Abstract
Purpose
Psychological stress plays a role in the exacerbation of functional lower urinary tract disorders such as painful bladder syndrome and overactive bladder. To better understand the mechanism underlying this relationship, we characterized changes in micturition, anxiety-related behavior, and bladder pathology in rats exposed to repeated water avoidance (WA) stress.
Methods
Twenty-four Wistar rats were subjected to WA stress or sham. Immediately following acute (day 1) and chronic (day 10) stress or sham, rats were placed in a metabolic cage for a 2-hour voiding behavior assessment. Voiding parameters were compared to baseline values obtained prior to stress. Four animals from each group were sacrificed on day 10 and bladders harvested for histologic and gene expression studies. The remaining 8 animals per group underwent repeated voiding assessment every 3 days for 1 month followed by 10 days of repeat WA stress or sham. Bladder histology and gene expression were studied.
Results
Rats exposed to WA stress developed a significant increase in micturition frequency and decrease in latency to void, voiding interval and volume of first void compared to sham and baseline. Alterations in micturition persisted for approximately 1 month. Stressed rats showed increased fecal pellet excretion and anxiety-like behavior. Additionally, bladder specimens from stressed animals revealed increased angiogenesis, and increased total and activated mast cells.
Conclusions
In rats, repeated psychological stress results in lasting alterations in micturition frequency, interval, and volume. This rodent model may represent a valid tool for studying syndromes characterized by increased urinary frequency.
Keywords: Water avoidance stress, overactive bladder, painful bladder syndrome/interstitial cystitis, animal model, urinary frequency
Introduction
Functional lower urinary tract disorders such as overactive bladder (OAB) and painful bladder syndrome/interstitial cystitis (PBS/IC) can be viewed as part of a spectrum of bladder hypersensitivity syndromes sharing the common symptom of urinary frequency. Stress appears to play a role in the exacerbation and possibly the development of these disorders and previous studies have identified physical and emotional stress as potential triggers for symptom aggravation.1-3 Stress induced visceral hypersensitivity to intestinal stimuli has been well documented in preclinical and clinical models of irritable bowel syndrome, a syndrome often comorbid with urinary tract disorders.4 In rodent models, corticotropin releasing factor (CRF) and CRF1 receptor signaling have been implicated as major mechanisms mediating stress induced changes in gastrointestinal motility and sensitivity;4,5 CRF related neuropeptides are also abundantly expressed in areas involved in the control of micturition including Barrington's nucleus and the lumbosacral spinal cord.6 Conditions characterized by increased urinary frequency may share complex interactions of neuronal and hormonal factors. Despite the evidence that symptoms in voiding disorders are exacerbated by stress, the pathophysiology underlying the effect of stress on urinary frequency and/or other voiding disorders remains unclear.
Rodent models of key components of complex human disorders, such as voiding behavior, provide us with the opportunity to study underlying pathophysiological mechanisms and identify targets for possible therapeutic interventions. Animal models of urinary frequency often involve intravesical administration of inflammatory agents7 or surgical creation of bladder outlet obstruction;8 however, these models have poor face validity for human functional disorders. In previous studies from our group, we developed a model of chronic water avoidance (WA) stress-induced visceral hyperalgesia in rats. This paradigm has been shown to increase anxiety-like behavior, fecal pellet output and visceral nociception in the colon. 2,9 Although bladder abnormalities such as epithelial ulceration, mast cell proliferation, and impairment of gap junctions have been reported in the WA stress model,10 to our knowledge functional voiding dysfunction has not been evaluated. In the current study, using a validated rodent model of chronic mild psychological stress, we sought to identify stress-related changes in 1) behavior, 2) bladder function, and 3) bladder tissue.
