Abstract
The effect of bioactive plant natural products on the expression and functional activity of P-glycoprotein (P-gp) is poorly understood. Interactions of bioactive plant-based food and dietary supplements with P-gp can cause significant alteration of pharmacokinetic properties of P-gp substrate drugs when used in combination. This can augment toxicity and/or interfere with the drug’s therapeutic outcomes. This study investigated the effects of diverse commonly used plant natural products on the expression and activity of P-gp in human adenocarcinoma cells (LS-180). These natural products included the tobacco cembranoid (1S,2E,4R,6R,7E,11E)-2,7,11-cembratriene-4,6-diol (cembratriene), the palm oil-derived γ-tocotrienol, the extra-virgin olive oil-derived secoiridoid oleocanthal, and the triterpene acid asiatic acid derived from Melaleuca ericifolia and abundant in several other common plant dietary supplements. Treatment with 25 μM of cembratriene, oleocanthal, γ-tocotrienol, or asiatic acid showed 2.3-3.0-fold increase in P-gp expression as demonstrated by Western blotting. These results were consistent with those obtained by quantitative analysis of fluorescent micrographs for P-gp. Accumulation studies demonstrated 31-38% decrease in rhodamine 123 intracellular levels when LS-180 cells were treated with the investigated compounds as a result of P-gp induction. Bioactive natural products can up-regulate the P-gp expression and functionality, which may induce herb/food-drug interactions when concomitantly used with P-gp substrate drugs.
Keywords: P-glycoprotein, oleocanthal, cembratriene, γ-tocotrienol, asiatic acid, intracellular accumulation
1. Introduction
Dietary supplements, also known as food supplements, are an important part of a balanced health regimen, as they can provide essential vitamins, minerals, antioxidants, fibers, amino acids and other nutrients that may be missing or not be consumed in sufficient quantities in a person’s diet (Prior and Cao, 2000).
Yet, there is a general misconception that natural products as health food and dietary supplements are absolutely safe while in fact they can cause health problems due, in part, to herb-drug and food-drug interactions (Bailey and Dresser, 2004). These interactions are considered a major problem in clinical practice as they are responsible for altering important pharmacokinetic processes such as absorption, distribution, metabolism, and elimination, resulting in therapeutic failure or increase toxicity of some prescription drugs (Bailey and Dresser, 2004). Clinical trials have shown that many over-the-counter dietary supplements can modulate the activity of drug metabolizing enzymes and/or drug transporters and further influence the bioavailability of co-administrated drugs (Gurley et al., 2004; Lin, 2007).
Herb-drug and food-drug interactions can arise from the modulation of cytochrome P450 (CYP) isoenzymes such as CYP3A4, multidrug efflux transporters like P-glycoprotein (P-gp), or a combination of both (Gurley et al., 2004; Lin, 2007). P-gp, the product of MDR1 (ABCB1) gene, is an efflux protein that plays a major role in drug disposition and has been reported to be involved in drug-drug and food/herb-drug interactions (Deferme and Augustijns, 2003; Zhang et al., 2009). P-gp is widely expressed in barrier and excretory tissues (Thiebaut et al., 1987). P-gp contributes to the disposition of a wide variety of drugs of different therapeutic categories due to its extensive tissue distribution and broad substrate specificity (Lin, 2007). The transporter is also expressed in other tissues such as liver and blood-brain barrier where it also plays an important efflux role (Thiebaut et al., 1987). Consequently, inhi bition or induction of P-gp may lead to adverse drug interactions at these sites due to altered pharmacokinetics and ultimately have a negative impact on therapeutic response. Nevertheless, in other cases such interactions can lead to therapeutically beneficial effects, as in the case of P-gp inhibition to enhance anti-cancer drugs delivery to the tumor cells, or P-gp induction in the case of Alzheimer’s disease (Lee et al., 2010; Abuznait et al., 2011).
Dietary supplements containing bioactive natural products are the most frequently used of all complementary and alternative therapies, primarily because they are widely available without the need to consult a health care practitioner. Recent reports showed that 18% of adults in the United States use prescription drugs concurrently with herbal or dietary products, placing an estimated 15 million patients at risk of potential drug-supplement interactions (LaValle, 2000). In addition to dietary supplements, bioactive natural products exist in food such as the extra-virgin olive oil derived oleocanthal and the tobacco cembranoids.
Several studies reported the ability of bioactive natural products found in traditional medicine and dietary supplements to modulate P-gp activity (Marchetti et al., 2007). For example, naturally-occurring flavonoids were found to bind P-gp with high affinity and inhibit its activity (Chieli et al., 1995). Also, in vitro studies demonstrated the ability of St. John’s wort to induce P-gp expression and functional activity (Tian et al., 2005). The administration of St. John’s wort extract to rats or humans for 14 days resulted in a 3.8- and 1.4-fold increase of intestinal P-gp expression, respectively (Durr et al., 2000). The 1.4-fold increase in intestinal P-gp expression in the healthy subjects resulted in an 18% decrease in digoxin exposure (Durr et al., 2000). Therefore, special care should be taken when drugs that are P-gp substrates are used by patients who are exposed to bioactive dietary natural products.
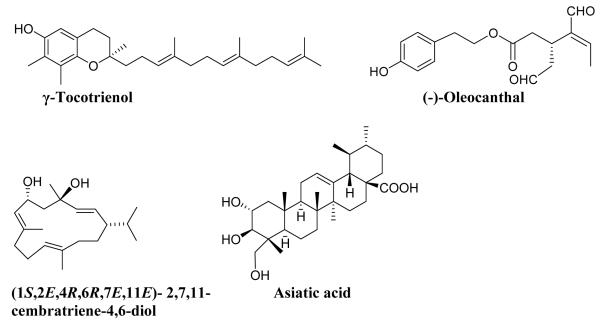
In the present investigation, studies were conducted to examine the effects of various bioactive natural compounds found in commonly used dietary and herbal supplements including γ-tocotrienol (a vitamin E isoform abundant in palm oil), asiatic acid (common in gotu kola and tea tree oil), oleocanthal (extra-virgin olive oil), and (1S,2E,4R,6R,7E,11E)-2,7,11-cembratriene-4,6-diol (tobacco leaves; hereafter cembratriene) on the induction of P-gp in LS-180 human colon adenocarcinoma cells grown in culture. These bioactive natural products (Fig. 1) were selected due to their high consumption and reported therapeutic benefits.
