Abstract
The neuronal ceroid lipofuscinoses (NCLs) are autosomal recessive lysosomal storage diseases characterized by progressive neurodegeneration and by accumulation of autofluorescent storage material in the central nervous system and other tissues. One of the most prominent clinical signs of NCL is progressive decline in cognitive function. We previously described a frame shift mutation of TPP1 in miniature long-haired Dachshunds which causes an early-onset form of NCL analogous to classical late-infantile onset NCL (CLN2) in children. Dogs homozygous for the TPP1 mutation exhibit progressive neurological signs similar to those exhibited by human patients. In order to establish biomarkers for evaluating the efficacy of ongoing therapeutic studies in this canine model, we characterized phenotypic changes in 13 dogs through 9 months of age. Cognitive function was assessed using a T-maze reversal learning task. Cognitive dysfunction was detected in affected dogs as early as 6 months of age and worsened as the disease progressed. Physical and neurological examination, funduscopy, and electroretinography (ERG) were performed at regular intervals. Only changes in ERG responses revealed signs of disease progression earlier than the reversal learning task. In the later stages of the disease clinical signs of visual and motor deficits became evident. The visual and motor deficits were not severe enough to affect the performance of dogs in the T-maze. Declining performance on the reversal learning task is a sensitive measure of higher order cognitive dysfunction which can serve as a useful biomarker of disease progression.
Keywords: ceroid lipofuscinosis, CLN2, cognitive decline, reversal learning, canine model, neurodegeneration
Introduction
The neuronal ceroid lipofuscinoses (NCLs) are autosomal recessively inherited lysosomal storage disorders characterized by progressive neurodegeneration accompanied by accumulation of autofluorescent lysosomal storage granules in the central nervous system and other tissues (Wisniewski et al., 2001). To date mutations in 8 different genes (PPTI, CLN2, CLN3, CLN5, CLN6, CLN8, CTSD, and MFSD8) have been found to underlie different forms of human NCL (Vesa et al., 1995; Sleat et al., 2009; International Batten Disease Consortium, 1995; Savukoski et al., 1998; Wheeler et al., 2002; Ranta et al., 1999; Siintola et al., 2006; Siintola et al., 2007). All forms of NCL result in progressive degeneration of the central nervous system. Characteristic neurological signs of NCL include vision loss, progressive motor and cognitive decline, and seizures. The disease usually culminates in a persistent vegetative state and, in all of the childhood-onset forms, is uniformly fatal (Wisniewski et al., 2001). Currently there are no known treatments to alter the progression of the NCLs, but a number of therapeutic approaches are under investigation including stem cell implantation and gene therapy.
We previously described a frame shift mutation of TPP1 in miniature long-haired Dachshunds which causes an early-onset form of NCL. The disease in the Dachshunds is analogous to classical late-infantile onset NCL (CLN2) in humans (Awano et al., 2006). Affected dogs exhibit progressive neurological signs similar to most of those characteristic of human CLN2 (Katz et al., 2008; Hainsworth et al., 2009). We have established a dog colony at the University of Missouri for use as a model to assess the efficacy of therapeutic interventions.
Measuring the efficacy of clinical intervention requires well-defined biomarkers at all stages of the disease. In order to establish biomarkers for evaluating experimental therapies in this canine model, we characterized phenotypic changes in affected dogs up to 9 months of age. The dogs received weekly physical and neurological examinations and monthly ophthalmological evaluations to document the clinical progression of the disease. In addition, electroretinography (ERG) was performed to document impairment of retinal function. One of the most devastating hallmarks of human NCL disease is progressive decline in cognitive function (Steinfeld et al., 2002). Therefore, dogs that were homozygous for the TPP1 mutation were objectively assessed for impaiment of learning and cognition. A number of tasks have been used previously to evaluate cognitive decline as a result of aging in dogs (Milgram et al., 2002; Cotman et al., 2002; Tapp et al., 2003). We chose a serial reversal learning task to evaluate learning ability and memory at monthly time points. Higher-order cognitive processes underlie serial reversal learning during maturity, involving high memory interference and detection of transitions between reversal phases (Watson et al., 2006). This test enabled us to assess the dogs for the types of cognitive decline characteristic of the human NCLs, including CLN2.
