Abstract
Soluble guanylyl cyclase (sGC) is a key protein in the nitric oxide (NO)/-cGMP signaling pathway. sGC activity is involved in a number of important physiological processes including smooth muscle relaxation, neurotransmission and platelet aggregation and adhesion. Regulation of sGC expression and activity emerges as a crucial factor in control of sGC function in normal and pathological conditions. Recently accumulated evidence strongly indicates that the regulation of sGC expression is a complex process modulated on several levels including transcription, post-transcriptional regulation, translation and protein stability. Presently our understanding of mechanisms governing regulation of sGC expression remains very limited and awaits systematic investigation. Among other ways, the expression of sGC subunits is modulated at the levels of mRNA abundance and transcript diversity. In this review we summarize available information on different mechanisms (including transcriptional activation, mRNA stability and alternative splicing) involved in the modulation of mRNA levels of sGC subunits in response to various environmental clues. We also summarize and cross-reference the information on human sGC splice forms available in the literature and in genomic databases. This review highlights the fact that the study of the biological role and regulation of sGC splicing will bring new insights to our understanding of NO/cGMP biology.
Keywords: nitric oxide, soluble guanylyl cyclase, splicing, regulation
1. Introduction
1a. Role of soluble guanylyl cyclase in nitric oxide signaling
It has been more than 30 years since nitric oxide (NO), a gaseous free radical, was recognized as a critical physiologic signaling molecule. Since that time the list of known NO-directed functions has grown substantially and includes the regulation of smooth muscle function in vascular and gastrointestinal systems, inhibition of platelet aggregation and adhesion, neurotransmission and neuromodulation, regulation of cellular respiration and cytotoxicity, mitochondrial biogenesis and immune defense [1]. In living organisms NO is synthesized enzymatically from L-arginine by the family of nitric oxide synthases (NOS), including endothelial (eNOS), neuronal (nNOS) and inducible (iNOS) isoforms. Generated NO is connected to downstream signaling by the enzyme soluble guanylyl cyclase (sGC), which produces the secondary messenger cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP). NO binding to sGC heme moiety increases the production of cGMP more than a hundred fold [2]. Increases in cGMP concentrations regulate activity of a number of cGMP-dependent proteins, including cGMP-binding protein kinases, cGMP-dependent phosphodiesterases and cyclic nucleotide gated ion channels [3].
The use of organic nitrates in the treatment of angina pectoris and heart failure dates back to the 19th century [4]. However, it was only when the mechanism of action of these nitrovasodilators was demonstrated to depend on sGC function, that the enzyme attracted the deserved attention from the scientific community [5]. Studies examining the regulatory mechanisms directly modulating sGC activity and/or expression, which could be used to circumvent the direct use of NO-donors, have been motivated further by the discovery of NO cytotoxicity and by the negative side-effects of prolonged use of nitro-vasodilators [6].
1b. sGC properties and its expression pattern
sGC is a heme-containing obligatory heterodimer composed of α and β subunits [7]. Its expression has been detected in all studied tissues, albeit at varying levels and with different subunit compositions [8]. Although sGC was originally purified from a cytosolic fraction, it was subsequently found associated with cellular membranes in a variety of cell types [9–15]. Each sGC subunit has two isoforms (α1/α2 and β1/β2) encoded by a total of 4 separate genes. Only sGC heterodimers containing the β1 subunit have been shown to possess catalytic activity in vivo [16]. While α subunits (α1 and α2) have similar catalytic properties, they differ in their tissue and subcellular distribution [8, 13, 16]. The functional role of the β2 subunit still remains unclear since native functional heterodimers containing this β2 subunit have yet to be isolated from tissues [17]. Recombinant β2 homodimer is reported to have relatively low enzymatic activity [18] while overexpression of β2 was shown to reduce NO-sensitive sGC activity by outcompeting β1 for heterodimerization with the α1 subunit [19].
1c. sGC function in mammalian physiology
With the generation and characterization of sGC knock-out (GC-KO) mouse strains has come a greater understanding of these genes roles in mammalian physiology (for an excellent review see [20]). Mouse knock-out studies have demonstrated that NO-dependent vascular relaxation and platelet aggregation depend exclusively on the functional expression of α1/β1 and α2/β1 sGC heterodimers [21, 22]. Moreover, targeted disruption in vascular smooth muscle cells (VSMC) is sufficient to induce hypertension in mice, underscoring the importance of sGC in the maintenance of normal blood pressure [23]. Surprisingly, GC-KO mice also present with severe developmental and gastrointestinal (GI) disorders, revealing important roles for sGC in embryonic development and GI function [22]. The significance of individual sGC subunits in overall enzyme activity was demonstrated by analysis of knockout strains for β1, α1 and α2 sGC subunits. The expression of β1 subunit was found to be non-redundant for sGC enzymatic function [21]. The α1 and α2 sGC subunits demonstrated partially overlapping roles in vascular and gastric nitrergic smooth muscle relaxation [8, 24, 25]. However, several important physiological functions, such as development of testosterone-dependent hypertension, NO-mediated pulmonary vasodilation and vascular remodeling in chronic hypoxia appeared to be mediated specifically by α1 and not by the α2 sGC subunit, pointing to divergent roles of α sGC genes [26–29]. Recent data demonstrating disrupted long term potentiation (LTP) in visual cortex and hippocampal slices isolated from α2 knock-out mice suggests a non-redundant role for this subunit in LTP expression [30, 31].
2. Regulation of sGC expression
2a. Why is it important?
Despite its critical role in NO signaling, there is rather limited information regarding how the regulation of expression of individual sGC subunits affects NO-dependent processes. The importance of sGC expression for regulation of activity is underscored by numerous studies which demonstrate that changes in mRNA and protein levels are associated with the impairment of vascular function in cardiovascular disorders. Decreased sGC expression is proposed to be one of the mechanisms underlying the development of endothelial dysfunction and tolerance to organic nitrates [32–35]. Impaired sGC expression is also implicated in the development of hypertension [36, 37] and atherosclerosis [38]. Down-regulation of sGC occurs both with aging and in response to a high salt diet in aortas of spontaneously hypertensive (SHR) rats [34, 39]. Finally, age-dependent decreases in estrogen level and associated increased vascular stiffness of aortas were shown to correlate with decreased levels of α1 and β1 sGC [40].
Detailed studies of sGC-KO mice additionally highlight the importance of regulating individual sGC subunit expression. Tamoxifen-induced tissue-specific knockout of the β1 subunit in vascular smooth muscle demonstrated that NO-stimulated cGMP production was reduced in a dose-dependent manner that correlated with gradual decreases in β1 sGC protein levels [23]. Deletion of α1 sGC, has been shown to significantly reduce basal and NO-stimulated sGC activity in mouse aortas [26, 41], left ventricular myocardium [26] and gastric tissues [25, 27, 28, 42]. This decreased sGC function was sufficient to compromise myocardial function [26] and promote hormone-dependent hypertension in hypoxic stress conditions [29]. Yet the severity of these phenotypes was partially compensated by changes in the expression of the α2 sGC subunit. Thus, all accumulated evidence directly indicates that regulation of the expression of α1, α2 and β1 genes is vital to maintain the integrity of NO/cGMP signaling in mammalian physiology.