Fig. 1.
Chemical structures for th e bioactive plant natural products examined in the present study.
2. Methods and materials
2.1 Chemicals and reagents
Verapamil, rifampicin, HEPES, and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO). RIPA buffer was purchased from Thermo Scientific (Rockford, IL). The reagents and supplements required for Western blotting were purchased from Bio-Rad (Hercules, CA). The mouse monoclonal P-gp antibody (C-219) was obtained from Covance Research Products (Dedham, MA). Anti-β-actin (C-11) and HRP-labeled secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Normal donkey serum, normal goat serum, and Rhodamine Red™-X-conjugated AffiniPure donkey anti-mouse IgG (H+L) secondary antibody against P-gp were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Formaldehyde 16% (Methanol Free, Ultrapure EM Grade) was purchased from Polysciences, Inc. (Warrington, PA). All other reagents, chemicals and supplies were purchased from VWR (West Chester, PA).
2.2 Bioactive natural products
γ-Tocotrienol was isolated from palm oil-derived tocotrienol-rich fraction (Palm TRF 70%, Davos, Singapore) on Si gel 60 using n-hexane-ethyl acetate as previously reported (Elnagar et al., 2010). (-)-Oleocanthal was isolated from extra-virgin olive oil (Member’s Mark®, Batch No. VF1_US102808, Italy) on lipophilic Sephadex LH20 (Sigma Aldrich, bead size 25-100 μ) using n-hexane-CH2Cl2 (1:9) and finally purified on C-18 reversed-phase Bakerbond octadecyl (40 μm; Mallinckrodt Baker, Inc.) using isocratic CH3CN-H2O (40-60) (Elnagar et al., 2011). Cembratriene was isolated from fresh tobacco leaf powder (Custom Blends, New York) using normal phase and finally on reversed phase silica gel (MeOH-H2O, 40:60, isocratic) (El Sayed et al., 2008). Asiatic acid was obtained from the leaves of Melaleuca ericifolia by repeated column chromatography on normal phase (n-hexane-EtOAc, 90:10) and finally on Sephadex LH20 using CHCl3-MeOH (95:5, isocratic) (Abdel Bar et al., 2008).
A purity of >95% was established for each compound as assessed by TLC, 1H NMR spectroscopy, and/or HPLC analysis (Abdel Bar et al., 2008, El Sayed et al., 2008, Elnagar et al. 2010 and 2011).
2.3 Cell culture
Human colorectal adenocarcinoma cell line (LS-180) and all cell culture reagents, as recommended for the growth and maintenance of the cells were obtained from the American Type Culture Collection (Manassas, VA). LS-180 cells were cultured in RPMI-1640 growth medium supplemented with 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 4500 mg/L D-glucose, 1500 mg/L NaHCO3, 10% FBS, 50 IU/mL penicillin and 50 μg/mL streptomycin. Cells were grown to confluence in 75-cm2 cell culture flasks for 3-6 days at 37°C/5% CO2 and used between passage numbers 5-20.
2.4 In vitro induction study
Induction of P-gp was carried out as following. Briefly, cells were seeded in 10 mm-cell culture dishes at a density of 5×106 cells/dish and allowed to attach and grow up to 50-60% confluence at 37°C and 5% CO2. Stock solutions of oleocanthal and γ-tocotrienol (in DMSO), in addition to cembratriene and asiatic acid (in methanol) were diluted to a final concentration of 50 μM in growth medium before use. Rifampicin stock solution, used as positive control, was prepared in DMSO and similarly was diluted to 50 μM with medium. After 48 h of cell seeding, control and compound-containing media at different concentrations were added to the respective treatment cells. The cells were then incubated for additional 48 h at 37°C/5% CO2. The media were not renewed for the duration of the experiment. Cells were viable at the conclusion of the treatment period as confirmed visually under the microscope and by protein levels analysis that was comparable to untreated cells.
2.5 Western blotting
After 48 h of drug treatment, total cell lysate were prepared and 16 μg of proteins were resolved on 7.5% SDS-polyacrylamide gel and transferred electrophoretically onto a nitrocellulose membrane. The membrane was blocked with 2% BSA in PBS for 1 h at room temperature and then incubated overnight at 4°C with the primary antibodies for P-gp (C-219) and β-actin (C-11) at dilutions 1:200 and 1:3000, respectively. For proteins detection, the membrane was incubated with HRP-labeled secondary IgG antibody for P-gp (anti-mouse) and β-actin (anti-goat) at dilution 1:5000. The bands were visualized by Pierce chemiluminescence detection kit (Thermo Scientific). Quantitative analysis of the immunoreactive bands was performed using Syngene luminescent image analyzer (Scientific Resources Southwest, Inc., Stafford, TX). The results of protein quantification were expressed as the ratio of P-gp to β-actin levels.
