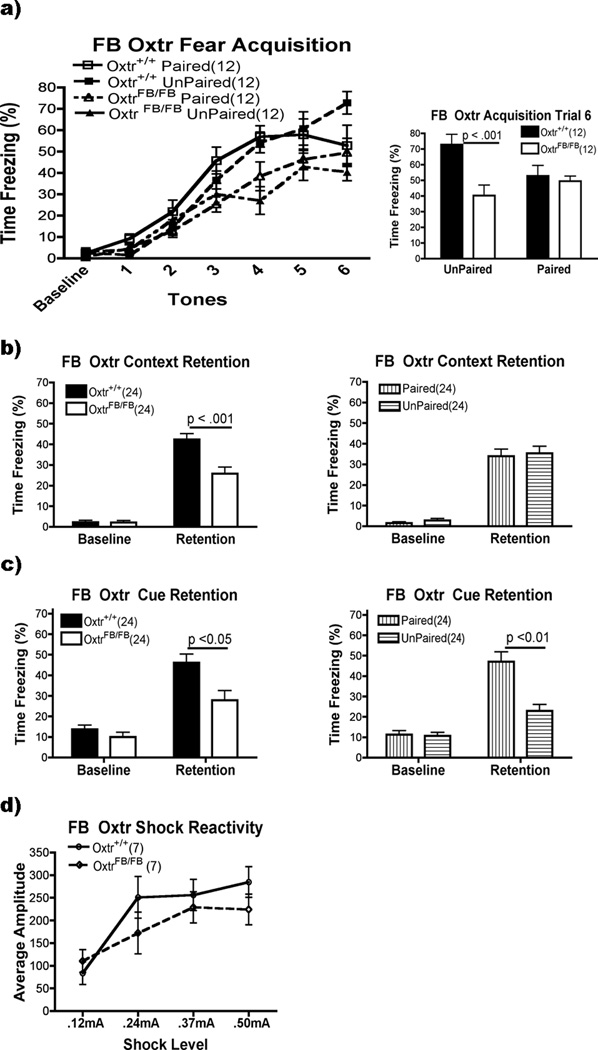
OxtrFB/FB mice
The OxtrFB/FB mice performed worse than the WT on acquisition (Fig. 3a, left). There were main effects for Genotype (F(1,44)=11.50, p<0.01) and Acquisition (F(6,264)=110.78, p<0.001). Post hoc analysis of the Acquisition variable demonstrated that freezing was equal during the baseline and first tone, rose until the fourth tone and plateaued (p<0.05). There were also interactions between Genotype × Acquisition (F(6,264)=4.71, p<0.01) and Genotype × Group × Acquisition (F(6,264)=3.30, p<0.05). Post hoc analysis of the Genotype × Acquisition interaction showed that the KO froze less on trials 4 and 6 (p<0.05)(Fig. 3a, right). The three-way interaction was assessed by running separate Genotype × Group ANOVAs for each trial of Acquisition. Trial 6 had the only Genotype × Group interaction. Post hoc analysis showed that the unpaired OxtrFB/FB differed from the WT on trial 6 (p>0.001), but the paired groups did not differ across genotype (p>0.05).
Figure 3.
FB Oxtr performance on fear conditioning and shock reactivity. a) OxtrFB/FB mice were worse overall on acquisition (p<0.01; left panel). On trial 6 (right panel), OxtrFB/FB in the unpaired group froze less than Oxtr+/+; freezing was equal in the paired group. b) While OxtrFB/FB mice froze to the context following training (p<0.001), they froze less than the Oxtr+/+ (p<0.001). c) Mice receiving unpaired tones/shocks froze less than those that received paired training (p<0.01). OxtrFB/FB froze more during tone presentation than during the baseline period (p<0.001), but still froze less than Oxtr+/+ mice (p<0.05). d) Shock reactivity was the same in OxtrFB/FB and Oxtr+/+ mice.