2b. Regulation of sGC expression by changes in mRNA steady-state levels
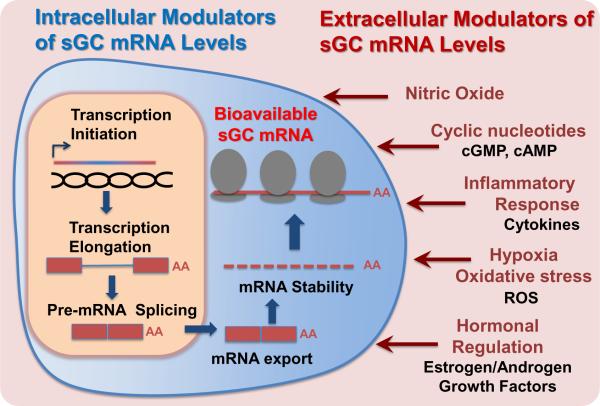
It has been demonstrated that the expression of sGC subunits may be modulated at the levels of mRNA abundance and/or protein stability (for excellent reviews on this subject see [43, 44]). Our review focuses primarily on regulation occurring at the mRNA level, emphasizing the specific role of the alternative splicing of sGC subunit genes. Multiple reports demonstrate that the regulation of steady-state sGC mRNA levels occurs in response to environmental clues. Clear differences in steady-state mRNA levels of individual sGC subunits have been observed in response to a number of extracellular and intracellular stimuli (see Figure 1). The α1 and β1 sGC mRNAs are affected by inflammatory regulators such as lipopolysaccharide (LPS), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), NO, reactive oxygen species (ROS), and by increased cAMP and cGMP levels [45–56]. sGC mRNA expression is also regulated by the nerve growth factor (NGF) [57]. Changes in the levels of various sex hormones also alter mRNA steady-state levels. Estrogen is known to rapidly down-regulate the α1 and β1 sGC in rat uterus [59], while in anterior pituitary gland it exerts a dual effect by decreasing β1 and increasing α1 sGC mRNA levels [58]. The α1 sGC mRNA is also increased upon exposure to androgen, which is thought to contribute to the proliferation of prostate cancer cells [60]. Thus, it is clear that sGC mRNA levels are regulated by multiple alternative mechanisms in a tissue-specific (cell-type specific) and a stimuli-specific manner. What remains unclear is the precise role of alternative RNA splicing in this process, given that the majority of studies have been done without quantification of individual RNA transcript levels. Instead, primers and probes typically have been targeted to the most conserved parts of sGC genes sequences, which are present in the majority of splice variants generated from the specific gene.
Figure 1. Factors modulating sGC steady state mRNA levels: environmental clues and regulatory mechanisms.
Several mediators associated with physiological and pathophysiological conditions influence the variety, stability, processing and localization of individual sGC transcripts, resulting in changes of steady-state transcript levels.
The maintenance of steady-state mRNA levels is achieved not only through transcription, but also through regulation of pre-mRNA splicing and mRNA stability (see Figure 1). Furthermore, the amount of mRNA available for translation is also dependent on polyadenylation, nuclear to cytoplasmic transport, subcellular localization/sequestration, and miRNA targeting. Below we will summarize the existing data related to the regulation of steady-state levels of sGC mRNA while highlighting the role of alternative splicing in this process.
2b-1. Transcription Initiation
For many years the rate of transcription initiation was considered to be a principal mode of regulation of mRNA abundance. We now know that post-transcriptional mechanisms like mRNA stability, splicing, intracellular localization/sequestration, and miRNAs play critical roles in gene regulation [61–63]. However, the regulation of transcription initiation remains an important and, from a metabolic point of view, the most economical way to modulate the expression of a particular gene.
The first study regarding α1 and β1 sGC gene transcriptional regulation was an examination of promoters from the Medaka fish. The α1 and β1 genes from Medaka fish are localized on the same chromosome and separated by less than 1000 base pairs. Their transcription appears to be cis-regulated in a polycistronic manner [64]. Similar regulation was proposed for human α1 and β1 sGC genes [64], as they are also localized to a single small chromosomal region [65]. However, the results from several laboratories demonstrated that all four mammalian sGC genes (α1, α2, β1 and β2) are transcribed independently. In humans the α2 and β2 sGC genes are localized to different chromosomes, 11 and 13 respectively, most definitely precluding a cis mode of transcriptional regulation. Promoter regions of α1 and β1 sGC in human and rodents (mice and rats) have been mapped ectopically to define specific sequences required for transcriptional activity [66–70]. The promoters for α2 and β2 sGC genes remain to be examined.
Several conserved features exist between α1 and β1 sGC promoters in humans and rodents. The core promoter regions of α1 and β1 sGC genes are TATA-less and contain a number of similar transcription factor (TF) binding sites. Importantly, each has a CCAAT-binding site, which is associated with the CBF (NF-Y) transcriptional factor, and shown to be essential for house-keeping promoter activity in all species investigated to date [66, 67, 69, 70]. The presence of these common elements suggests a potential for coordinated regulation of α1 and β1 sGC genes transcription.
Consistent with the reports indicating that sGC expression is regulated during embryonic development [71–75], TF binding sites involved in morphogenesis and differentiation, such as c-Myb, AP1, GATA1, Nkx-2.5 and Lyf-1, have been identified in the α1 and β1 sGC promoters [66, 67, 69]. Additionally, human and rodent α1 sGC promoters possess a variety of binding sites for stress response TFs, including NFAT, NF-kB, AP1 and SP1. Reporter construct studies in primary human aortic smooth muscle cells revealed regulation by pro-inflammatory stimuli (combination of LPS, γ-INF, IL-1β, TNFα or NO donors) mapped to NF-kB and CBF binding sites [67]. Finally, NFATc3 (nuclear factor of activated T cells isoform c3) has been shown to promote the transcription of the α1 sGC gene in response to pulmonary hypoxia in mice [76].
Thus, accumulated evidence indicates that transcription plays an important role in regulation of sGC mRNA levels, especially in response to inflammation and during embryonic development. Still, our understanding of the mechanisms governing transcription of all four sGC genes remains very limited and awaits further investigation.
2b-2. mRNA stability
The bioavailability of newly transcribed RNA for translation depends on several important factors including accurate processing, export to the cytoplasm, and mRNA stability. It is the balance of these events that determines overall steady-state mRNA levels. For sGC transcripts, mRNA stability is known to play an important role and is probably the best studied among these regulatory mechanisms. The rate of mRNA degradation is determined by intrinsic properties of each individual RNA primary sequence, and a number of mechanisms regulate this process. Multiple studies indicate that stability of sGC mRNAs is tightly regulated in mammalian cells and varies in response to different stimuli. For example, elevated levels of NO, cyclic nucleotides and growth factors have been shown to modulate the stability of α1 and β1 sGC mRNAs [50, 51, 57, 77]. Exposure to N-methyl-D-aspartate (NMDA) was found to increase the stability of α2 sGC mRNA in rat cerebellar granule cells [78].
The feedback mechanism that destabilizes sGC transcripts in response to elevated cAMP/cGMP levels following cell stimulation with NO-donors or forskolin was recently unraveled [51, 77]. HuR (Human-Antigen R) protein acts to stabilize α1 and β1 sGC transcripts by binding Adenine- and Uracil-rich elements (ARE) located in the 3'-untranslated regions (UTRs). It has been demonstrated that HuR down-regulation in response to elevated cAMP/cGMP levels increases the rate of α1 and β1 mRNAs degradation in rat aortic cells [51]. In the spontaneously hypertensive rat (SHR) model, reductions in HuR protein are associated with decreased α1 and β1 sGC mRNA levels and contribute to the development of age-dependent hypertension [52]. It was also shown that the exposure to chronic hypoxia decreases cytosolic levels of HuR protein with concomitant reduction of α1 sGC mRNA in murine pulmonary vascular smooth-muscle cells [76].
In neuronal cells, α2 sGC mRNA degradation is found to be regulated by Adenosine-Uridine binding factor 1 (AUF1). AUF1, along with HuR protein, is known to bind ARE sites located in the 3'UTR of the α2 sGC [79]. Interestingly, N-methyl-D-aspartic acid (NMDA) treatment of rat cerebellar granule cells elevates α2 sGC mRNA levels, which is associated with a decrease in AUF1, but not HuR protein. These data suggest that multiple RNA-binding proteins are involved in the regulation of sGC mRNA stability, and that they might act in both tissue-specific and stimuli-specific manners.