Materials and Methods
Animals
Experimental protocols were approved by the UCLA Animal Review Committee. Adult female Wistar rats (200-300g, WKY strain 008, Charles River, Wilmington, MA), a strain genetically predisposed to enhanced levels of anxiety,11 were subjected to the WA stress (n=12) or sham protocol (n=12). This model represents a well characterized, potent psychological stressor with elevations of stress hormones including adrenocorticotropin and corticosterone.4 This model best translates the working hypothesis in the human condition that genetic polymorphisms contribute to susceptibility to stressors. Females were chosen since the severity of OAB syndrome is greater in women.12 Estrous cycles were not controlled for. Animals were maintained in a 12:12-hour light dark cycle and housed in pairs. Standard chow and water were available ad libitum. The animal had two weeks to adjust to their environment before the stress protocol began.
WA stress protocol
Animals were exposed to WA stress, as originally described by Monnikes et al, between 8 am and 12 pm to minimize circadian effect.13 Briefly, rats were placed on a glass platform (diameter, 9.5 cm) in the center of a plastic container (56×25 cm). The container was filled with 25°C water to 1 cm below the glass platform. For the sham group, rats were placed on a glass platform in a waterless plastic container. The animals remained in the containers for 1 hour a day for 10 consecutive days. Rats avoided the aversive stimulus (water) by remaining on the glass platform. If they fell into the water they were gently replaced on the glass pedestal. One day of exposure to the WA stress protocol was considered acute stress and ten consecutive days of exposure was considered chronic stress.
Measurement of fecal pellet output
The number of fecal pellets excreted in the plastic container during the 1 hour WA stress or sham was recorded as a measure of stress induced colonic motility.13 Additionally, fecal pellets were counted during the baseline, acute and chronic voiding assessments in the metabolic cages.
Light-dark box (LDB) transition test
To quantify anxiety-like behavior on day 10, the LDB transition test was used.14 Previous published work has established parameters of anxiety-like behavior such as shorter latency to enter the dark, less time in the lighted chamber, less activity or transitions between chambers, and less rearing (rising on hind legs) behavior.15,16 The apparatus used consisted of a 30×76×40 cm glass aquarium (Petco, San Gabriel, CA) divided into two sections with a 7.5×7.5 cm opening for the animal to easily pass. The light chamber was brightly illuminated and the dark chamber was painted black and covered with a lid. The rats were placed on the light side and allowed to move freely between the two chambers for 10 minutes while being recorded by video camera. Scoring and coding of the parameters listed above were performed.
Voiding assessment
Baseline micturition parameters in all 24 animals were obtained using a metabolic cage (Tecniplast USA, Exton, PA) for two separate, 2-hour sessions prior to exposure to WA stress or sham. Animals had free access to food and water. Voiding frequency, interval, and volume, as well as, fecal pellet excretion and water intake were recorded by an investigator blinded to the animals' exposure to WA or sham. On days 1 and 10, immediately following WA stress or sham, animals were placed in metabolic cages for two hours and voiding parameters recorded. In order to evaluate the durability of voiding alterations, eight animals in each group underwent repeat voiding assessment every three days until the voiding habits normalized to pre-study baseline.
Histology
Four animals from each group were sacrificed on day 10 and the remaining eight animals from each group who underwent repeated voiding assessment were sacrificed on day 10 of repeat WA stress or sham and their bladders harvested. A strip of bladder, extending from base to dome, was fixed in 10% formalin, embedded in paraffin wax, sectioned and stained with hematoxylin and eosin. Sections were imaged using light microscopy coupled with a digital camera. Three non-contiguous sections were evaluated for structural changes including mucosal integrity, thickness, and angiogenesis. Vascularity was further quantified using CD 31 antibody (Dako, Carpinteria, CA) staining for endothelial cells and a systematic grid for counting.17 Trichrome stains were performed and the area of smooth muscle to connective tissue ratio analyzed with Adobe Photoshop CS4. The total number of pixels in pink (smooth muscle) and blue (connective tissue) was determined using the magic wand function (selecting the similar color range and pixel count within the selected area) in the histogram display. The ratio was determined for each image taken at 25× magnification.