Freezing to the context twenty-four hours later was also reduced in the OxtrFB/FB mice (Fig 3b). There were main effects of Genotype (F(1,44)=12.04, p<0.01) and of Context Retention (F(1,44)=272.55, p<0.001). There was also an interaction between the two variables (F(1,44)=15.46, p<0.001). Post hoc analysis showed that both groups froze more to the context after training (p<0.001). While both groups had equivalently low levels of freezing during the baseline period (p>0.05), the WT froze more following training (p<0.001).
Freezing to the tone was also reduced in the OxtrFB/FB mice, though for both genotypes, freezing in the paired group was higher than the unpaired group (Fig. 3c). There were main effects for Genotype (1,44)=9.49, p<0.01), Group (F(1,44)=13.21, p<0.001) and Cue Retention (1,44)=98.65, p<0.001). There were also interactions between Genotype × Cue Retention (F(1,44)=6.57, p<0.05) and Group × Cue Retention (F(1,44)=23.75, p<0.001). Post hoc analysis of the Genotype × Cue Retention interaction indicated that both groups froze more following training (p<0.001) and while freezing was the same during the baseline periods (p>0.05), the WT mice froze more than the OxtrFB/FB mice during tone presentation (p<0.001). For the Group × Cue Retention interaction, the paired group (p<0.001) and the unpaired group (p<0.05) froze more during tone presentation than during the baseline period. While the two groups had equal levels of freezing during the baseline period (p>0.05), the paired group froze more during the tones (p<0.001).
Shock Reactivity
We observed decreased freezing in the OxtrFB/FB mice relative to the controls during the Acquisition, Context Retention and Cue Retention portions of the experiment. While the KO group showed learning, as indicated by the elevated levels of freezing compared to baseline in the later stages of acquisition and both retention tests, it was always less than the WT group. The reduction in freezing could have been due to altered shock perception.