2.6 In vitro activity study
LS-180 cells were seeded in 48-well plates at a density of 5×104 cells/well and allowed to attach and grow to 50-60% confluence at 37°C/5% CO2. The cells were then treated as previously described in the in vitro induction study section. Similar concentrations were used as for the expression studies. The activity of the induced P-gp in LS-180 cells was evaluated by an uptake study to measure the accumulation of P-gp substrate within the LS-180 cells as follows: After 48 h the treatment medium was aspirated and the cells were incubated in fresh growth medium for 4 h. Cells were then washed three times with a transport buffer (141 mM NaCl, 4 mM KCl, 2.8 mM CaCl2, 1 mM MgSO4, 10 mM D-glucose, and 10 mM HEPES). The cells were then pre-incubated with or without 100 μM verapamil in transport buffer for 30 min. The activity experiments were started by the addition of 1 μg/mL of rhodamine 123 in transport buffer with or without 100 μM verapamil for 2 h at 37°C/5% CO2. The activity experiment was then terminated by washing the cells three times with ice-cold PBS and then disrupting them with a lysis for 1 h at 37°C. The fluorescent intensity of rhodamine 123 accumulated inside the cells were measured using Synergy 2 microplate reader (Biotek, Winooski, VT) under the excitation wavelength 485 nm and emission wavelength 529 nm and data acquisition was achieved using Gene5 software (Biotek). Data was normalized for the protein content determined by Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific) according to the manufacturer’s instruction. Cellular accumulation of rhodamine 123 was used to calculate the inhibition ratio, which is the ratio of fluorescent intensity per mg protein of the treatment sample in the presence of verapamil divided by the fluorescent intensity per mg protein of the same sample in the absence of verapamil. P-gp activity for cells treated with rifampicin, used as positive control, was investigated in parallel. Results were expressed as means ± standard deviation (SD) for the inhibition ratios compared to control.
2.7 Immunofluorescence staining and imaging of P-gp
To confirm effect of natural products treatment on P-gp, its protein expression was visualized using confocal microscope as follows: LS-180 cells (5×104 cells) were seeded on 35-mm poly-D-lysine coated glass bottom plates no. 1.5 (MatTek Corporation, Ashland, MA) and treated with 25 μM of oleocanthal, cembratriene, γ-tocotrienol, and asiatic acid for 48 h as described above. Following treatment, cells were washed three times with PBS, fixed with 4% formaldehyde, and blocked for 30 min with 10% of normal donkey and goat sera in 0.3% Triton X-100/PBS. The cells were then incubated overnight at 4°C with a 1:50 dilution of primary antibody against P-gp in solution composed of 1% normal donkey and goat sera in PBS. The cells were washed with PBS and incubated for 30 min with Rhodamine Red goat anti-mouse secondary antibody at 1:250 dilution. Cell nuclei were stained with DAPI. Images for P-gp were captured using Zeiss LSM 5 Pascal confocal microscope equipped with 543 nm line of HeNe Laser and 63X oil immersion objective lens with numerical aperture = 1.4 (Carl Zeiss MicroImaging, LLC, Thornwood, NY). Negative controls for each treatment that were processed without primary antibody showed negligible background fluorescence. P-gp membrane immunofluorescence for each sample was quantified using ImageJ version 1.44 software (Research Services Branch, NIMH/NIH, Bethesda, MD).
2.8 Statistical analysis
Wherever possible, the experimental results were statistically analyzed for significant difference using Two-Tailed Student’s t-test to evaluate differences between controls and treated groups. Emax and EC50 were determined from non-linear regression of concentration versus fold increase curves using the four-parameter Hill equation by GraphPad Prism version 5.0 for Windows (GraphPad Software Inc., San Diego, CA). A p-value less than 0.05 was considered to be statistically significant. Three independent experiments were carried out for each compound.
3. Results
3.1 In vitro induction of P-gp expression by bioactive natural products
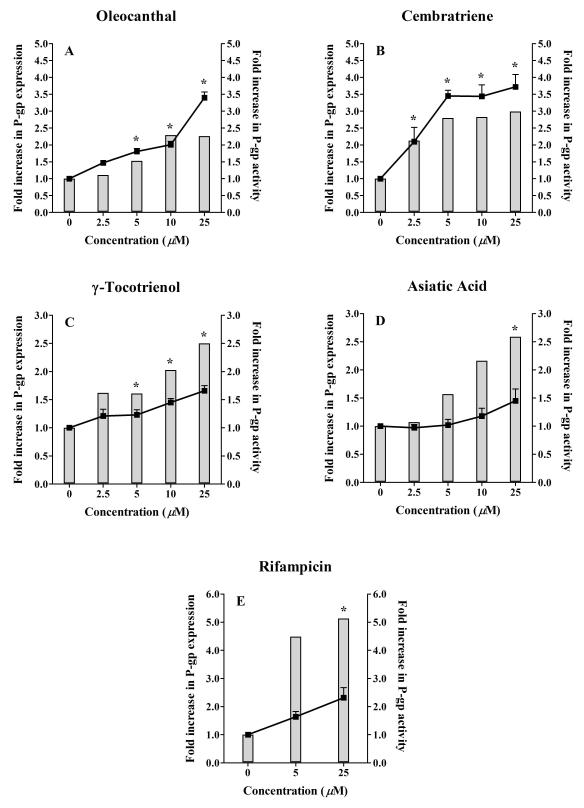
The ability of the investigated natural compounds to induce P-gp expression was assessed by Western blotting using the C-219 mouse monoclonal antibody. Fig. 2 and 3 (solid columns, left Y axis) illustrate Western blots for P-gp expression in LS-180 cells treated with increasing concentrations of oleocanthal, cembratriene, γ-tocotrienol, asiatic acid, and rifampicin as positive control, and fold increase in P-gp expression quantified by densitometry of the immunoblots. All investigated compounds resulted in an increase in P-gp expression following 48 h treatments. A 25 μM treatment of oleocanthal and cembratriene resulted in 2.3 and 3.0-fold increase in the expression of P-gp protein compared to control, respectively. Similarly, at 25 μM concentration γ-tocotrienol and asiatic acid induced P-gp expression by 2.5- and 2.6-fold, respectively, compared to the control. At 25 μM concentration, the positive control rifampicin increased P-gp expression by 5-fold.
Fig. 2.