Mast Cell Quantification
Paraffin sections were stained with Toluidine blue stain. In each bladder cross sectional specimen, total number of mast cells, resting (not degranulated), partially activated (released <20% of granules), and fully activated (released >20% of granules) were counted under light microscopy. A total of 2-4 sections were counted for each bladder specimen and the mean was calculated. The area of each bladder specimen was measured, and the mean number of mast cells per bladder area in mm2 was calculated.
Real Time PCR
A second strip of bladder, extending from base to dome, was placed in RNA-later (Qiagen, Valencia, CA) at -20C. RT-PCR was used to quantify α-2 adrenergic receptor (AR), tumor necrosis factor (TNF)-α, β-2AR, and interleukin (IL)-1b mRNA. Duplicate determinations on each tissue sample were quantified. RT-PCR:100 ng of RNA isolated from whole cell homogenates from bladder wall tissue was reverse-transcribed into cDNA using the Taqman One-step RT-PCR kit (Applied Biosystems Foster City, CA). GAPDH (rRNA) was used as an endogenous control and was detected using dual labeled fluorogenic probe (5′-FAM/3′-MGB probe, Applied Biosystems, Foster City, CA). mRNA levels were quantified using a fluorogenic 5′-nuclease PCR assay with a 7500 Fast RT-PCR sequence detection system (Applied Biosystems, Foster City, CA). Duplicate reactions of each standard or sample were incubated for two minutes at 50°C, denatured for 10 minutes at 95°C, and subjected to 40 cycles of annealing at 55°C for 20 seconds, extension at 60°C for one minute' followed by denaturation at 95°C for 15 seconds.
Nerve Sprouting
A third strip of extracted bladder tissue, extending from base to dome, was snap frozen in liquid nitrogen and the distribution of catecholamoinergic nerve sprouting was mapped in 18μm cryostat sections using glyoxylic acid chemoflourescence.18
Statistical analysis
Statistical testing was performed using student's t-test and 2-way ANOVA where appropriate, p<0.05 was considered significant. Results are expressed as mean ± standard deviation.
Results
Evidence for stress induced behavioral changes
Animals subjected to WA stress showed a significant decrease in episodes of rearing behavior (37.8 ±16.2 vs. 54.5±8.6 episodes, p=0.05), and a notable but non-significant decrease in latency to enter the dark (36±27 vs. 176±194 seconds, p=0.11) and total number of transitions between light and dark chambers (13.5±8.0 vs. 20.6±6.7 transitions, p=0.14) compared to sham. All animals were classified as displaying or not displaying “anxiety-like behavior” based on cumulative LDB observer ratings. WA stress rats showed greater anxiety-like behaviors with 83% (10/12) of these animals categorized as such. Only 17% (2/12) of the sham animals were rated as displaying “anxiety like behavior”. In addition, acute and chronically stressed animals showed a marked increase in fecal pellet excretion compared to baseline and compared to sham (Figure 1).
Figure 1.
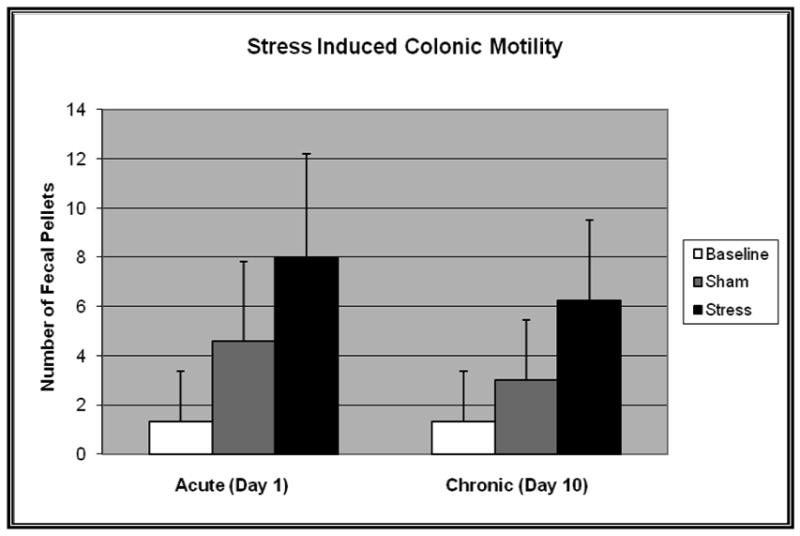
Fecal pellet output at baseline and following acute and chronic water avoidance stress (n=12) or sham (n=12). Two-way ANOVA revealed a significant difference between stressed and sham animals, both acutely and chronically (F=12.4, p=0.002). Statistical significance was not achieved between sham and baseline.