It seems likely, that a plethora of factors involved in the regulation of sGC mRNA stability remains to be discovered. Among these factors, regulation by small inhibitory RNAs and microRNAs is an intriguing and yet unexplored possibility.
2b-3. sGC splicing: literature and database analysis
Splicing allows the generation of different transcripts from the same precursor RNA by incorporation or exclusion of various exon sequences; a process termed alternative splicing. Changes in the un-translated (UTR) regions of RNA introduced by splicing have been shown to affect the stability, export, localization, and translational capacity of the transcript, either through modifications of RNA structure or by introducing/eliminating binding sites for various RNA-binding regulatory proteins. Variations involving coding sequence lead to alteration in encoded protein properties, thus increasing the functional diversity of the genome. Introduction of pre-mature stop codons by splicing regulates RNA stability through the activation of nonsense-mediated decay (NMD) pathways. Recent transcriptome analysis indicates that more than 90% of human genes are alternatively spliced, making it one of the most important post-transcriptional regulatory mechanisms [63].
Several studies have demonstrated that transcripts derived from all sGC genes undergo alternative splicing [73, 80–84]. Furthermore, there is a remarkable diversity of sGC mRNA transcripts deposited in the GenBank databases. However, the mechanisms involved in the regulation of sGC splicing and the biological role of different splice forms remains, for the most part, unknown. There is also a lack of a systematic unifying nomenclature for sGC transcripts. The recent sorting and validation of α1 sGC gene transcripts by NCBI (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov) was a welcome first step in the classification of splice forms of sGC genes [85]. However, new transcript and protein isoform names have been assigned by NCBI without taking into account the names in already published reports. To fill this gap, we summarize and cross-reference the information available in the literature and genomic databases for all genes of human sGC subunits. We have relied extensively on the UCSC Browser tool, which provides cross annotations from both NCBI and Ensembl databases [86].
α1 sGC (GUCY1A3 - Chromosome 4q32.1)
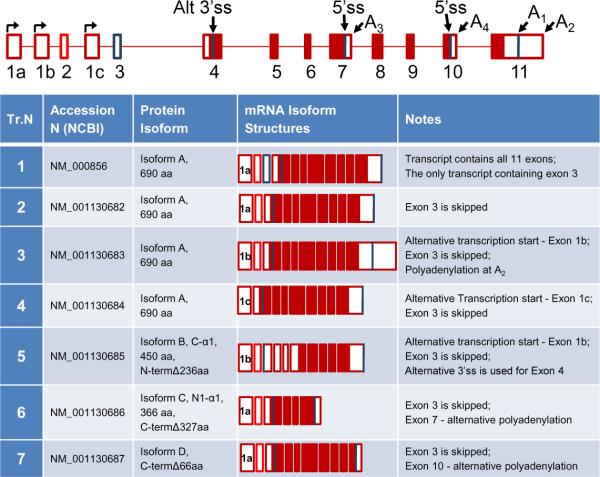
The α1 gene is found to be expressed in all examined tissues, with the highest levels detected in vasculature [8]. To date, α1 splice variants are the most diverse and best characterized group in comparison with other sGC genes. Recently, the variety of human α1 transcripts existing in the NCBI database was revised and consolidated into seven transcript types, which encode four different protein isoforms named A, B, C and D. We have cross-referenced and assembled them, highlighting their resulting mRNA structure relative to the genomic organization, and indicated their corresponding protein isoforms in Figure 2. All α1 transcripts can be sub-divided into two groups based on the encoded protein isoforms. The first group includes transcripts 1 to 4, which encode an identical 690 aa protein, isoform A (canonical α1 sGC). These transcripts differ from each other only in their 5'- and 3'-UTR sequences which, as discussed previously, likely play a role in regulation of mRNA stability. In the second group, transcripts 5 to 7 each encodes a unique polypeptide named isoforms B, C and D. Several of these splice forms have been assigned conventional names at the time of their original biochemical characterization (Figure 2).
Figure 2. Alternative splice forms of α1 sGC.
Annotated RefSeq mRNA isoforms are shown. The figure provides a schematized diagram of the α1 sGC gene. Three alternative transcription start sites are denoted. Alternative splice sites (Alt ss), polyadenylation sites (A), and exons are indicated. The “canonical” α1 sGC transcript (NCBI Transcript 1) is presented at top of the table. “Open” regions indicate untranslated (UTR) areas of the exons, “dark” regions – coding sequence.
Within the second group, alternative splice variants lacking the canonical translational start site were first reported by Ritter et al [87]. Through an analysis of transcripts present in the NCBI database, we identified additional splice forms encoding polypeptides with deletions in C- and N-termini of the α1 protein [84]. Using RT- and qRT-PCR analyses we demonstrated that transcripts encoding truncated α1 proteins are differentially expressed in normal human tissues. Characterization of these proteins performed in two different cell models, BE2 human neuroblastoma cells and Sf9 insect cells, demonstrated that encoded deletions generate sGC enzymes with altered functional properties. The N1-α1 variant (Transcript 6, isoform C) encodes α1 sGC protein with extensive deletion in the catalytic domain. It displays properties of a dominant negative mutant and inhibits activity of the α1/β1 sGC heterodimer. In contrast, the C-α1 sGC splice form (Transcript 5, isoform B), encodes a fully functional protein despite a 236 aa deletion of the N-terminal regulatory domain. sGC heterodimers containing C-α1 sGC are much less sensitive to ODQ-induced oxidative protein degradation and partially protect α1 sGC levels when co-expressed together in BE2 cells [84]. In a recent report, we established that C-α1 sGC splice form is expressed at high levels during differentiation of human embryonic stem cell (hES) and its intracellular distribution varies from canonical α1 sGC subunit [73].
The last isoform in this group (Transcript 7, isoform D) is a recently validated referenced sequence (RefSeq, NCBI) derived from human placenta and intestine libraries (http://www.ncbi.nlm.nih.gov/nuccore/NM_001130687). This transcript is generated through the use of an alternative polyadenylation in exon 10. The mRNA isoform is predicted to encode a polypeptide with a 66 aa deletion at the C-terminus. The deletion is located in close proximity to the catalytic domain and thus is likely to affect the catalytic properties of the generated spliced α1 polypeptide.
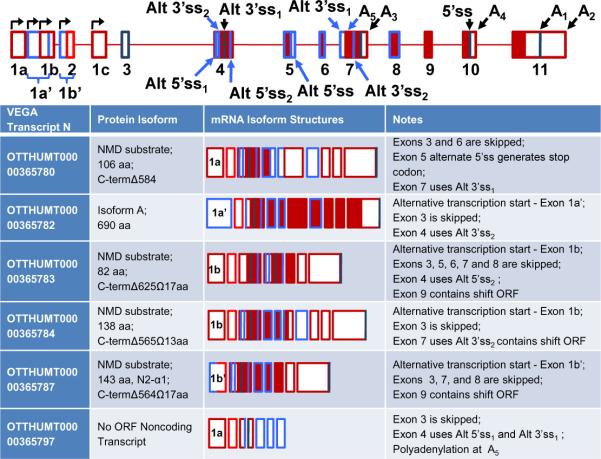
Finally, at least 6 additional alternative α1 sGC transcripts not found in the NCBI Referenced Sequences (RefSeq) database, including N2-α1 described earlier by our group ([84], Figure 3, VEGA Transcript N OTTHUMT000000365787), exist in the newly updated ENCODE Genecode database [88]. These splice variants encode a variety of short N-terminal polypeptides of the α1 protein. Four out of six transcripts (VEGA Transcript N OTTHUMT000000365780, 83, 84, and 87) represent potential substrates for nonsense mediated decay (NMD) due to the presence of premature stop codons within internal exons. NMD is a mechanism that detects and eliminates aberrant mRNAs whose expression would result in truncated proteins [89]. Splicing that favors creation of transcripts targeted to NMD when the transcription rate remains unchanged would result in quick depletion of total mRNA levels (activate an “RNA sink”). This process may function as an alternative mechanism to quickly down-regulate steady-state α1 mRNA levels. Whether this mechanism truly modulates α1 sGC levels remains to be determined. Regarding the remaining transcripts, one encodes the canonical protein isoform A (OTTHUMT00000365780) and the other is a small RNA with no appreciable ORFs (Figure 3). It should be noted that the reported exon 11 polyadenylation sites vary greatly among these transcripts suggesting that regulation at the 3' UTR may be more complex than currently recognized.