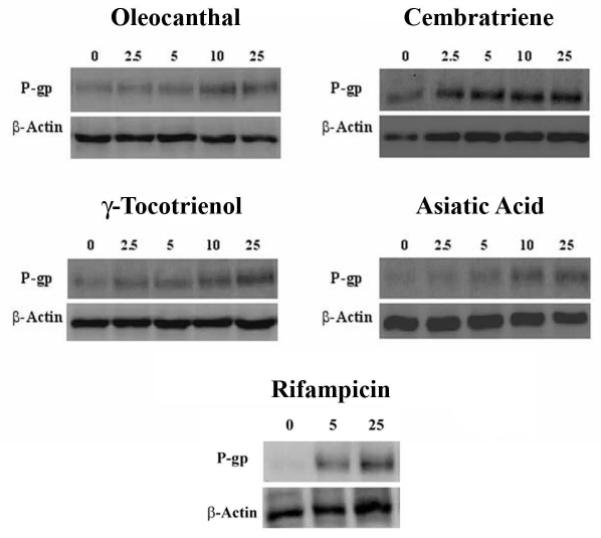
Representative Western blots for P-gp in LS-180 cells treated with oleocanthal, cembratriene, γ-tocotrienol, and asiatic acid. Rifampicin is included as a positive control. Cells were treated for 48 h with increasing concentrations of the indicated compounds in the range of 2.5-25 μM. P-gp protein expression was analyzed by immunoblotting with C-219 mouse monoclonal antibody.
Fig. 3.
Effect of treatment of LS-180 cells with increasing concentrations of (A) oleocanthal, (B) cembratriene, (C) γ-tocotrienol, (D) asiatic acid, and (E) rifampicin on P-gp expression and activity. Cells were treated for 48 h followed by Western blot analysis for P-gp expression (shown as column bars) and uptake study for P-gp activity (shown as lines). P-gp activity was measured as the ratio of intracellular rhodamine 123 fluorescence in the presence and absence of 100 μM verapamil as P-gp inhibitor. The data is expressed as mean ± SD for P-gp activity (n=3). P-gp expression was determined for n=2. *P < 0.05 compared P-gp activity in untreated and treated cells.
3.2 Evaluation of P-gp functional activity following its induction
The functional significance of the increase in P-gp expression was determined by evaluating the cellular uptake of P-gp substrate rhodamine 123 following treatments with increasing concentrations of the compounds ranging from 2.5 to 25 μM in the presence and absence of 100 μM verapamil as a P-gp inhibitor. Figure 3 (A-E) demonstrates the fold increase in P-gp activity (lines, right Y axis) following LS-180 cells treatment with oleocanthal, cembratriene, γ-tocotrienol, asiatic acid, and rifampicin. Figure 4 illustrates the effect of P-gp induction, by the compounds at 25 μM concentrations, on the intracellular accumulation of rhodamine 123 in the absence and presence of verapamil. The increase in P-gp expression was associated with an increase in P-gp transport activity, measured as the ratio of intracellular rhodamine 123 fluorescence in the presence and absence of 100 μM verapamil, as P-gp inhibitor. In the absence of compound treatments (control), LS-180 cells exhibit a low level of P-gp expression and function that was significantly increased following treatment with the examined compounds (p < 0.05, Fig. 2 and 3). The effect of oleocanthal on P-gp activity in LS-180 cells increased progressively (47-240%) over the range 2.5-25 μM (Fig. 3A). A similar pattern was seen with cembratriene over the same concentration range (110-270% increase, Fig. 3B). Cells treated with γ-tocotrienol and asiatic acid increased P-gp activity by > 45% at 10 and 25 μM, respectively (Fig. 3C, D). The reason for the discrepancy between induction of P-gp expression and P-gp activity observed with γ-tocotrienol, asiatic acid and rifampicin is not clear, however it could be related to that a fraction of the induced protein is not functioning. Also, despite washing of the cells for 4 h, residual amounts of rifampicin, and possibly γ-tocotrienol and asiatic acid in the cells could have inhibited P-gp activity (Abuznait et al, 2011; Gupta et al, 2008).
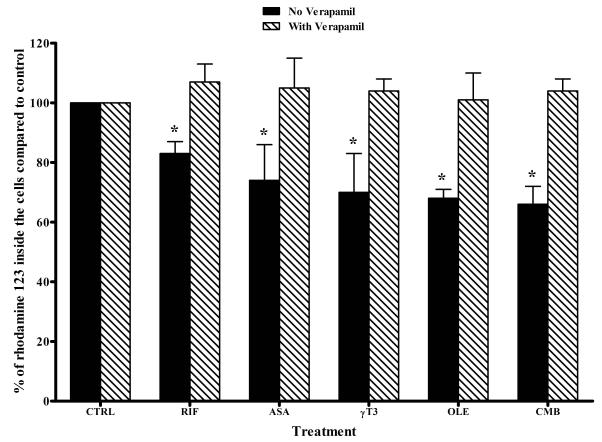
Fig. 4.
Effect of treatment of LS-180 cells with 25 μM of rifampicin (RIF), oleocanthal (OLE), cembratriene (CMB), γ-tocotrienol (γT3), or asiatic acid (ASA), on the intracellular accumulation of rhodamine 123. Percent change in the accumulation of rhodamine 123 was evaluated in the presence (dashed bars) and absence (solid bars) of verapamil, a specific P-gp inhibitor. The data are expressed as mean ± SD (n = 3-4). *P < 0.05 compared to control untreated cells.
Rifampicin at the concentrations 5 and 25 μM increased P-gp activity by 180 and 230%, respectively (Fig. 3E). At 25 μM concentrations of the compounds including rifampicin, the increase in P-gp activity was associated with a significant reduction in the intracellular accumulation of rhodamine 123 by 18-38%, which was restored to the control levels (around 100%) by P-gp inhibition with verapamil (Fig. 4).