Stress induced changes in bladder function
Rats exposed to acute and chronic WA stress, but not sham, developed a significant increase in micturition frequency and a decrease in latency to first void, voiding interval and volume of first void when compared to sham and to baseline voiding parameters (Table 1). Two-way ANOVA with repeated measures allowed evaluation of the effect of stress as well as time (baseline, acute stress/day 1, and chronic stress/day 10) on voiding parameters and discovered the main effect was due to the WA stress intervention (F statistics and p-values reported in Table 1). The effect of stress (or sham) on micturition parameters was similar in the acute in chronic setting. Only the stressed animals showed a significant difference in voiding parameters compared to baseline. Alterations in voiding parameters following chronic stress persisted for approximately one month (mean duration 24 days, data not shown) before returning to baseline. Water intake and voided volume showed no significant difference between stress and sham animals. Pearson's correlation between fecal pellet frequency and urinary frequency was weakly positive (r=0.25).
Table 1.
Micturition parameters (mean ± standard error) at baseline and following acute and chronic water avoidance stress (n=12) or sham, (n=12). Two-way ANOVA F Statistic and p-value for effect of stress vs. sham shown.
| Baseline | Acute Stress | Acute Sham | Chronic Stress | Chronic Sham | F | p-value | |
|---|---|---|---|---|---|---|---|
| Total # Voids/2 Hours | 2.9 +/- 0.6 | 5.8 +/- 0.8 | 3.4 +/- 0.8 | 7.2 +/- 1.0 | 3.8 +/- 1.0 | 5.74 | 0.026 |
| Latency to 1st Void (min) | 42.0 +/- 7.4 | 9.6 +/- 4.5 | 35.4 +/- 4.5 | 8.0 +/- 4.4 | 29.2 +/- 4.4 | 15.49 | 0.001 |
| # Minutes between Voids | 42.1 +/- 7.0 | 20.4 +/- 4.6 | 38.6 +/- 4.6 | 18.3 +/- 6.8 | 34.4 +/- 6.8 | 4.76 | 0.040 |
| Volume of 1st Void (ml) | 0.92 +/- 0.1 | 0.5 +/- 0.1 | 1.0 +/- 0.1 | 0.5 +/- 0.1 | 0.8 +/- 0.1 | 6.79 | 0.016 |
Stress induced tissue changes
The gross architecture of the bladder did not appear different between stress and sham groups with similar smooth muscle to connective tissue content as demonstrated by Trichrome staining. No difference in bladder wall thickness was noted between stress and sham (1,551±429 um vs. 1,814±662 um, p=0.16). Harvested bladder tissues of chronically stressed animals showed increased number of total and activated mast cells when compared to sham (Figure 2) but no evidence for an increase in inflammatory markers (TNFα:3.25 vs. 3.19 units, p=0.94; IL-1β:6.59 vs. 7.42 units, p=0.74). Bladders of chronically stressed animals also showed increased vascularity and vessel number as shown in Figure 3 (28.1±9.0 vs. 7.8±2.4 vessels, 25× magnification, p<0.001). There was no change in overall sympathetic innervation, as determined by catecholaminergic neural sprouting in the majority of stressed animals when compared to sham, and no changes in adrenergic receptor expression (α-2AR: 7.2±0.5 vs. 7.4±0.9 units, p=0.82; β-2AR: 11.3±2.1 vs. 9.6±1.4 units, p=0.50).