OxtrFB/FB mice did not differ in their reaction to shock at any of the four shock intensities tested (Fig. 3d). In an ANCOVA using weight as a covariate, there was no effect of Genotype on average startle amplitude (F(4,8)=2.21, p>0.05). A repeated measures ANOVA indicated a difference in reactivity for the different shock intensities (F(3,36)=13.03, p<0.001). Mice reacted more to shock levels of 0.24mA, 0.37mA and 0.50mA than to 0.12mA (p<0.05), but reacted equally to the higher levels of shock intensity (0.24mA=0.37mA=0.50mA, p>0.05).
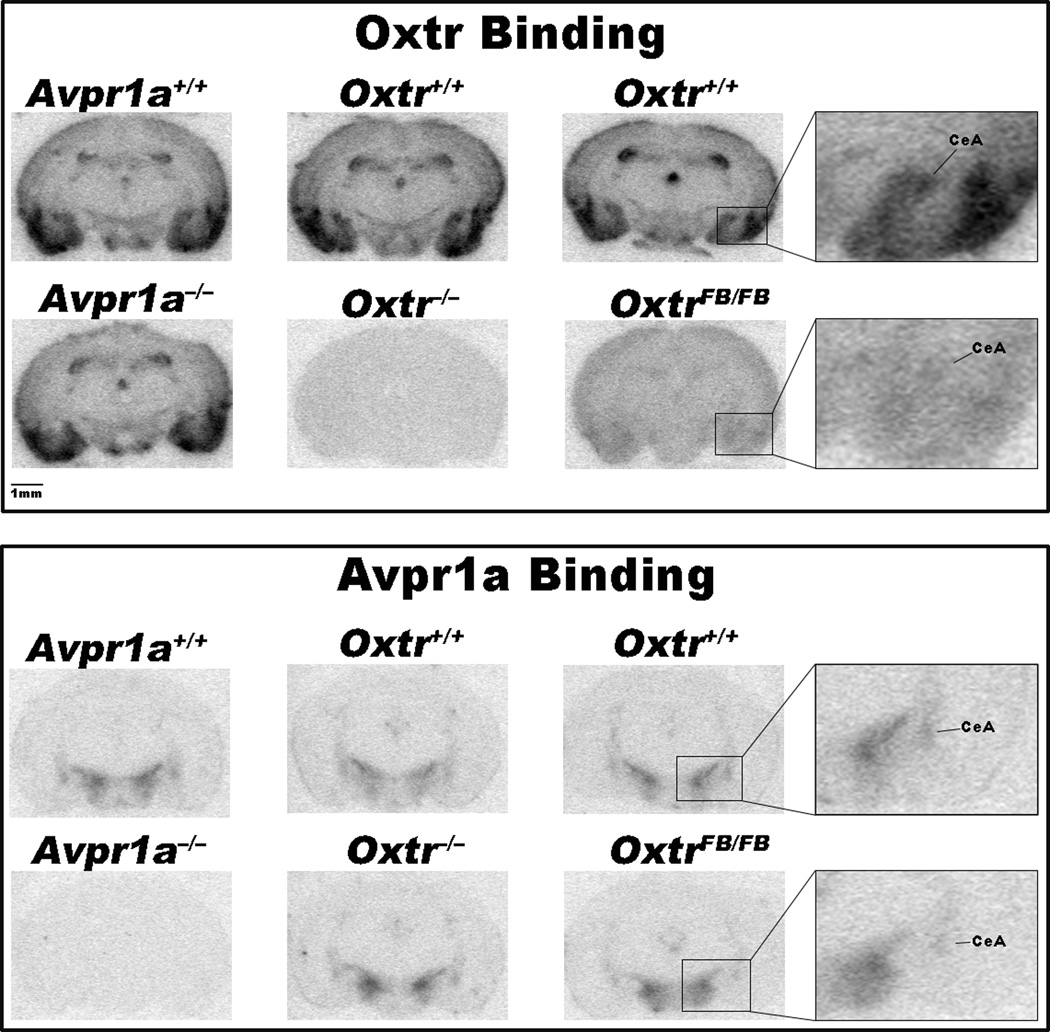
In vitro receptor autoradiography
The total KOs showed no binding of their respective ligands in any area examined (see Table 1 and Fig. 4). Binding to Avpr1a and Oxtr was unaffected in Oxtr−/− and Avpr1a−/− mice, respectively. The exception was in the BNST of the Oxtr line, where there is a difference between KO and WT. This difference appears to be due to spuriously low levels of binding in the Oxtr+/+, as binding in the Oxtr−/− mice was comparable to binding in WTs from other lines (p>0.1). The OxtrFB/FB had reduced levels of Oxtr in every area examined (to between 12 and 17% of wildtype levels). Avpr1a were also reduced in the CeA of OxtrFB/FB mice by about 50% (see Table 1).
Table 1.
Avpr1a and Oxtr binding in the forebrain. Binding to Avpr1a and Oxtr expressed as a fold increase above background minus 1; 0 equals no binding (mean ± SEM). OxtrFB/FB had decreased Oxtr binding throughout the forebrain. Avpr1a binding was also reduced in the CeA, and marginally in the ZI. The total KOs showed no biding in any area examined. Binding to Avpr1a and Oxtr was unaffected in Oxtr−/− and Avpr1a−/− mice, respectively. BNST levels of Oxtr−/− are comparable to WTs in other lines; Oxtr+/+ mice, Avpr1a levels are lower than other lines, perhaps due to sampling error.