Figure 3. NonRefSeq Alternative splice forms of α1 sGC.
ENCODE mRNA isoforms not present in the RefSeq database are shown. The figure is based upon the gene structure provided in Figure 2, but adds additional alternative RNA processing events denoted by blue. “Open” regions indicate untranslated (UTR) areas of the exons, “dark” regions – coding sequence. Note that for the sake of simplicity the 3'UTR of exon 11 was kept a constant size.
Interestingly, only a single α1 sGC transcript with altered ORF exists in the mouse NCBI RefSeq database. It encodes a spliced polypeptide identical to human isoform C (Accession AK031305). The rest of the mouse transcripts encode the canonical α1 protein (isoform A) and differ only in the 5'- or 3'-UTR sequences. Evolutional conservation of isoform C between human and rodents suggests that it might play an important biological function.
α2 sGC (GUCY1A2 – Chromosome 11q22.3)
The α2 transcript has a more restricted pattern of expression than the α1 sGC and it is highly abundant in neuronal tissues [8]. The canonical α2 sGC transcript contains 8 exons (NM_000855 derived from Accession X63282, BC130488, BC130484, GenBank). The first evidence that the human α2 pre-mRNA undergoes alternative splicing was reported when the α2i splice variant was described [80]. This transcript is generated by the insertion of an alternative cassette exon after the coding exon 6 (Accession N Z50053, NCBI). Co-expression of recombinant α2i with the α2 and β1 subunits demonstrated that this splice form possess properties of a dominant negative mutant [80], very similar to the N1-α1 variant of the α1 sGC [84]. Analysis of database sequence also reveals the existence of a third type of splice transcript (Accession BC144033, GenBank), which is generated by an in-frame insertion of alternative exon after the coding exon 4. This exon encodes a unique 22 amino acid residue insertion in the proximity to the catalytic domain. Functional properties of this new α2 splice variant await further investigation.
Recently, the existence of a splice variant homologous to the human α2i has been demonstrated in the rat pituitary and liver tissues [82]. In this report semi-quantitative RT-PCR demonstrated that α2i, but not α2 sGC transcript, is up-regulated in response to estrogen in rat adult pituitary gland. This interesting observation suggests that the splicing of the α2 sGC subunit might be regulated by sex hormones in rodents. However, in the mouse non-referenced RNA database α2i is not present. Instead, three different transcripts encoding putative α2 proteins with deletions in N- and C- termini generated through exon exclusion are present (Accessions AK165150, AK147527 and AK034879, GenBank). These transcripts, which currently do not have human homologs in humans, remain an interesting subject for further investigation.
β1 sGC (GUCY1B3 Chromosome 4q31.1)
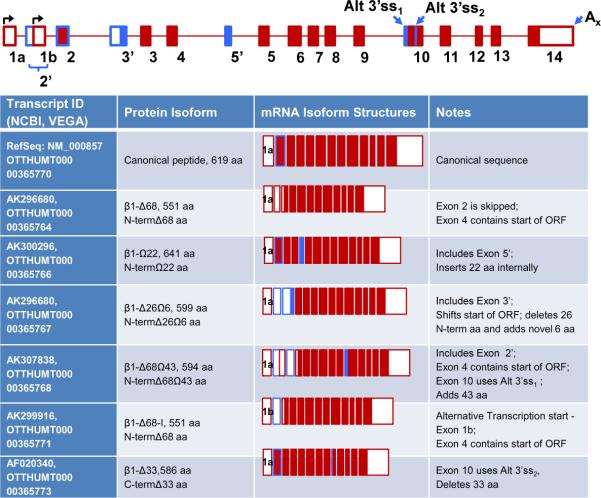
The ubiquitously expressed α1 sGC, is the primary “small” sGC subunit and is indispensable to support sGC activity [8, 22, 23]. Changes in α1 sGC mRNA steady-state expression levels have been demonstrated in multiple reports; however, the precise regulatory mechanisms are not well studied. Similar to α1 sGC, there is much evidence supporting a potential regulatory role for alternative splicing despite the fact that only a single RefSeq currently exists for the gene in NCBI database (NM_000857). Heterogeneity of β1 sGC transcripts was first demonstrated by Chhajlani et al. who showed the presence of two mRNA species in human lung tissue [83]. In addition to the canonical transcript, they observed a shorter mRNA (Accession AF020340, GenBank) predicted to encode a non-functional peptide containing 33 aa deletion in the proximity of catalytic domain (see Figures 4 and 5). No biochemical characterization of this isoform has been performed so far.
Figure 4. Alternative splice forms of β1 sGC.
The figure provides a schematized diagram of the β1 sGC gene. Two alternative transcription start sites are denoted. Alternative splice sites (Alt ss), polyadenylation sites (A), and exons are indicated. The single “canonical” RefSeq α1 sGC transcript is presented at top of the table. The remaining transcripts are derived from the ENCODE database. “Open” regions indicate un-translated (UTR) areas of exons, “dark” regions – coding sequence. Note that for the sake of simplicity the 3'UTR of exon 14 was kept a constant size.
Figure 5. Insertions and deletions introduced by the alternative splicing in β1 sGC protein sequence.
Analysis of encoded polypeptides was performed using CLUSTAL W (1.83) multiple sequence alignment (SDSC Biology Workbench).
Several additional non-referenced human β1 sGC isoforms are present in available sequence databases. These transcripts, their genomic organization, and changes in protein sequences are summarized in Figure 4. To simplify the recognition of the different α1 sGC splice forms, they are labeled based on corresponding changes in encoded polypeptides.
As shown in Figure 4, there are splice isoforms generated by use of an alternate promoter 1b (that removes the canonical start codon), skipping of exon 2, inclusions of alternative exons (2', 3' or 5') or changes involving 3' splice site selection for exon 10. These sequence rearrangements introduce changes in the ORF of α1 sGC and encode several polypeptides with unique deletions and insertions in N- and C-termini (Figure 5).
Alternative 3' splice site selection for exon 10 either introduces an in-frame 33 amino residue deletion, called by us β1-Δ33 variant (OTTHUMT00000365773) [83] or generates the β1-Δ68Ω43 variant (OTTHUMT00000365768) by 43 aa insertion (Figure 4). The location of these changes in the primary protein sequence suggests that these splice forms might have different catalytic properties. The β1-Δ68Ω43 variant (Accession AK307838, GenBank) also contains an extensive 68 aa deletion in the N-terminal regulatory region, resulting from insertion of a noncoding exon 2', which leads to a shift in the start of the ORF to exon 3. Loss of this N-terminal region is likely to lead to altered sGC regulatory properties. We observed expression of this splice form by RT-PCR analysis in a number of normal human tissues and several cancer cell lines (Sharina IG and Shah N, unpublished observation) suggesting that this variant is widely expressed, at least in humans.
Two β1 splice isoforms are generated through the inclusion of alternative exons downstream of exons 2 and 4 (β1-Δ26Ω6 and β1-Ω22, Figure 4). A third alternative isoform (β1-Ω68, Figure 4), derives from the use of an alternative downstream promoter. As a result, the ORF now begins in exon 4 (Figure 4, protein sequence alignment in Figure 5). The majority of α1 sGC transcripts encoding alternative splice variants were identified by the NEDO human cDNA sequencing project group from Tokyo, Japan (http://www.nbrc.nite.go.jp/hflcdna/cgi-bin). Changes in protein sequence of the β1 sGC encoded by these splice variants are likely to affect the regulatory properties of the sGC heterodimers containing them (specific coding changes are summarized in Figure 5).