3.3 Determination of EC50 and potency
The potencies of the compounds to up-regulate P-gp activity were estimated from the average of fold increase in P-gp activity. The Hill equation was fit to the fold increase in P-gp activity as a function of increasing inducer concentration to estimate the EC50 and Emax, from which the induction activity (or potency) was estimated (Potency = Emax/EC50). To better estimate the EC50, additional concentration points were investigated up to 50 μM; the compounds were toxic to the cells above 50 μM. γ-Tocotrienol was toxic to the cells at concentrations > 25 μM, thus its EC50 was estimated from the concentration range 0-25 μM. The Rifampicin’s EC50 to up-regulate P-gp activity was estimated from the concentration range 0-100 μM. Table 1 illustrates the in vitro potencies of the inducers to up-regulate P-gp activity as follows: cembratriene > oleocanthal > γ-tocotrienol > rifampicin > asiatic acid. The concentrations required to increase P-gp activity by 50% (EC50) were much lower for the examined natural products (2.8-14 μM) compared to rifampicin (26.8 μM). Thus, cembratriene, oleocanthal, and γ-tocotrienol potencies to up-regulate P-gp activity would be at much lower concentrations compared to rifampicin. However, unlike rifampicin that increased P-gp activity by 4.4-fold, asiatic acid demonstrated a relatively high EC50 and low Emax (1.5-fold, Table 1).
Table 1.
In vitro EC50, Emax and Potencies of the Tested Natural Products to Up-regulate P-gp Activity in LS-180 Cells.
| Natural Product | EC50 (μM) | Emax (fold ↑ in P-gp activity) | Emax/EC50 |
|---|---|---|---|
| Rifampicin | 26.8 | 4.4 | 0.17 |
| (-)-Oleocanthal | 14 | 4.5 | 0.32 |
| Cembratriene | 2.8 | 3.7 | 1.32 |
| γ-Tocotrienol | 5.9 | 1.7 | 0.29 |
| Asiatic Acid | 11.3 | 1.5 | 0.13 |
3.4 Immunofluorescence detection of P-gp up-regulation in LS-180 cells
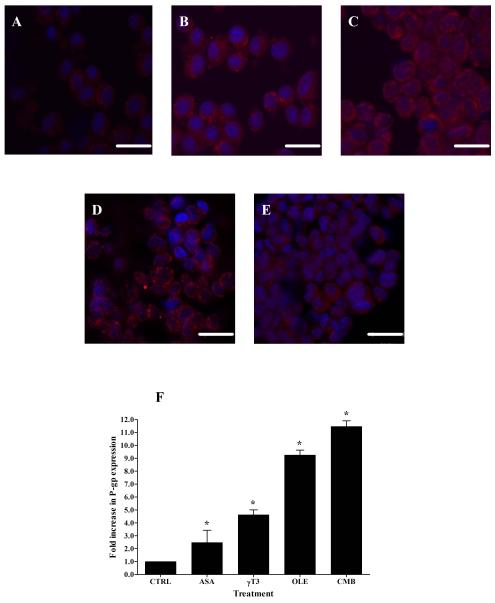
Fig. 5A-E show fluorescent micrograph images of untreated P-gp expression (control, Fig. 5A), oleocanthal (Fig. 5B), cembratriene (Fig. 5C), γ-tocotrienol (Fig. 5D), and asiatic acid (Fig. 5E)-treated cells (25 μM). Fluorescence of secondary antibody against P-gp was located mainly on the cell membrane. The quantitative analysis of P-gp expressed in LS-180 cells showed a significant increase in membrane P-gp expression of compound-treated cells compared to control. For example, oleocanthal and cembratriene resulted in 9.3 ± 0.37 and 11.5 ± 0.44 fold-increase, respectively, in P-gp expression compared to the control (p < 0.05; Fig. 5F). Treatments with γ-tocotrienol and asiatic acid induced membrane P-gp expression by 4.6 ± 0.39 and 2.5 ± 0.95 folds, respectively.
Fig. 5.
Representative fluorescent micrographs of P-gp for control (A) and treated LS-180 cells with 25 μM of oleocanthal (B), cembratriene (C), asiatic acid (D), and γ-tocotrienol (E). Quantitative folds change in the P-gp expression were measured using ImageJ version 1.44. The data are expressed as mean ± SD (n=4). *P < 0.05 compared to control untreated cells. Scale bar = 20 μm.
4. Discussion
P-gp, an efflux transporter, is highly expressed in body tissues and can form a biological barrier against xenobiotic agents. Thus, it can be a major determinant of drug pharmacokinetic behavior and response. Inhibition or induction of P-gp has been reported to be one of the causes of drug-drug interactions in animals and humans. Besides, it has been reported that natural products found in traditional medicine and dietary supplements can modulate P-gp activity (Marchetti et al., 2007). Excluding St John’s wort, to date there are limited reports on the effect of chronic intake of bioactive natural products on P-gp activity and their role in drug interactions. Therefore, we aimed in this study to investigating the in vitro effect of bioactive natural plant products on P-gp expression and activity in LS-180 human colon adenocarcinoma cell line (Tom et al., 1976). Rifampicin is a well established inducer of P-gp (Ballent et al., 2010; Harmsen et al., 2009), thus in this study it has been utilized as a positive control.