| Avpr1a | Oxtr | FB Oxtr | ||||
|---|---|---|---|---|---|---|
| Avpr1a+/+ | Avpr1a−/− | Oxtr+/+ | Oxtr−/− | Oxtr+/+ | OxtrFB/FB | |
| Oxtr Binding | ||||||
| CeA | 3.19 ± 0.15 | 3.37 ± 0.24 | 2.82 ± 0.15 | 0.02 ± 0.02 | 2.47 ± 0.19 | 0.29 ± 0.07*** |
| MeA | 4.18 ± 0.18 | 5.57 ± 0.70 | 4.75 ± 0.66 | −0.03 ± 0.03 | 2.98 ± 0.15 | 0.50 ± 0.09*** |
| Hipp & DG | 1.26 ± 0.05 | 1.45 ± 0.12 | 1.59 ± 0.08 | 0.01 ± 0.02 | 1.36 ± 0.16 | 0.22 ± 0.03*** |
| PRh | 4.36 ± 0.29 | 4.82 ± 0.22 | 4.31 ± 0.14 | −0.02 ± 0.03 | 3.66 ± 0.30 | 0.60 ± 0.08*** |
| Pir | 6.09 ± 0.23 | 6.42 ± 0.21 | 5.26 ± 0.31 | −0.06 ± 0.03 | 4.76 ± 0.48 | 0.56 ± 0.07*** |
| Avpr1a Binding | ||||||
| LS | 4.88 ± 0.43 | 0.00 ± 0.01 | 4.42 ± 0.29 | 4.42 ± 0.21 | 5.76 ± 0.53 | 6.03 ± 0.53 |
| BNST | 2.36 ± 0.17 | 0.16 ± 0.02 | 1.86 ± 0.13** | 2.59 ± 0.14 | 2.62 ± 0.12 | 2.45 ± 0.12 |
| CeA | 2.15 ± 0.02 | 0.13 ± 0.02 | 1.98 ± 0.22 | 1.88 ± 0.04 | 2.61 ± 0.43 | 1.39 ± 0.30* |
| ZI | 4.63 ± 0.41 | 0.03 ± 0.02 | 4.15 ± 0.27 | 4.24 ± 0.22 | 4.62 ± 0.63 | 3.11 ± 0.40^ |
| LH | 9.03 ± 0.61 | 0.05 ± 0.01 | 7.67 ± 0.38 | 8.14 ± 0.59 | 9.09 ± 1.22 | 6.93 ± 0.44 |
BNST = bed nucleus of the stria terminalis; CeA = central nucleus of the amygdala; DG = dentate gyrus; Hipp = hippocampus; LH = lateral hypothalamus; LS = lateral septum; MeA = medial nucleus of the amygdala; Pir = piriform cortex; PRh = perirhinal cortex; ZI = zona incerta.
p=0.069,
p<0.05,
p<0.01,
p<0.001
Figure 4.
Images from in vitro receptor autoradiography of the Avpr1a, Oxtr and FB Oxtr lines (columns). Coronal sections are approximately 2mm behind bregma.
Discussion
We examined the effect of total Oxtr and Avpr1a deletion, and post-weaning forebrain Oxtr alteration on fear behavior. Total elimination of Oxtr or Avpr1a had no effect on fear conditioning. We found no alteration of Avpr1a binding in the Oxtr−/−, or of Oxtr binding in the Avpr1a−/− mice, despite elimination of the targeted receptor. Only the OxtrFB/FB mice, with reduced Oxtr expression throughout the forebrain and concomitant Avpr1a reduction in the CeA, had altered fear behavior. Post-shock, context and cue retention freezing were all reduced relative to the WT mice. This did not reflect a perceived difference in shock intensity, as OxtrFB/FB and Oxtr+/+ have similar levels of shock reactivity. It is not due to hyperactivity, as OxtrFB/FB do not display increased locomotion in an open field or in an empty cage (Lee et al., 2008). These data show that the timing of receptor loss/pattern of elimination and sparing of Oxtr receptors are critically important for fear conditioning. The OxtrFB/FB’s freezing deficit across experimental phases is consistent with a model in which the acquisition of fear is reduced due to alterations in the CeA circuit that contains Oxtr and Avpr1a. Spatial and temporal targeting of receptors in specific nuclei will determine their role in other stages of memory.
That Oxtr and Avpr1a KOs have fear expression equal to their WT counterparts is surprising. Administration of exogenous Oxt and Avp has effects on many forms of memory and anxiety (see (Caldwell et al., 2008; Landgraf and Neumann, 2004; Lee et al., 2009; Neumann, 2008; Ross and Young, 2009). Intra-amygdalar administrations of Oxt and Avp have opposite effects on freezing and heart rate (Roozendaal et al., 1992). Similarly, subcutaneous injections of Oxt C-terminal fragments decreases freezing while Avp fragments increase freezing following foot shock (Stoehr et al., 1992). Recent work by Huber et al. (2005) points to a circuit within the CeA where Oxtr are expressed on inhibitory neurons in the lateral CeA (CeL) that project to Avrp1a-expressing neurons in the medial CeA (CeM). Our expectation that Oxtr and Avpr1a deletion would have opposite effects on fear behavior were not born out by our experiments.