Figure 2.
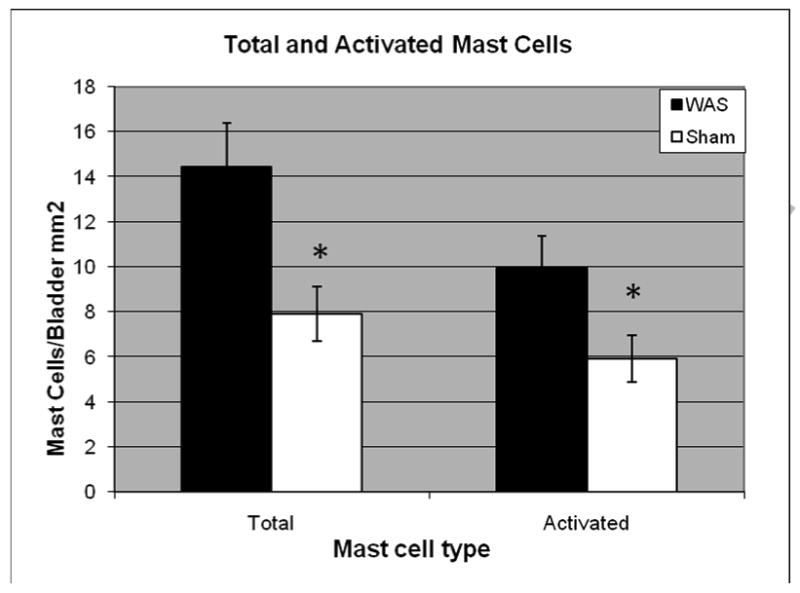
Mean (± SEM) total mast cells per bladder area (mm2) and activated (partially + fully activated) mast cells in Wistar rats exposed to chronic water avoidance stress (n=12) compared to sham (n=10). Statistical analysis by student's t-test. (* denotes p<0.05)
Figure 3.
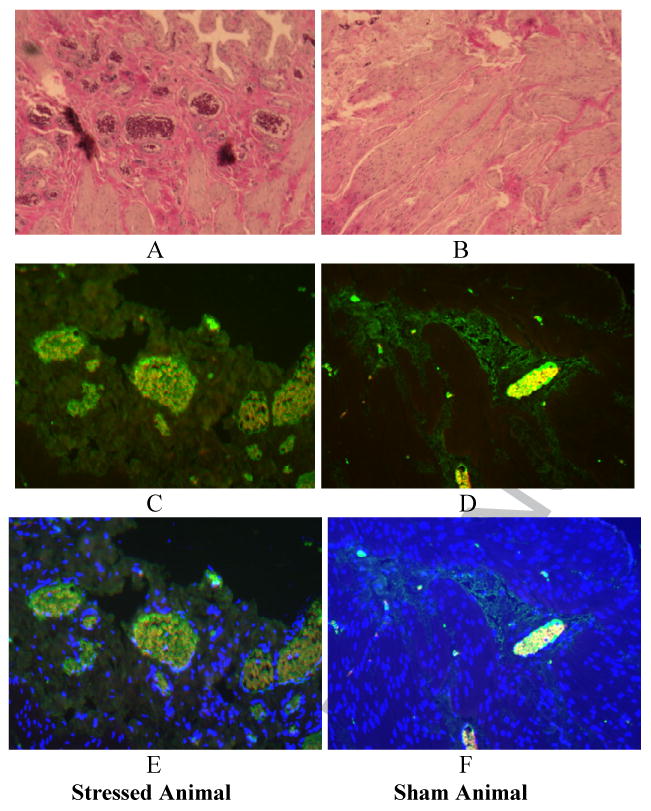
Increased angiogenesis in stress versus sham animals. A. Representative H&E section showing significantly greater number of red blood cell filled vessels (on end). B. Representative H&E section showing normal blood vessel architecture in the sham bladder. D. CD-31 antibody allows staining of endothelial lined cells as well as red blood vessels. D. Minimal stain appreciated in the sham animal. E. DAPI allows identification of nucleated cells (endothelial cells) and does not identify non nucleated red blood cells. F. No nuclear positive, CD-31 positive cells identified in the sham specimen.