Terpenes and phenolics are among the most abundant plant bioactive secondary metabolites (Newman and Cragg, 2009). Therefore, the terpenes cembratriene and asiatic acid and the phenolics γ-tocotrienol and oleocanthal were selected as representatives of these important bioactive plant secondary metabolite classes. These bioactive natural products, which present in dietary supplements and food, have shown to provide potential therapeutic benefits. However, the effect of their chronic intake on P-gp expression and/or activity and their potential association with drug interactions has not been investigated yet. Oleocanthal, a phenolic ester secoiridoid found in extra-virgin olive oil, is a key ingredient of the Mediterranean diet. It has a natural pain relief effect similar to ibuprofen, and exerts anti-inflammatory activity via inhibition of COX-1 and COX-2 activities (Beauchamp et al., 2005). Oleocanthal is widely believed to be useful for cancer (Elnagar et al., 2011), stroke and heart disease (Colomer and Menendez, 2006). In addition, other studies on oleocanthal have shown potentia l anti-Alzheimer’s activity (Pitt et al., 2009). On the other hand, cembratriene is a natural cembranoid diterpene found in the leaf and flower cuticular wax of most Nicotiana species (Ferchmin et al., 2005). In rats, tobacco cembranoids were reported to block the expression of the behavioral sensitization to nicotine and inhibit neuronal acetylcholine receptors, suggesting a possible use for the treatment of nicotine addiction (Ferchmin et al., 2005). It is found in therapeutic quantities as part of Nicotiana supplements such as SmokeHalt and considered one of the main flavor ingredient in tobacco smoke (Herbasanté, 2011). Similar to oleocanthal, cembranoids have also an anticancer activity and were reported to inhibit tumorigenesis (Saito et al., 1985). γ-Tocotrienol is one form of naturally occurring vitamin E present in palm oil, wheat germ and rice bran (Sundram et al., 2002). It exhibits significant health benefits including anticancer (Shah and Sylvester, 2005), anticholesterolemic (Song and DeBose-Boyd, 2006), and potent antioxidant activities (Tomeo et al., 1995). γ-Tocotrienol has been reported to activate the nuclear receptors PXR, which regulates the expression of P-gp encoding gene MDR1 (Zhou et al., 2004). Finally, asiatic acid is a pentacyclic triterpenoid derived from the tropical medicinal plants Centella asiatica and Melaleuca ericifolia (Hsu et al., 2005; Singh and Agarwal, 2007). Several studies have also shown asiatic acid to be cytotoxic in a wide variety of cancer cell lines, including breast cancer, melanoma and myeloma (Hsu et al., 2005), and was reported to have anti-angiogenic and anti-Alzheimer’s activities (Singh and Agarwal, 2007).
In the literature, few studies have evaluated the exposure consequences of the crude extract of cembratriene (tobacco smoke extract) (Pan et al., 2009), and a mixture of terpenic compounds isolated from Euphorbia species (Duarte et al., 2009) on P-gp, and tocotrienols on P-gp and PXR (Zhou et al., 2004); and to our knowledge this is the first study identifying individual components of different bioactive natural products includi ng oleocanthal, cembratriene, γ-tocotrienol, and asiatic acid following in vitro chronic exposure to up-regulate P-gp expression and activity. For example, Pan et al. (2009) investigated the effect of tobacco smoke extract on P-gp in an epidermal carcinoma cell line. The authors reported that the efflux of rhodamine 123 was reduced in a concentration-dependent manner. However, these authors did not identify the specific compounds in the tobacco smoke extract responsible for such effect (Pan et al., 2009). Similarly, taraxastane-type triterpenes isolated from Euphorbia lagascae and E. tuckeyana exhibited significant P-gp modulation and apoptosis induction activities (Duarte et al., 2009), verifying the role of triterpenes including asiatic acid as P-gp inducers. In addition, Zhou et al. (2004) demonstrated that tocotrienols up-regulated MDR1 in LS-180 cells but not in primary hepatocytes (Zhou et al., 2004). Consistent with their findings our results further confirmed γ-tocotrienol as an inducer of P-gp protein expression and activity in a concentration-dependent manner.
Similar to rifampicin, the exposure of LS-180 cells to oleocanthal, cembratriene, γ-tocotrienol, and asiatic acid for 48 h resulted in a concentration-dependent increase in P-gp expression, which was confirmed by both Western blotting and immunofluorescence imaging. Quantitative analysis of P-gp expression obtained from immunofluorescence images was higher than those obtained from Western blotting, which could be related to the method sensitivity; overall, the results from both procedures were parallel (Figs. 2, 3, and 5). In addition, consistent with the expression studies, the activity studies demonstrated an increase in P-gp activity (Figs. 3 and 4). The functionality of P-gp in these cells was demonstrated by rhodamine 123 accumulation, which decreased by compounds treatment and increased by P-gp inhibition with verapamil, a specific competitive inhibitor for P-gp and is commonly used to evaluate the functionality of P-gp efflux transporter (Pham et al., 2000), si gnifying the increase in P-gp activity as the explanation of the reduced intracellular accumulation of rhodamine 123.
Interestingly, our data revealed that cembratriene, oleocanthal and γ-tocotrienol were more potent than rifampicin (Table 1). Clinical studies in human subjects investigated the effect of rifampicin’s oral administration on the pharmacokinetics of different P-gp substrates including digoxin and risperidone (Greiner et al., 1999; Kim et al., 2008). The P-gp inducer rifampicin significantly altered the pharmacokinetics of both drugs. Rifampicin decreased digoxin and risperidone area under the plasma curves (AUC) by 30 and 51%, respectively (Greiner et al., 1999; Kim et al., 2008). Besides, following 23 days of rifampicin treatment, Greiner et al. (1999) have reported that the decrease in digoxin bioavailability was associated with a significant increase in the human subjects’ intestinal P-gp expression by 3.5-fold, quantified by Western blotting. Accordingly, as our in vitro results showed that cembratriene, oleocanthal and γ-tocotrienol can up-regulate P-gp functionality more potently compared to rifampicin, it is expected that these compounds could have the potential to alter the pharmacokinetics, hence therapeutic effects, of drugs that are substrate of P-gp in vivo. Future experiments to examine the in vivo effect of oleocanthal, cembratriene, γ-tocotrienol, and asiatic acid on P-gp expression and activity, and their potential for drug-herb/food interactions remain to be investigated in order to elucidate the clinical significance of such combinations.