A perceived decrease in US strength could explain the OxtrFB/FB performance on acquisition, context and cued fear. Oxt and Avp have both been implicated in pain perception in rats (Ge et al., 2002; Yang et al., 2007). The OxtrFB/FB receptor loss late in development may have influenced perceived shock intensity (and thus US strength) in way that the constitutive receptor loss in the Oxtr line did not. The OxtrFB/FB and OxtrWT/WT did not differ in shock reactivity, however. Moreover, in mice, Oxt’s analgesic effect appears to be mediated by Avpr1a. Oxtr−/− mice have pain sensitivity identical to WT mice, including Oxt-induced analgesia, while Oxt administration has no effect on pain thresholds in Avpr1a−/− mice (Schorscher-Petcu et al., 2010). The reduced freezing reported in the OxtrFB/FB mice is not likely a performance effect, either: while they showed lower levels of freezing than WTs, their levels of freezing did increase across acquisition. Reduced OxtrFB/FB freezing is not due to the inability to freeze. Future work examining behavioral endpoints not dependent on freezing, or measuring freezing to an unconditioned fear stimulus, will provide additional insight.
Oxtr binding was reduced throughout the forebrain of the OxtrFB/FB mice; it is difficult to pinpoint exactly the structures for which Oxtr loss critically affected behavior. Oxtr and Avpr1a are expressed in several areas involved in fear conditioning. Lesions of the dorsal hippocampus attenuate contextually conditioned freezing, cued fear, under conditions that support robust conditioning (Kim and Jung, 2006; Phillips and LeDoux, 1992). This may reflect the association strength and the nature of the cues (Maren, 2008), as dorsal hippocampal lesions reduce cued fear with fewer trials or a weaker US (Quinn et al., 2008). Lesion of dorsal and ventral hippocampus can also reduce cued fear, however (Burman et al., 2006). Oxtr are robustly expressed throughout the hippocampus (Insel et al., 1991; Lee et al., 2008; Veinante and Freund-Mercier, 1997) and may have influenced OxtrFB/FB fear. Oxtr reduction in the perirhinal cortex (PRh) also may have influenced the OxtrFB/FB deficit through direct involvement in memory retrieval (Burwell et al., 2004) or by providing additional cues that aid retrieval (Corodimas and LeDoux, 1995). Lesions of the PRh block fear potentiated startle to a visual cue (Rosen et al., 1992) while inactivation impairs freezing to discrete auditory and contextual cues (Sacchetti et al., 2002). Lesions of the amygdala also decrease freezing to both context and discrete cues (Goosens and Maren, 2001; LeDoux et al., 1990; Maren, 1999; Wallace and Rosen, 2001), a finding also seen with inactivation of just the CeA (Wilensky et al., 2006). Huber et al. (2005) described a population of Oxtr-expressing neurons in the CeL that inhibit neurons in the CeM. CeM neurons are excited by Avp, through Avpr1a, and likely project to areas mediating fear responses. This is consistent with local inhibitory connections within the CeL and between the CeL and CeM that are crucial for plasticity related to conditioned fear (Ciocchi et al., 2010; Haubensak et al., 2010). While each of these areas, or interactions between them, may have contributed to the OxtrFB/FB deficit, it is interesting to note that only the OxtrFB/FB had both a behavioral deficit and Oxtr/Avpr1a reduction. Other receptor levels in the CeA may be modulated following Avpr1a or Oxtr deletion, and may be differently modulated by our constitutive versus conditional receptor deletions. Future research will focus on changes in CeA gene expression that may occur as a result of receptor deletions in the KOs.