It is important to note that when examined in detail, the human α1 sGC alternative cDNA encoding isoforms have been isolated from a variety of different tissues including: lung, fetal brain, placenta, tongue tumor tissue, etc. This wide diversity of tissues involved might suggest a complex tissue specific splicing of the α1 sGC gene. Needless to say, the functional roles of α1 sGC splice forms, their expression profiles and biochemical properties await systematic investigation.
β2 sGC (GUCY1B2 – Chromosome 13q14.3)
More than 20 years after the cloning of the β2 sGC subunit gene its biological role remains a mystery [80, 90]. According to several publications the α1/β2 heterodimer possesses very low, if any, enzymatic activity and was suggested to act as a dominant negative isoform to other subunits [19, 91]. It is the only known sGC subunit which is reported to possess activity as homodimer [18], resembling the particulate guanylyl cyclases. UniGene NCBI EST profile demonstrate that the β2 sGC transcript is expressed in several tissues, including kidney, intestine, lung, stomach, connective tissue, skin and mouth with highest level found in esophagus. The expression of β2 is detected in several tumors including esophageal and gastrointestinal (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer).
Similar to other sGC subunits, the β2 pre-mRNA undergoes alternative splicing. Several β2 splice variants have been described in the literature [17, 18, 81, 92]. The human canonical RefSeq gene is comprised of 17 exons (NR_003923) and surprisingly is listed as a non-coding RNA. The longest ORF (617 aa) derives from exons 4 through 15. Thus, the presence of a stop codon in exon 14 would predict that this transcript is a potential substrate for NMD. Two alternative transcripts encode the N-terminally truncated splice isoforms corresponding to the splice variants originally described by Behrends' group (Acc. Ns CR615161 and AK296746). The β2SV sGC, which is generated by the skipping of exons encoding the N-terminal heme-binding domain of the protein, was isolated from human corpus cavernosum [17]. Based on the size and location of the deletion, it was suggested that sGC enzyme containing this 2SV splice form should be NO-insensitive. Another splice form encoding an N-terminally truncated β2 sGC protein was isolated from human gastric carcinoma [81]. Both transcripts are predicted to contain C-terminal truncations resulting from alternative polyadenylation involving exon 12.
Finally, in a recent report, a new splice variant encoding an ORF with additional amino acids at the N-terminus was described [92]. Interestingly, the same report demonstrated that 5'UTRs of the two alternative β2 sGC transcripts (`canonical' and new splice form) possess an active internal ribosome entry site (IRES). Moreover, the activity of the 5'UTR from alternative transcript was much higher than that of `canonical' one, supporting the concept that alternative splicing plays a role in modulation of β2 sGC expression.
3. Conclusions
Why is it important to study sGC splicing?
The regulation of expression is crucially important to support sGC function in normal and pathological conditions. Data strongly indicate that the regulation of sGC expression is a complex process modulated on many levels including transcription, post-transcriptional regulation, translation and protein stability.
For some time we have known that sGC functions primarily as a heterodimer, comprised of two types of independent α and β subunits. A search for gene homologues uncovered the existence of four gene family members, allowing for the generation of four different heterodimers (α1/β1, α1/β2, α2/β1 and α2/β2), and perhaps a single homodimer (α2/β2), where once only a single enzyme form was described. It was the examination of these canonical sGC isoforms that first revealed differential expression and the unique functional consequences resulting from the choice of subunit composition. With the discovery of alternatively spliced sGC transcripts, we now know that enzyme complexity extends well beyond 5 types of sGC dimers. If one considers all theoretical peptide products generated by splicing: four α1, three β2, six α1, and three β2 subunits; there is the potential to generate a large number of unique heterodimers.
Splicing of sGC fundamentally contributes to sGC expression not only by giving rise to splice variants encoding sGC subunits with different biochemical properties, but also by producing transcripts with different regulatory sequences, which may affect mRNA stability, transport and translation. Alternative mRNA isoforms that differ in their 3' and 5' UTR regions, are likely to play critical roles in regulation of mRNA steady-state levels through stabilization mechanisms and/or inhibitory RNA targeting. The diversity of expressed sGC transcripts allows for dynamic spatio-temporal regulation in response to multiple physiological signals tailoring enzyme properties to better suit the molecular environment of the cell. Consideration of this observation alone is sufficient to provide compelling evidence for a crucial role of alternative splicing in regulation of sGC function.
The importance of splicing in the physiology of the NO/cGMP pathway is emphasized by the fact that other enzymes of this pathway also undergo alternative splicing. Accumulated evidence demonstrates that not only sGC, but also all NOS genes [93–96], cGMP-dependent protein kinase I [97] and several cGMP-dependant cyclic nucleotide phosphodiesterase (PDE) genes [98–101] express a variety of alternatively spliced transcripts. Moreover, aberrant splicing regulation of the eNOS gene has been associated with increased risk of coronary artery disease [95], while alternative splicing of PKGI has been implicated in the development of nitrate tolerance in vascular smooth muscle [97]. These data suggest that coordinated regulation of splicing of the enzymes participating in the NO/cGMP signaling may be involved in the development of cardiovascular disorders. Therefore, advancement in the understanding of the mechanisms of splicing will facilitate the development of new therapeutic strategies to combat vascular disorders and/or tailor the existing ones based on individual differences in the expression of splice isoforms of NO/cGMP pathway enzymes.
Studies of the regulation of sGC expression still remain in initial stages of development. All four sGC genes undergo splicing and demonstrate remarkable diversity of the transcripts. Though the biological role(s) of sGC splice forms are poorly understood, unraveling them will likely be fundamental to our understanding of sGC biology. Currently the components of splicing machinery participating in the regulation of sGC splicing remain completely unknown. A major challenge will be to define the mechanisms and splicing regulators participating in control of the exon selection on constitutive, cell-specific or stimulus-specific levels.
Acknowledgments
The authors would like to thank Mrs. Beverly Smulevitz for critical reading of the manuscript. This work was supported by in part by NIH grants HL088128, 3RO1HL088128-02S1 (to E.M.) and AHA grant 09GRNT2060182 (to E.M.) and startup funds from The University of Texas (to I.S.).