In addition to P-gp, LS-180 cells express pregnane X receptor (PXR) and the metabolizing enzyme CYP3A (Pfrunder et al., 2003). P-gp and CYP3A are regulated via PXR and act coordinately at the intestinal barrier in determining drugs disposition, thus the modulation of intestinal P-gp, by inhibition or induction, is expected to modulate CYP3A as in the case of rifampicin (Pfrunder et al., 2003). In the current study, rhodamine 123, used to evaluate P-gp enhanced activity, is a substrate for P-gp but not CYP3A. Thus, the above data would not demonstrate the tested compounds as CYP3A up-regulators. However, their potential to up-regulate CYP3A is very likely, which may present an additional mechanism for food-drug interactions that requires further consideration.
In conclusion, our data have confirmed the ability of the bioactive natural compounds examined in this study to in vitro up-regulate both P-gp activity and expression. Thus, concomitant use of these bioactive natural products with P-gp substrate drugs might induce potential drug interactions and interfere with their pharmacokinetics and therapeutic effects. The tobacco-derived cembratriene was the most potent while the triterpene asiatic acid was the least.
Highlights.
- Bioactive plant natural products including oleocanthal, γ-tocotrienol, cembratriene and asiatic acids up-regulate P-gp
- Oleocanthal, γ-tocotrienol and cembratriene are more potent in vitro inducers of P-gp when compared to rifampicin
- Concomitant use of these natural products with P-gp substrate drugs might alter these drugs kinetics thus response
Acknowledgments
This research was supported by the National Center for Research Resources (Grant P20RR016456). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institute of Health. The Louisiana Board of Regents is acknowledged for supporting the new NMR facility (LEQSF(2010-11)-ENH-TR-41).
Abbreviations
- (AUC)
Area under the curve
- CYP
Cytochrome P450
- MDR
Multidrug resistance
- P-gp
P-glycoprotein
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel Bar FM, Zaghloul AM, Bachawal SV, Sylvester PW, Ahmad KF, El Sayed KA. Antiproliferative triterpenes from Melaleuca ericifolia. J Nat Prod. 2008;71:1787–1790. doi: 10.1021/np800360a. [DOI] [PubMed] [Google Scholar]
- Abuznait AH, Cain C, Ingram D, Burk D, Kaddoumi A. Up-regulation of P-glycoprotein reduces intracellular accumulation of beta amyloid: Investigation of P-glycoprotein as a novel therapeutic target for Alzheimer’s disease. J Pharm Pharmacol. 2011;63:1111–1118. doi: 10.1111/j.2042-7158.2011.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuznait AH, Patrick SG, Kaddoumi A. Exposure of LS-180 cells to drugs of diverse physicochemical and therapeutic properties up-regulates P-glycoprotein expression and activity. J Pharm Pharmaceut Sci. 2011;14:236–248. doi: 10.18433/j36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DG, Dresser GK. Natural products and adverse drug interactions. CMAJ. 2004;170:1531–1532. doi: 10.1503/cmaj.1031558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballent M, Lifschitz A, Virkel G, Mate L, Lanusse C. Pretreatment with the inducers rifampicin and phenobarbital alters ivermectin gastrointestinal disposition. J Vet Pharmacol Ther. 2010;33:252–259. doi: 10.1111/j.1365-2885.2009.01129.x. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, Lee CH, Smith AB, Breslin PA. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437:45–46. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- Chieli E, Romiti N, Cervelli F, Tongiani R. Effects of flavonols on P-glycoprotein activity in cultured rat hepatocytes. Life Sci. 1995;57:1741–1751. doi: 10.1016/0024-3205(95)02152-9. [DOI] [PubMed] [Google Scholar]
- Colomer R, Menendez JA. Mediterranean diet, olive oil and cancer. Clin Transl Oncol. 2006;8:15–21. doi: 10.1007/s12094-006-0090-0. [DOI] [PubMed] [Google Scholar]
- Deferme S, Augustijns P. The effect of food components on the absorption of P-gp substrates: a review. J Pharm Pharmacol. 2003;55:153–162. doi: 10.1211/002235702603. [DOI] [PubMed] [Google Scholar]
- Duarte N, Ramalhete C, Varga A, Molnar J, Ferreira MJ. Multidrug resistance modulation and apoptosis induction of cancer cells by terpenic compounds isolated from Euphorbia species. Anticancer Res. 2009;29:4467–4472. [PubMed] [Google Scholar]
- Durr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K. St John’s Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- El Sayed KA, Laphookhieo S, Baraka HN, Yousaf M, Hebert A, Bagaley D, Rainey FA, Muralidharan A, Thomas S, Shah GV. Biocatalytic and semisynthetic optimization of the anti-invasive tobacco (1S,2E,4R,6R,7E,11E)-2,7,11-cembratriene-4,6-diol. Bioorg Med Chem. 2008;16:2886–2893. doi: 10.1016/j.bmc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- Elnagar AY, Sylvester PW, El Sayed KA. Oleocanthal as a c-Met inhibitor for the control of metastatic breast and prostate cancers. Planta Med. 2011;77:1013–1019. doi: 10.1055/s-0030-1270724. [DOI] [PubMed] [Google Scholar]
- Elnagar AY, Wali VB, Sylvester PW, El Sayed KA. Design and preliminary structure-activity relationship of redox-silent semisynthetic tocotrienol analogues as inhibitors for breast cancer proliferation and invasion. Bioorg Med Chem. 2010;18:755–768. doi: 10.1016/j.bmc.2009.11.054. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Hao J, Perez D, Penzo M, Maldonado HM, Gonzalez MT, Rodriguez AD, de Vellis J. Tobacco cembranoids protect the function of acute hippocampal slices against NMDA by a mechanism mediated by alpha4beta2 nicotinic receptors. J Neurosci Res. 2005;82:631–641. doi: 10.1002/jnr.20666. [DOI] [PubMed] [Google Scholar]
- Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, Kroemer HK. The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest. 1999;104:147–153. doi: 10.1172/JCI6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mugundu GM, Desai PB, Thummel KE, Unadkat JD. Intestinal human colon adenocarcinoma cell line LS180 is an excellent model to study pregnane X receptor, but not constitutive androstane receptor, mediated CYP3A4 and multidrug resistance transporter 1 induction: studies with anti-human immunodeficiency virus protease inhibitors. Drug Metab Dispos. 2008;36:1172–1180. doi: 10.1124/dmd.107.018689. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Carrier J, Khan IA, Edwards DJ, Shah A. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Ther. 2004;76:428–440. doi: 10.1016/j.clpt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother Pharmacol. 2009 doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbasanté Inc [Accessed: 2/18/2011];SmokeHalt. http://www.natural-remedies.com/products/72-smokehalt.