Oxt has organizational effects during gestation and the neonatal period. Male Oxtr−/− mice (Takayanagi et al., 2005) and Oxt KO mice born to homozygous KO parents (Winslow et al., 2000) have elevated levels of aggression, a phenomenon not seen in KOs from non-obligate Oxt KO pairings (both parents heterozygotes). Thus prenatal Oxt has an important organizational effect that influences adult aggressive behavior. In C57BL/6 mice, maternal separation during the first two weeks of life increases anxiety behavior and maternal aggression in females, effects that are mirrored by a concomitant decrease in Oxt immunoreactivity in the paraventricular nucleus of the hypothalamus (Veenema et al., 2007). A high amount of maternal grooming increases Oxtr in the CeA of adult female mice, while the same grooming increases Avpr1a binding in the CeA of male mice (Francis et al., 2002). Oxt also influences the expression of other receptors. Neonatal Oxt increases the expression of estrogen receptors (ER) in hypothalamic regions of adult female prairie voles, while Oxt antagonists increase ER in the bed nucleus of the stria terminalis of males (Kramer et al., 2007). A single injection of Oxt or an Oxt antagonist given to prairie voles on the first postnatal day alters the expression of Avpr1a in several brain regions of adults (Bales et al., 2007).
The opposing actions of neuropeptides like Oxt and Avp in the CeA are part of balanced, individually tuned circuits acting in concert to control behavior (Viviani and Stoop, 2008). The timing of receptor deletion could alter this balance differently, leading to different phenotypes. Oxtr−/− mice constitutively lack Oxtr and are not subject to Oxt’s pre- and postnatal effects. The effects of lifelong Oxtr deletion may be obscured by compensatory mechanisms yet to be identified. Oxtr deletion in the OxtrFB/FB mice begins after PND 21 (Lee et al., 2008) when expression of cre recombinase, under control of the Camk2a promoter, begins. In OxtrFB/FB, receptor loss may occur after the organizing effect of Oxt and beyond the point at which compensation can occur; the critical period for Oxt’s organizational effects remains to be determined. Future work focusing on spatially restricted elimination of Oxtr across developmental time points will be useful in teasing apart the contribution of Oxt’s organizational and activational effects.
Fear is a basic emotion that strongly motivates behavior. Its maladaptation is associated with a variety of emotional disorders. Reduction of Oxtr and Avpr1a within the CeA of OxtrFB/FB mice may upset the balance of a circuit critical for specific fear responses (Huber et al., 2005; Viviani and Stoop, 2008). We hypothesized that a reduction in Oxtr would reduce inhibition of Avpr1a neurons in the CeA and would result in potentiated fear responding. This had presumed normal levels of Avpr1a that the OxtrFB/FB mice do not have: it is apparent from the binding results that the post-weaning loss of Oxtr affected Avpr1a levels as well. Reduction of Avpr1a may have reduced activation of the CeM and consequently diminished behavioral responding. Homozygous Brattleboro rats, known for their absence of Avp, show decreased freezing when re-exposed to a context associated with footshock (Stoehr et al., 1993). While there is evidence to suggest a causal link between early Oxt manipulations and effects on Avpr1a (this paper; Bales et al., 2007) the mechanism is currently unknown. Understanding this relationship is necessary for comprehending how perturbation of the amygdalar circuit is involved in the etiology of affective disorders.
Acknowledgements
The authors would like to thank Kevin Brown, Michael Burman and Michael Lehmann for their comments on the original document and Emily Shepard and June Song for their technical assistance. This research was supported by the NIMH Intramural Research Program (Z01-MH-002498-22).
Footnotes
The authors state that they have no conflicts of interest.