Abbreviations
- NO
nitric oxide
- eNOS
endothelial
- nNOS
neuronal
- iNOS
inducible nitric oxide synthases
- sGC
soluble guanylyl cyclase
- cGMP
cyclic guanosine monophosphate
- cAMP
cyclic adenosine monophosphate
- TF
transcription factor
- UTR
untranslated region
- ORF
open reading frame
- aa
amino acid
- NMD
nonsense-mediated decay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355(19):2003–11. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 2.Hoenicka M, et al. Purified soluble guanylyl cyclase expressed in a baculovirus/Sf9 system: stimulation by YC-1, nitric oxide, and carbon monoxide. J Mol Med. 1999;77(1):14–23. doi: 10.1007/s001090050292. [DOI] [PubMed] [Google Scholar]
- 3.Waldman SA, Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987;39(3):163–96. [PubMed] [Google Scholar]
- 4.Brunton TL. Use of nitrite of amyl in angina patient. Lancet. 1867;2:97–98. [Google Scholar]
- 5.Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol Sci. 1997;18(12):484–91. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- 6.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol. 2009;(191):277–308. doi: 10.1007/978-3-540-68964-5_13. [DOI] [PubMed] [Google Scholar]
- 7.Kamisaki Y, et al. Soluble guanylate cyclase from rat lung exists as a heterodimer. J Biol Chem. 1986;261(16):7236–41. [PubMed] [Google Scholar]
- 8.Mergia E, et al. Major occurrence of the new alpha2beta1 isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal. 2003;15(2):189–95. doi: 10.1016/s0898-6568(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 9.Bellingham M, Evans TJ. The alpha2beta1 isoform of guanylyl cyclase mediates plasma membrane localized nitric oxide signalling. Cell Signal. 2007;19(10):2183–93. doi: 10.1016/j.cellsig.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Feussner M, et al. Association of soluble guanylate cyclase with the sarcolemma of mammalian skeletal muscle fibers. Acta Histochem. 2001;103(3):265–77. doi: 10.1078/0065-1281-00602. [DOI] [PubMed] [Google Scholar]
- 11.Lin LH, Talman WT. Soluble guanylate cyclase and neuronal nitric oxide synthase colocalize in rat nucleus tractus solitarii. J Chem Neuroanat. 2005;29(2):127–36. doi: 10.1016/j.jchemneu.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Pifarre P, et al. NO-sensitive guanylyl cyclase beta1 subunit is peripherally associated to chromosomes during mitosis. Novel role in chromatin condensation and cell cycle progression. Int J Biochem Cell Biol. 2009;41(8–9):1719–30. doi: 10.1016/j.biocel.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Russwurm M, Wittau N, Koesling D. Guanylyl cyclase/PSD-95 interaction: targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J Biol Chem. 2001;276(48):44647–52. doi: 10.1074/jbc.M105587200. [DOI] [PubMed] [Google Scholar]
- 14.Venema RC, et al. Novel complexes of guanylate cyclase with heat shock protein 90 and nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2003;285(2):H669–78. doi: 10.1152/ajpheart.01025.2002. [DOI] [PubMed] [Google Scholar]
- 15.Zabel U, et al. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat Cell Biol. 2002;4(4):307–11. doi: 10.1038/ncb775. [DOI] [PubMed] [Google Scholar]
- 16.Russwurm M, et al. Functional properties of a naturally occurring isoform of soluble guanylyl cyclase. Biochem J. 1998;335(Pt 1):125–30. doi: 10.1042/bj3350125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrends S, et al. Expression of nitric oxide-sensitive guanylyl cyclase subunits in human corpus cavernosum. Biochem Pharmacol. 2000;59(6):713–7. doi: 10.1016/s0006-2952(99)00381-0. [DOI] [PubMed] [Google Scholar]
- 18.Koglin M, et al. Nitric oxide activates the beta 2 subunit of soluble guanylyl cyclase in the absence of a second subunit. J Biol Chem. 2001;276(33):30737–43. doi: 10.1074/jbc.M102549200. [DOI] [PubMed] [Google Scholar]
- 19.Gupta G, et al. The beta2 subunit inhibits stimulation of the alpha1/beta1 form of soluble guanylyl cyclase by nitric oxide. Potential relevance to regulation of blood pressure. J Clin Invest. 1997;100(6):1488–92. doi: 10.1172/JCI119670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friebe A, Koesling D. The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide. 2009;21(3–4):149–56. doi: 10.1016/j.niox.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Dangel O, et al. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J Thromb Haemost. 2010;8(6):1343–52. doi: 10.1111/j.1538-7836.2010.03806.x. [DOI] [PubMed] [Google Scholar]
- 22.Friebe A, et al. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci U S A. 2007;104(18):7699–704. doi: 10.1073/pnas.0609778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groneberg D, et al. Smooth muscle-specific deletion of nitric oxide-sensitive guanylyl cyclase is sufficient to induce hypertension in mice. Circulation. 2010;121(3):401–9. doi: 10.1161/CIRCULATIONAHA.109.890962. [DOI] [PubMed] [Google Scholar]
- 24.Nimmegeers S, et al. Functional role of the soluble guanylyl cyclase alpha(1) subunit in vascular smooth muscle relaxation. Cardiovasc Res. 2007;76(1):149–59. doi: 10.1016/j.cardiores.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Vanneste G, et al. Gastric motility in soluble guanylate cyclase alpha 1 knockout mice. J Physiol. 2007;584(Pt 3):907–20. doi: 10.1113/jphysiol.2007.140608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buys ES, et al. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res. 2008;79(1):179–86. doi: 10.1093/cvr/cvn068. [DOI] [PubMed] [Google Scholar]
- 27.De Backer O, et al. Role of the soluble guanylyl cyclase alpha1/alpha2 subunits in the relaxant effect of CO and CORM-2 in murine gastric fundus. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(5):493–502. doi: 10.1007/s00210-008-0315-6. [DOI] [PubMed] [Google Scholar]
- 28.Dhaese I, et al. Involvement of soluble guanylate cyclase alpha(1) and alpha(2), and SK(Ca) channels in NANC relaxation of mouse distal colon. Eur J Pharmacol. 2008;589(1–3):251–9. doi: 10.1016/j.ejphar.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Vermeersch P, et al. Soluble guanylate cyclase-alpha1 deficiency selectively inhibits the pulmonary vasodilator response to nitric oxide and increases the pulmonary vascular remodeling response to chronic hypoxia. Circulation. 2007;116(8):936–43. doi: 10.1161/CIRCULATIONAHA.106.677245. [DOI] [PubMed] [Google Scholar]
- 30.Haghikia A, et al. Long-term potentiation in the visual cortex requires both nitric oxide receptor guanylyl cyclases. J Neurosci. 2007;27(4):818–23. doi: 10.1523/JNEUROSCI.4706-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taqatqeh F, et al. More than a retrograde messenger: nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation. J Neurosci. 2009;29(29):9344–50. doi: 10.1523/JNEUROSCI.1902-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melichar VO, et al. Reduced cGMP signaling associated with neointimal proliferation and vascular dysfunction in late-stage atherosclerosis. Proc Natl Acad Sci U S A. 2004;101(47):16671–6. doi: 10.1073/pnas.0405509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulsch A, et al. Effects of in vivo nitroglycerin treatment on activity and expression of the guanylyl cyclase and cGMP-dependent protein kinase and their downstream target vasodilator-stimulated phosphoprotein in aorta. Circulation. 2001;103(17):2188–94. doi: 10.1161/01.cir.103.17.2188. [DOI] [PubMed] [Google Scholar]
- 34.Ruetten H, et al. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res. 1999;85(6):534–41. doi: 10.1161/01.res.85.6.534. [DOI] [PubMed] [Google Scholar]
- 35.Schermuly RT, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J. 2008;32(4):881–91. doi: 10.1183/09031936.00114407. [DOI] [PubMed] [Google Scholar]