Materials and Methods
Animals
Thirteen long-haired miniature Dachshunds were bred and housed at the University of Missouri Office of Animal Resources (OAR) facilities. All of the dogs were closely related. The colony was established from a mating between two dogs that were heterozygous for the TPP1 mutation. The dogs used in this study were produced by matings between descendents from this original pair or by mating the founder male and his descendents. In addition to the standard care provided by the OAR, the dogs were socialized at least 5 days per week by laboratory staff and research assistants. This consisted of walking the dogs outside of their cages, talking to them, petting them, bathing them, and playing with them with and without toys. Genotyping indicated 4 puppies were homozygous for the mutant allele (TPP1 −/−), 3 were heterozygous (TPP1 +/−) and 6 puppies were normal homozygous (TPP1 +/+). Control dogs included the normal homozygous and heterozygous genotypes. Previous assessments of Dachshunds that were heterozygous for the TPP1 mutation did not reveal any neurological abnormalities in these dogs up to at least 5 years of age. As with human CLN2, only dogs homozygous for the mutant allele manifest signs of NCL. Of the four affected dogs, two were male and two were female. Puppies began acclimatization and training in the T-maze at 2 months of age. Socialization and training in the testing room was performed five days/week until the puppies reached 4 months of age and began the data collection phase of the T-maze study. In the testing room, socialization consisted of playful interactions with the staff who subsequently conducted the testing. These interactions included talking to and petting the dogs, providing them with food treats, and playing with them using toys.
Clinical Evaluations
Neurological and physical examinations were performed weekly on all affected dogs beginning at 5 weeks of age. Neurologic dysfunction was assessed subjectively by standard clinical neurologic examination (Lorenz et al., 2011). Components of the neurologic examination include observation (mentation, posture, gait, and movement abnormalities); cranial nerve evaluation; postural reaction testing (proprioception, hopping, wheelbarrow, tactile placement and extensor postural thrust); segmental reflexes (myotatic and withdrawal); and sensory evaluation (nociception and hyperesthesia). Gait evaluation was assessed as normal, ataxic and paretic (ambulatory, nonambulatory). Postural reactions, segmental reflexes and sensory evaluation were assessed as normal, decreased or absent.
Electroretinography
Electroretinography was performed with a portable ERG unit (HMsERG model 1000; RetVet Corp., Columbia, MO) on four affected and four age-matched normal control dogs at 4, 6 and 7–8 months of age as previously described (Katz et al., 2008). Briefly, dogs were sedated by intramuscular injection of dexmedetomidine hydrochloride (0.03–0,04 mg/kg, Dexdomitor; Pfizer Animal Health, Exton, PA) and ketamine hydrochloride ( 5 mg/kg, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA). Heart and respiratory rates were closely monitored throughout the procedure, and dogs were temperature controlled with a heating pad. Maximum pupillary dilatation was achieved with topical application of 1% tropicamide (Alcaine; Alcon) and the cornea was topically anesthetized prior to insertion of an eyelid speculum. Scotopic, high-intensity responses were elicited using 10 cd-s/m2 white flashes of light, and averaged responses to four flashes administered at 20-second intervals were recorded. These recordings depicted combined retinal responses from both rod and cone photoreceptors. ERG recordings were evaluated, and the amplitudes and implicit times for the a-waves and b-waves were measured as previously described (Marmor et al., 2009). After termination of the ERG session, an injection of atipamezole (Antisedan; Pfizer Inc. St Louis, MO) was administered intramuscularly at a volume equal to that of dexmedetomidine to reverse sedation. The ERGs were performed during the periods when the dogs were being evaluated in the T-maze but on one of the days of the week when the dogs were not running the T-maze (for each time point, the dogs were run in the T-maze for a maximum of 5 consecutive days before being given a 2-day break; the ERGs were performed during these breaks). For each time point, the maximum difference in the ages of the dogs at which the ERGs were recorded was 2 weeks.