Discussion
Using a validated rodent model of chronic stress, we demonstrate that repeated psychological stress in female animals is associated with changes in voiding behavior which show homology with symptoms of increased micturition frequency often reported by patients with OAB and PBS/IC. We demonstrate evidence for increased mast cells and angiogenesis in the bladder tissue. As previously demonstrated, we confirm chronic stress induced increase in stress responsiveness in the form of anxiety-like behavior and increased fecal pellet output
Stress induced changes in behavior
In this study, animals exposed to WA stress exhibited increased anxiety-like behavior which correlated with previous reports using this model.2 In addition, increased fecal pellet excretion was seen in the stressed animals reproducing previous observations and validating our technique. Fecal pellet output is considered a reliable measure of sacral parasympathetic modulation of colonic motility19 and previous data suggests this model of chronic WAS shows excellent face validity for irritable bowel syndrome (which often overlaps with PBS/IC and OAB) with respect to anxiety like behavior, increased fecal pellet output, and stress induced colonic hyperalgesia.2
Changes in bladder function
Voiding behavior in animals subjected to WA stress showed remarkable similarity to humans with functional lower urinary tract disorders. Acute and chronically stressed animals had nearly double the voiding frequency as the sham animals and nearly half the voiding interval with no change in overall voided volume. This suggests that the stressed animals experienced a trigger to void at approximately half the capacity than the non-stressed animals, a common complaint among patients with OAB and PBS/IC. These findings suggest that the changes in sacral parasympathetic outflow associated with WA stress not only affect the motility of the distal colon,2 but also of the urinary bladder. Previous studies of social stress using exposure of a submissive male mouse to a larger, aggressive breeder mouse produced a voiding pattern of retention, increased volume at micturition, and increased bladder mass reminiscent of dysfunctional voiding in children.20 In contrast, our studied looked at female rats subjected to a different form of psychosocial stress previously shown to increase bowel motility. Differences in these models likely extend beyond gender and encompass the neurochemical pathways involved in different mechanisms of stress and stress response.
Changes in bladder tissue
Harvested bladder tissue showed that although there were no gross structural abnormalities in smooth muscle and connective tissue ratios, there were profound alterations in mast cell infiltration and angiogenesis in bladders of animals exposed to WA stress. Bladders of patients with PBS/IC show an increased number of mast cells with activation and degranulation of these cells and increased urinary mast cell mediators.21 Mast cells are thought to be involved in the mediation and development of bladder hypersensitivity and hyperalgesia via secretion of vasoactive substances, increased urothelial permeability, and subsequent sensory afferent nerve up-regulation which can further stimulate mast cell activity perpetuating this cycle.10,22 In the current study, rat bladder tissue exposed to chronic WA stress demonstrated increased total, resting, and activated mast cells numbers when compared to sham WA. The mechanism of this increase in mast cells is incompletely understood. Given that the WA stress model does not involve direct bladder insult and only psychological stress, central activation may play a significant role in peripheral change, supporting neuroimmune interactions between the bladder and stress response. This theory is supported by the known association between autonomic nervous system activity and mast cell activation.23 Further studies are underway looking at the role of mast cells in urinary frequency.