- 36.Gupta MP, Evanoff V, Hart CM. Nitric oxide attenuates hydrogen peroxide-mediated injury to porcine pulmonary artery endothelial cells. Am J Physiol. 1997;272(6 Pt 1):L1133–41. doi: 10.1152/ajplung.1997.272.6.L1133. [DOI] [PubMed] [Google Scholar]
- 37.Woods JD, Edwards JS, Ritter JM. Inhibition by nitroprusside of platelet calcium mobilization: evidence for reduced sensitivity to nitric oxide in essential hypertension. J Hypertens. 1993;11(12):1369–73. doi: 10.1097/00004872-199312000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Verbeuren TJ, et al. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986;58(4):552–64. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- 39.Kloss S, Bouloumie A, Mulsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension. 2000;35(1 Pt 1):43–7. [PubMed] [Google Scholar]
- 40.Stice JP, Eiserich JP, Knowlton AA. Role of aging versus the loss of estrogens in the reduction in vascular function in female rats. Endocrinology. 2009;150(1):212–9. doi: 10.1210/en.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mergia E, et al. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J Clin Invest. 2006;116(6):1731–7. doi: 10.1172/JCI27657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhaese I, et al. Small intestinal motility in soluble guanylate cyclase alpha1 knockout mice: (Jejunal phenotyping of sGCalpha1 knockout mice) Naunyn Schmiedebergs Arch Pharmacol. 2009;379(5):473–87. doi: 10.1007/s00210-008-0380-x. [DOI] [PubMed] [Google Scholar]
- 43.Andreopoulos S, Papapetropoulos A. Molecular aspects of soluble guanylyl cyclase regulation. Gen Pharmacol. 2000;34(3):147–57. doi: 10.1016/s0306-3623(00)00062-8. [DOI] [PubMed] [Google Scholar]
- 44.Pyriochou A, Papapetropoulos A. Soluble guanylyl cyclase: more secrets revealed. Cell Signal. 2005;17(4):407–13. doi: 10.1016/j.cellsig.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Baltrons MA, Garcia A. Nitric oxide-independent down-regulation of soluble guanylyl cyclase by bacterial endotoxin in astroglial cells. J Neurochem. 1999;73(5):2149–57. [PubMed] [Google Scholar]
- 46.Papapetropoulos A, et al. Downregulation of nitrovasodilator-induced cyclic GMP accumulation in cells exposed to endotoxin or interleukin-1 beta. Br J Pharmacol. 1996;118(6):1359–66. doi: 10.1111/j.1476-5381.1996.tb15545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pedraza CE, et al. Interleukin-1 beta and lipopolysaccharide decrease soluble guanylyl cyclase in brain cells: NO-independent destabilization of protein and NO-dependent decrease of mRNA. J Neuroimmunol. 2003;144(1–2):80–90. doi: 10.1016/j.jneuroim.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Scott WS, Nakayama DK. Escherichia coli lipopolysaccharide downregulates soluble guanylate cyclase in pulmonary artery smooth muscle. J Surg Res. 1998;80(2):309–14. doi: 10.1006/jsre.1998.5442. [DOI] [PubMed] [Google Scholar]
- 49.Gerassimou C, et al. Regulation of the expression of soluble guanylyl cyclase by reactive oxygen species. Br J Pharmacol. 2007;150(8):1084–91. doi: 10.1038/sj.bjp.0707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filippov G, Bloch DB, Bloch KD. Nitric oxide decreases stability of mRNAs encoding soluble guanylate cyclase subunits in rat pulmonary artery smooth muscle cells. J Clin Invest. 1997;100(4):942–8. doi: 10.1172/JCI119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kloss S, Furneaux H, Mulsch A. Post-transcriptional regulation of soluble guanylyl cyclase expression in rat aorta. J Biol Chem. 2003;278(4):2377–83. doi: 10.1074/jbc.M206453200. [DOI] [PubMed] [Google Scholar]
- 52.Kloss S, et al. Human-antigen R (HuR) expression in hypertension: downregulation of the mRNA stabilizing protein HuR in genetic hypertension. Hypertension. 2005;45(6):1200–6. doi: 10.1161/01.HYP.0000165674.58470.8f. [DOI] [PubMed] [Google Scholar]
- 53.Papapetropoulos A, et al. Mechanisms of tolerance to sodium nitroprusside in rat cultured aortic smooth muscle cells. Br J Pharmacol. 1996;117(1):147–55. doi: 10.1111/j.1476-5381.1996.tb15167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott WS, Nakayama DK. Sustained nitric oxide exposure decreases soluble guanylate cyclase mRNA and enzyme activity in pulmonary artery smooth muscle. J Surg Res. 1998;79(1):66–70. doi: 10.1006/jsre.1998.5382. [DOI] [PubMed] [Google Scholar]
- 55.Ujiie K, et al. Homologous and heterologous desensitization of a guanylyl cyclase-linked nitric oxide receptor in cultured rat medullary interstitial cells. J Pharmacol Exp Ther. 1994;270(2):761–7. [PubMed] [Google Scholar]
- 56.Takata M, et al. Cytokines decrease sGC in pulmonary artery smooth muscle cells via NO-dependent and NO-independent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;280(2):L272–8. doi: 10.1152/ajplung.2001.280.2.L272. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Force T, Bloch KD. Nerve growth factor decreases soluble guanylate cyclase in rat pheochromocytoma PC12 cells. J Biol Chem. 1997;272(9):6038–43. doi: 10.1074/jbc.272.9.6038. [DOI] [PubMed] [Google Scholar]
- 58.Cabilla JP, et al. 17 beta-estradiol modifies nitric oxide-sensitive guanylyl cyclase expression and down-regulates its activity in rat anterior pituitary gland. Endocrinology. 2006;147(9):4311–8. doi: 10.1210/en.2006-0367. [DOI] [PubMed] [Google Scholar]
- 59.Krumenacker JS, Hyder SM, Murad F. Estradiol rapidly inhibits soluble guanylyl cyclase expression in rat uterus. Proc Natl Acad Sci U S A. 2001;98(2):717–22. doi: 10.1073/pnas.98.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai C, et al. Androgen regulation of soluble guanylyl cyclasealpha1 mediates prostate cancer cell proliferation. Oncogene. 2007;26(11):1606–15. doi: 10.1038/sj.onc.1209956. [DOI] [PubMed] [Google Scholar]
- 61.Licatalosi DD, Darnell RB. RNA processing and its regulation: global insights into biological networks. Nat Rev Genet. 2010;11(1):75–87. doi: 10.1038/nrg2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris AR, Mukherjee N, Keene JD. Systematic analysis of posttranscriptional gene expression. Wiley Interdiscip Rev Syst Biol Med. 2010;2(2):162–80. doi: 10.1002/wsbm.54. [DOI] [PubMed] [Google Scholar]
- 63.Wang ET, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–6. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mikami T, Kusakabe T, Suzuki N. Tandem organization of medaka fish soluble guanylyl cyclase alpha1 and beta1 subunit genes. Implications for coordinated transcription of two subunit genes. J Biol Chem. 1999;274(26):18567–73. doi: 10.1074/jbc.274.26.18567. [DOI] [PubMed] [Google Scholar]
- 65.Giuili G, et al. Colocalization of the genes coding for the alpha 3 and beta 3 subunits of soluble guanylyl cyclase to human chromosome 4 at q31.3–q33. Hum Genet. 1993;91(3):257–60. doi: 10.1007/BF00218267. [DOI] [PubMed] [Google Scholar]
- 66.Jiang Y, Stojilkovic SS. Molecular cloning and characterization of alpha1-soluble guanylyl cyclase gene promoter in rat pituitary cells. J Mol Endocrinol. 2006;37(3):503–15. doi: 10.1677/jme.1.02180. [DOI] [PubMed] [Google Scholar]
- 67.Marro ML, et al. Characterization of the human alpha1 beta1 soluble guanylyl cyclase promoter: key role for NF-kappaB(p50) and CCAAT-binding factors in regulating expression of the nitric oxide receptor. J Biol Chem. 2008;283(29):20027–36. doi: 10.1074/jbc.M801223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharina IG, et al. Genomic organization of alpha1 and beta1 subunits of the mammalian soluble guanylyl cyclase genes. Proc Natl Acad Sci U S A. 2000;97(20):10878–83. doi: 10.1073/pnas.190331697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharina IG, et al. CCAAT-binding factor regulates expression of the beta1 subunit of soluble guanylyl cyclase gene in the BE2 human neuroblastoma cell line. Proc Natl Acad Sci U S A. 2003;100(20):11523–8. doi: 10.1073/pnas.1934338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vazquez-Padron RI, et al. Molecular dissection of mouse soluble guanylyl cyclase alpha1 promoter. Biochem Biophys Res Commun. 2004;314(1):208–14. doi: 10.1016/j.bbrc.2003.12.078. [DOI] [PubMed] [Google Scholar]