- Hsu YL, Kuo PL, Lin LT, Lin CC. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J Pharmacol Exp Ther. 2005;313:333–344. doi: 10.1124/jpet.104.078808. [DOI] [PubMed] [Google Scholar]
- Kim KA, Park PW, Liu KH, Kim KB, Lee HJ, Shin JG, Park JY. Effect of rifampin, an inducer of CYP3A and P-glycoprotein, on the pharmacokinetics of risperidone. J Clin Pharmacol. 2008;48:66–72. doi: 10.1177/0091270007309888. [DOI] [PubMed] [Google Scholar]
- LaValle JB. Natural therapeutics pocket guide: 2000-2001. Lexi-Comp; Hudson, OH: 2000. [Google Scholar]
- Lee CA, Cook JA, Reyner EL, Smith DA. P-glycoprotein related drug interactions: clinical importance and a consideration of disease states. Expert Opin Drug Metab Toxicol. 2010;6:603–619. doi: 10.1517/17425251003610640. [DOI] [PubMed] [Google Scholar]
- Lin JH. Transporter-mediated drug interactions: clinical implications and in vitro assessment. Expert Opin Drug Metab Toxicol. 2007;3:81–92. doi: 10.1517/17425255.3.1.81. [DOI] [PubMed] [Google Scholar]
- Marchetti S, Mazzanti R, Beijnen JH, Schellens JH. Concise review: Clinical relevance of drug-drug and herb-drug interactions mediated by the ABC transporter ABCB1 (MDR1, P-glycoprotein) Oncologist. 2007;12:927–941. doi: 10.1634/theoncologist.12-8-927. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural product scaffolds as leads to drugs. Future Med Chem. 2009;1:1415–1427. doi: 10.4155/fmc.09.113. [DOI] [PubMed] [Google Scholar]
- Pan WC, Chen RM, Shen YC, Chen CC, Ueng YF. Suppressive effect of tobacco smoke extracts on oral P-glycoprotein function and its impact in smoke-induced insult to oral epidermal cells. Toxicol Lett. 2009;185:116–123. doi: 10.1016/j.toxlet.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Pfrunder A, Gutmann H, Beglinger C, Drewe J. Gene expression of CYP3A4, ABC-transporters (MDR1 and MRP1-MRP5) and hPXR in three different human colon carcinoma cell lines. J Pharm Pharmacol. 2003;55:59–66. doi: 10.1111/j.2042-7158.2003.tb02434.x. [DOI] [PubMed] [Google Scholar]
- Pham YT, Regina A, Farinotti R, Couraud P, Wainer IW, Roux F, Gimenez F. Interactions of racemic mefloquine and its enantiomers with P-glycoprotein in an immortalised rat brain capillary endothelial ce ll line, GPNT. Biochim Biophys Acta. 2000;1524:212–219. doi: 10.1016/s0304-4165(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Pitt J, Roth W, Lacor P, Smith AB, 3rd, Blankenship M, Velasco P, De Felice F, Breslin P, Klein WL. Alzheimer’s-associated Abeta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol Appl Pharmacol. 2009;240:189–197. doi: 10.1016/j.taap.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior RL, Cao G. Analysis of botanicals and dietary supplements for antioxidant capacity: a review. J AOAC Int. 2000;83:950–956. [PubMed] [Google Scholar]
- Saito Y, Takizawa H, Konishi S, Yoshida D, Mizusaki S. Identification of cembratriene-4,6-diol as antitumor-promoting agent from cigarette smoke condensate. Carcinogenesis. 1985;6:1189–1194. doi: 10.1093/carcin/6.8.1189. [DOI] [PubMed] [Google Scholar]
- Shah SJ, Sylvester PW. γ-Tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor kB activity. Exp Biol Med. 2005;230:235–241. doi: 10.1177/153537020523000402. [DOI] [PubMed] [Google Scholar]
- Singh RP, Agarwal R. Inducible nitric oxide synthase-vascular endothelial growth factor axis: a potential target to inhibit tumor angiogenesis by dietary agents. Curr Cancer Drug Targets. 2007;7:475–483. doi: 10.2174/156800907781386632. [DOI] [PubMed] [Google Scholar]
- Song BL, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem. 2006;281:25054–25061. doi: 10.1074/jbc.M605575200. [DOI] [PubMed] [Google Scholar]
- Sundram K, Thiagarajan T, Gapor A, Basiron Y. Palm tocotrienols: new antioxidants for the new millennium. Inform. 2002;133:634–640. [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Koyabu N, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. Functional induction and de-induction of P-glycoprotein by St. John’s wort and its ingredients in a human colon adenocarcinoma cell line. Drug Metab Dispos. 2005;33:547–554. doi: 10.1124/dmd.104.002485. [DOI] [PubMed] [Google Scholar]
- Tom BH, Rutzky LP, Jakstys MM, Oyasu R, Kaye CI, Kahan BD. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976;12:180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML. Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids. 1995;30:1179–1183. doi: 10.1007/BF02536621. [DOI] [PubMed] [Google Scholar]
- Zhang W, Han Y, Lim SL, Lim LY. Dietary regulation of P-gp function and expression. Expert Opin Drug Metab Toxicol. 2009;5:789–801. doi: 10.1517/17425250902997967. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Sadatrafiei A, Grun F, Blumberg B. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metab Dispos. 2004;32:1075–1082. doi: 10.1124/dmd.104.000299. [DOI] [PubMed] [Google Scholar]