References
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis C, Balestre M-N, Jard S, Tibollet E, Arsenijevic Y, Dreifuss JJ, Bankowski K, Manning M, Chan WY, Schlosser SS, Holsboer F, Elands J. Characterization of a novel, linear radioiodinated vasopressin antagonist: an excellent radioligand for vasopressin V1a receptors. Neuroendocrinology. 1995;62:135–146. doi: 10.1159/000126998. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Breton JD, Poisbeau P, Darbon P. Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord. Mol Pain. 2009;5:63. doi: 10.1186/1744-8069-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16:103–113. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ. Perirhinal and postrhinal contributions to remote memory for context. J Neurosci. 2004;24:11023–11028. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an "original" neuropeptide. Prog Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, Ledoux JE. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behav Neurosci. 1995;109:613–619. doi: 10.1037//0735-7044.109.4.613. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elands J, Barberis C, Jard S, Tribollet E, Dreifuss JJ, Bankowski K, Manning M, Sawyer WH. 125I-labelled d(CH2)5[Tyr(Me)2,Thr4,Tyr-NH2(9)]OVT: a selective oxytocin receptor ligand. Eur J Pharmacol. 1988;147:197–207. doi: 10.1016/0014-2999(88)90778-9. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Ledoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Ge Y, Lundeberg T, Yu LC. Blockade effect of mu and kappa opioid antagonists on the anti-nociception induced by intra-periaqueductal grey injection of oxytocin in rats. Brain Res. 2002;927:204–207. doi: 10.1016/s0006-8993(01)03346-7. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SB, Zhao ZS, Yhap C, Grinberg A, Huang SP, Westphal H, Gold P. Vasopressin receptor 1a-mediated negative regulation of B cell receptor signaling. J Neuroimmunol. 2003;135:72–81. doi: 10.1016/s0165-5728(02)00442-3. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Yoshida S, Papademetriou E, Cushing BS. The organizational effects of oxytocin on the central expression of estrogen receptor alpha and oxytocin in adulthood. BMC neuroscience. 2007;8:71. doi: 10.1186/1471-2202-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ledoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav. 2009;8:558–567. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Phillips RG, Ledoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Ma QD, Tinsley MR, Fanselow MS. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus. 2008;18:640–654. doi: 10.1002/hipo.20424. [DOI] [PubMed] [Google Scholar]
- Rojas-Piloni G, Mejia-Rodriguez R, Martinez-Lorenzana G, Condes-Lara M. Oxytocin, but not vassopressin, modulates nociceptive responses in dorsal horn neurons. Neurosci Lett. 2010;476:32–35. doi: 10.1016/j.neulet.2010.03.076. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Schoorlemmer GH, Wiersma A, Sluyter S, Driscoll P, Koolhaas JM, Bohus B. Opposite effects of central amygdaloid vasopressin and oxytocin on the regulation of conditioned stress responses in male rats. Ann N Y Acad Sci. 1992;652:460–461. doi: 10.1111/j.1749-6632.1992.tb34384.x. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Miserendino MJ, Falls WA, Campeau S, Davis M. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. J Neurosci. 1992;12:4624–4633. doi: 10.1523/JNEUROSCI.12-12-04624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Baldi E, Lorenzini CA, Bucherelli C. Differential contribution of some cortical sites to the formation of memory traces supporting fear conditioning. Exp Brain Res. 2002;146:223–232. doi: 10.1007/s00221-002-1165-y. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Davis M. Visual pathways involved in fear conditioning measured with fear-potentiated startle: behavioral and anatomic studies. J Neurosci. 2001;21:9844–9855. doi: 10.1523/JNEUROSCI.21-24-09844.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci. 1996;93:3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Gallagher M, Stanton ME. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn Mem. 2007;14:597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr JD, Cheng SW, North WG. Homozygous Brattleboro rats display attenuated conditioned freezing responses. Neurosci Lett. 1993;153:103–106. doi: 10.1016/0304-3940(93)90087-2. [DOI] [PubMed] [Google Scholar]
- Stoehr JD, Cramer CP, North WG. Oxytocin and vasopressin hexapeptide fragments have opposing influences on conditioned freezing behavior. Psychoneuroendocrinology. 1992;17:267–271. doi: 10.1016/0306-4530(92)90067-h. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci. 2005;102:16096–17101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- Viviani D, Stoop R. Opposite effects of oxytocin and vasopressin on the emotional expression of the fear response. Prog Brain Res. 2008;170:207–218. doi: 10.1016/S0079-6123(08)00418-4. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci. 2001;21:3619–3627. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O'carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, Ledoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC. Central oxytocin enhances antinociception in the rat. Peptides. 2007;28:1113–1119. doi: 10.1016/j.peptides.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Kuhar MJ. A new method for receptor autoradiography: [3H]opioid receptors in rat brain. Brain Res. 1979;179:255–270. doi: 10.1016/0006-8993(79)90442-6. [DOI] [PubMed] [Google Scholar]