Angiogenesis has been found in the end organ of other stress-related models of disease and is felt to be important in many chronic inflammatory processes.24,25 Previous work in inflammatory bowel disease has shown concomitant angiogenesis with intestinal inflammation.26 It has been suggested that angiogenic components may actually exacerbate the specific disease conditions due to their resultant impact on the local tissues.25 In a mouse model of ovarian cancer, chronic stress resulted in enhanced angiogenesis with greater expression of tissue catecholamines mediated primarily through beta-adrenergic receptors, highlighting the importance of the sympathetic stress response in angiogenesis.27 Increased vascularity has also been documented in humans with increased urinary frequency and PBS/IC.25 Our model shows a profound increase in mature vessel formation in the bladder of animals exposed to WA stress. Although preliminary tissue microarray studies show alterations in expression of bladder tissue angiogenic factors in stressed animals compared to controls (data not shown), the mechanism and significance of the neovascularity remains unknown and subject to future studies. It is possible that the changes in micturition parameters seen in our study are related to sensitization of visceral afferent pathways possibly through mediators released after stress exposure.
Implications of findings for PBS/IC and OAB
Functional urinary tract disorders produce a significant burden of patient disease with various costly diagnostics and therapeutics administered with marginal success.28 Bothersome urinary frequency is the cornerstone of these disorders and symptom exacerbation has been noted after stressful life events. Several epidemiologic reports have linked stress, anxiety and depression to urinary tract symptomatology, including urinary frequency29 with the recent epidemiology of lower urinary tract symptoms (Epi-LUTS) study corroborating this association.30 The high prevalence of psychological stress in patients with LUTS, most profoundly in PBS/IC, suggests central neurochemical alterations may affect urinary tract function.2,3 Physiological changes accompanying these disorders may reflect alterations in serum and urine biomarkers, byproducts of stress factors, which have the potential to be cost effective adjuncts to current diagnostic tools. With better understanding of stress induced biochemical changes in lower urinary tract structure and function, it may be possible to intervene with novel diagnostic and treatment strategies for PBS/IC and OAB.
We report a novel animal model of urinary frequency induced by psychological stress rather than caustic chemicals or social fear. Numerous questions remain unanswered such as the role of mast cells and neovascularity in the development of urinary frequency. Studies are needed to evaluate animals for hyperalgesia since the literature suggests that stress has a profound effect on visceral and somatic pain and this may be the driving force behind urinary frequency. Lastly, bladder dynamics are needed to determine if this is a new model of urinary frequency alone or if detrusor overactivity or poor voiding efficiency may be present.
Conclusions
We present a novel animal model of urinary frequency in female rats subjected to repeated WA stress. Bladder specimens from stressed animals revealed pathologic changes including increased vascularity and increased total and activated mast cells. Similar effects on the gastrointestinal system argue for initiation of this response centrally. This animal model may represent a valid tool for studying functional urinary tract disorders as these changes may provide insight into the pathophysiology of complex voiding disorders.
Acknowledgments
Grants: NIH/NIDDK P50 DK64539, NIH/NIDDK 1RC1 DK 086150-01 (CP and SB), the Perkins Foundation
Footnotes
Disclosures: The authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Macaulay AJ, Stern RS, Holmes DM, et al. Micturition and the mind: psychological factors in the aetiology and treatment of urinary symptoms in women. Br Med J (Clin Res Ed) 1987;294:540–3. doi: 10.1136/bmj.294.6571.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer EA, Naliboff BD, Chang L, et al. Stress and the gastrointestinal tract: V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 3.Rothrock NE, Lutgendorf SK, Kreder KJ. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology. 2001;57:422–7. doi: 10.1016/s0090-4295(00)00988-2. [DOI] [PubMed] [Google Scholar]