- 71.Bloch KD, et al. Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period. Am J Physiol. 1997;272(3 Pt 1):L400–6. doi: 10.1152/ajplung.1997.272.3.L400. [DOI] [PubMed] [Google Scholar]
- 72.Harumi T, et al. Expression of membrane-bound and soluble guanylyl cyclase mRNAs in embryonic and adult retina of the medaka fish Oryzias latipes. Zoolog Sci. 2003;20(2):133–40. doi: 10.2108/zsj.20.133. [DOI] [PubMed] [Google Scholar]
- 73.Sharin VG, et al. Nitric Oxide Receptor Soluble Guanylyl Cyclase Undergoes Splicing Regulation in Differentiating Human Embryonic Cells. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smigrodzki R, Levitt P. The alpha 1 subunit of soluble guanylyl cyclase is expressed prenatally in the rat brain. Brain Res Dev Brain Res. 1996;97(2):226–34. doi: 10.1016/s0165-3806(96)00162-9. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto T, et al. Localization of the nitric oxide/cGMP signaling pathway-related genes and influences of morpholino knock-down of soluble guanylyl cyclase on medaka fish embryogenesis. Zoolog Sci. 2003;20(2):181–91. doi: 10.2108/zsj.20.181. [DOI] [PubMed] [Google Scholar]
- 76.de Frutos S, et al. Regulation of soluble guanylyl cyclase-alpha1 expression in chronic hypoxia-induced pulmonary hypertension: role of NFATc3 and HuR. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L475–86. doi: 10.1152/ajplung.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kloss S, Srivastava R, Mulsch A. Down-regulation of soluble guanylyl cyclase expression by cyclic AMP is mediated by mRNA-stabilizing protein HuR. Mol Pharmacol. 2004;65(6):1440–51. doi: 10.1124/mol.65.6.1440. [DOI] [PubMed] [Google Scholar]
- 78.Jurado S, Sanchez-Prieto J, Torres M. Differential expression of NO-sensitive guanylyl cyclase subunits during the development of rat cerebellar granule cells: regulation via N-methyl-D-aspartate receptors. J Cell Sci. 2003;116(Pt 15):3165–75. doi: 10.1242/jcs.00620. [DOI] [PubMed] [Google Scholar]
- 79.Jurado S, et al. NMDA induces post-transcriptional regulation of alpha2-guanylyl-cyclase-subunit expression in cerebellar granule cells. J Cell Sci. 2006;119(Pt 8):1622–31. doi: 10.1242/jcs.02867. [DOI] [PubMed] [Google Scholar]
- 80.Behrends S, et al. A variant of the alpha 2 subunit of soluble guanylyl cyclase contains an insert homologous to a region within adenylyl cyclases and functions as a dominant negative protein. J Biol Chem. 1995;270(36):21109–13. doi: 10.1074/jbc.270.36.21109. [DOI] [PubMed] [Google Scholar]
- 81.Behrends S, Vehse K. The beta(2) subunit of soluble guanylyl cyclase contains a human-specific frameshift and is expressed in gastric carcinoma. Biochem Biophys Res Commun. 2000;271(1):64–9. doi: 10.1006/bbrc.2000.2596. [DOI] [PubMed] [Google Scholar]
- 82.Cabilla JP, et al. Nitric oxide sensitive-guanylyl cyclase subunit expression changes during estrous cycle in anterior pituitary glands. Am J Physiol Endocrinol Metab. 2009;296(4):E731–7. doi: 10.1152/ajpendo.90795.2008. [DOI] [PubMed] [Google Scholar]
- 83.Chhajlani V, et al. Heterogeneity in human soluble guanylate cyclase due to alternative splicing. FEBS Lett. 1991;290(1–2):157–8. doi: 10.1016/0014-5793(91)81248-7. [DOI] [PubMed] [Google Scholar]
- 84.Sharina IG, et al. Alpha1 soluble guanylyl cyclase (sGC) splice forms as potential regulators of human sGC activity. J Biol Chem. 2008;283(22):15104–13. doi: 10.1074/jbc.M710269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sayers EW, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2011;39(Database issue):D38–51. doi: 10.1093/nar/gkq1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fujita PA, et al. The UCSC Genome Browser database: update 2011. Nucleic Acids Res. 2011;39(Database issue):D876–82. doi: 10.1093/nar/gkq963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ritter D, et al. Alternative splicing for the alpha1 subunit of soluble guanylate cyclase. Biochem J. 2000;346(Pt 3):811–6. [PMC free article] [PubMed] [Google Scholar]
- 88.Raney BJ, et al. ENCODE whole-genome data in the UCSC genome browser (2011 update) Nucleic Acids Res. 2011;39(Database issue):D871–5. doi: 10.1093/nar/gkq1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shyu AB, Wilkinson MF, van Hoof A. Messenger RNA regulation: to translate or to degrade. EMBO J. 2008;27(3):471–81. doi: 10.1038/sj.emboj.7601977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuen PS, Potter LR, Garbers DL. A new form of guanylyl cyclase is preferentially expressed in rat kidney. Biochemistry. 1990;29(49):10872–8. doi: 10.1021/bi00501a002. [DOI] [PubMed] [Google Scholar]
- 91.Gibb BJ, Wykes V, Garthwaite J. Properties of NO-activated guanylyl cyclases expressed in cells. Br J Pharmacol. 2003;139(5):1032–40. doi: 10.1038/sj.bjp.0705318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vazquez-Padron RI, et al. An internal ribosome entry site mediates the initiation of soluble guanylyl cyclase beta2 mRNA translation. FEBS J. 2008;275(14):3598–607. doi: 10.1111/j.1742-4658.2008.06505.x. [DOI] [PubMed] [Google Scholar]
- 93.Boissel JP, Schwarz PM, Forstermann U. Neuronal-type NO synthase: transcript diversity and expressional regulation. Nitric Oxide. 1998;2(5):337–49. doi: 10.1006/niox.1998.0189. [DOI] [PubMed] [Google Scholar]
- 94.Eissa NT, et al. Alternative splicing of human inducible nitric-oxide synthase mRNA. tissue-specific regulation and induction by cytokines. J Biol Chem. 1996;271(43):27184–7. doi: 10.1074/jbc.271.43.27184. [DOI] [PubMed] [Google Scholar]
- 95.Lorenz M, et al. Alternative splicing in intron 13 of the human eNOS gene: a potential mechanism for regulating eNOS activity. FASEB J. 2007;21(7):1556–64. doi: 10.1096/fj.06-7434com. [DOI] [PubMed] [Google Scholar]
- 96.Schodel J, et al. Expression of neuronal nitric oxide synthase splice variants in atherosclerotic plaques of apoE knockout mice. Atherosclerosis. 2009;206(2):383–9. doi: 10.1016/j.atherosclerosis.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gerzanich V, et al. Alternative splicing of cGMP-dependent protein kinase I in angiotensin-hypertension: novel mechanism for nitrate tolerance in vascular smooth muscle. Circ Res. 2003;93(9):805–12. doi: 10.1161/01.RES.0000097872.69043.A0. [DOI] [PubMed] [Google Scholar]
- 98.O'Connor V, et al. Differential amplification of intron-containing transcripts reveals long term potentiation-associated up-regulation of specific Pde10A phosphodiesterase splice variants. J Biol Chem. 2004;279(16):15841–9. doi: 10.1074/jbc.M312500200. [DOI] [PubMed] [Google Scholar]
- 99.Rentero C, Monfort A, Puigdomenech P. Identification and distribution of different mRNA variants produced by differential splicing in the human phosphodiesterase 9A gene. Biochem Biophys Res Commun. 2003;301(3):686–92. doi: 10.1016/s0006-291x(03)00021-4. [DOI] [PubMed] [Google Scholar]
- 100.Richter W, Jin SL, Conti M. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem J. 2005;388(Pt 3):803–11. doi: 10.1042/BJ20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuasa K, et al. Isolation and characterization of two novel phosphodiesterase PDE11A variants showing unique structure and tissue-specific expression. J Biol Chem. 2000;275(40):31469–79. doi: 10.1074/jbc.M003041200. [DOI] [PubMed] [Google Scholar]