- 4.Taché Y, M V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–30. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am J Physiol. 1998;274:G827–31. doi: 10.1152/ajpgi.1998.274.5.G827. [DOI] [PubMed] [Google Scholar]
- 6.Imaki T, Nahan JL, Rivier C, et al. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–99. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charrua A, Cruz CD, Narayanan S, et al. GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol. 2009;181:379–86. doi: 10.1016/j.juro.2008.08.121. [DOI] [PubMed] [Google Scholar]
- 8.Fry CH, Daneshgari F, Thor K, et al. Animal models and their use in understanding lower urinary tract dysfunction. Neurourol Urodyn. 2010;29:603–8. doi: 10.1002/nau.20903. [DOI] [PubMed] [Google Scholar]
- 9.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 10.Cetinel S, Ercan F, Cikler E, et al. Protective effect of melatonin on water avoidance stress induced degeneration of the bladder. J Urol. 2005;173:267–70. doi: 10.1097/01.ju.0000145891.35810.56. [DOI] [PubMed] [Google Scholar]
- 11.Paré WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992;51:1051–6. doi: 10.1016/0031-9384(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 12.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–36. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 13.Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716–23. doi: 10.1016/0016-5085(93)91006-4. [DOI] [PubMed] [Google Scholar]
- 14.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharrnacol Biochem Behav. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 15.Kilfoil T, Michel A, Montgomery D, et al. Effects of anxiolytic and anxiogenic drugs on exploratory activity in a simple model of anxiety in mice. Neuropharmacology. 1989;28:901–5. doi: 10.1016/0028-3908(89)90188-3. [DOI] [PubMed] [Google Scholar]
- 16.Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 17.Cameron IL, Sun LZ, Short N, et al. Therapeutic Electromagnetic Field (TEMF) and gamma irradiation on human breast cancer xenograft growth, angiogenesis and metastasis. Cancer Cell Int. 2005;26:5–23. doi: 10.1186/1475-2867-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidry G. A method for counterstaining tissues in conjunction with the glyoxylic acid condensation reaction for detection of biogenic amines. J Histochem Cytochem. 1999;47:261–4. doi: 10.1177/002215549904700215. [DOI] [PubMed] [Google Scholar]
- 19.Robbins MT, DeBerry J, Ness TJ. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol Behav. 2007;91:544–50. doi: 10.1016/j.physbeh.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang A, Butler S, Sliwoski J, et al. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol. 2009;297:F1101–8. doi: 10.1152/ajprenal.90749.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sant GR, Kempuraj D, Marchand JE, et al. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology. 2007;69:34–40. doi: 10.1016/j.urology.2006.08.1109. [DOI] [PubMed] [Google Scholar]
- 22.Parsons CL, Greene RA, Chung M, et al. Abnormal urinary potassium metabolism in patients with interstitial cystitis. J Urol. 2005;173:1182–85. doi: 10.1097/01.ju.0000148361.82074.77. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia V, Tandon RK. Stress and the gastrointestinal tract. J Gastroenterol Hepatol. 2005;20:332–9. doi: 10.1111/j.1440-1746.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- 24.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature Medicine. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 25.Kiuchi H, Tsujimura A, Takao T, et al. Increased vascular endothelial growth factor expression in patients with bladder pain syndrome/interstitial cystitis: its association with pain severity and glomerulations. BJU Int. 2009;104:826–31. doi: 10.1111/j.1464-410X.2009.08467.x. [DOI] [PubMed] [Google Scholar]
- 26.Scaldaferri F, Vetrano S, Sans M, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585–95. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 27.Thaker PH, Lutgendorf SK, Sood AK. The neuroendocrine impact of chronic stress on cancer. The Cell Cycle. 2007;6:430–3. doi: 10.4161/cc.6.4.3829. [DOI] [PubMed] [Google Scholar]
- 28.Hu TW, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123–8. doi: 10.1016/s0090-4295(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 29.Litman HJ, Steers WD, Wei JT, et al. Relationship of lifestyle and clinical factors to lower urinary tract symptoms: results from Boston Area Community Health survey. Urology. 2007;70:916–21. doi: 10.1016/j.urology.2007.06.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coyne KS, Wein AJ, Tubaro A, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103:4–11. doi: 10.1111/j.1464-410X.2009.08371.x. [DOI] [PubMed] [Google Scholar]