Cognitive Testing Apparatus
The testing apparatus was a wooden T-maze (4 foot wide × 8 foot long × 3 foot high) acquired from CanCog Technologies (Toronto, Ontario Canada). The maze consisted of a start box, runway and two reward arms. Sliding doors on all sides of the start box controlled access to the runway and ingress to the start box from the ends of the reward arms. Additionally, two spring-loaded doors held open by electromagnets and controlled by a pair of switches adjacent to the operator’s position allowed the reward arms to be sealed behind dogs after a choice was made (Figure 1). Clear acrylic panels covered the top of the maze except for the start box which was covered by an opaque panel. During testing, the overhead lights in the testing room were turned off and the maze was interiorly illuminated by six, evenly-spaced fluorescent fixtures. The end of each reward arm contained a small, metal food reward dish. The experimenter was positioned adjacent to the start box during testing such that the opaque start box cover and darkened room prevented dogs from seeing the human operator during testing.
Fig. 1.
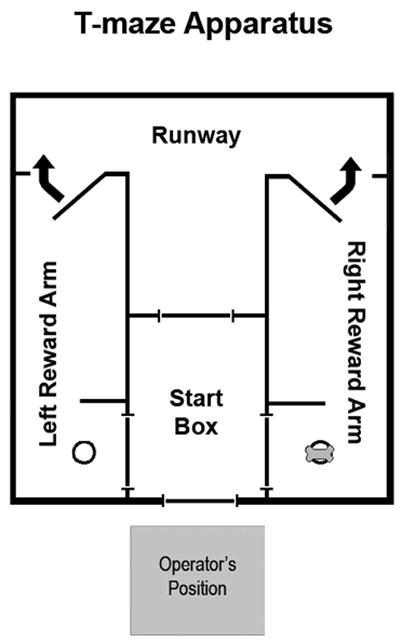
Diagram of the T-maze apparatus used in this study. Dogs exit the start box through the door leading to the runway. Dogs enter either the left or right Reward Arm, where a spring-loaded door is closed behind them by the operator to prevent revision of their choice. Dogs are then returned to the start box through one of the side doors. Food rewards are placed in dishes at the end of a reward arm.
Cognitive Testing Protocol
All dogs underwent a standard training protocol beginning at 2 months of age. T-maze training consisted of acclimatization to the testing room, experimenters and the testing apparatus, reward approach learning and evaluation for motivational response to the food reward. Reward approach learning consisted of training the dogs to explore the maze for food rewards. Initially, rewards were placed throughout the maze and the dogs were allowed full access to all areas. As training progressed, the movements of the dogs became more restricted and the number of food rewards was reduced until the dogs were replicating the reward approach behaviors that were subsequently used during the data collection phase of the experiment.
Food rewards consisted of approximately 1g of Hill’s Science Diet adult formula canned dog food (Hill’s Pet Nutrition, Topeka, KS). Dogs received only dry kibble in their home cages, making the reward used in the testing procedure novel. The canned food served as an effective motivator for all dogs in the study in the absence of food deprivation.
Each reversal learning session consisted of 10 daily trials. During each trial, the dog made a choice between the left and right reward arms. Correct choices resulted in a food reward. The choice made, whether correct or incorrect, was recorded immediately after each trial. The times required for the dogs to reach the reward box after being released from the start box were also recorded for each trial. After a dog entered one of the reward arms, dogs were allowed to re-enter the start box through the appropriate side door. Between each trial, a 30 second reset interval allowed the T-maze apparatus to be prepared for the next trial. An additional 5 second rest interval in which the experimenters were completely quiet occurred immediately before the start of each trial.
Each data collection time point consisted of a Preference Determination (PD) phase, a Preference Reinforcement (PR) phase and three Reversal Learning (RL) phases. During the PD phase, the experimenters determined individual preferred sides for each dog. Dogs received food rewards on both sides of the maze during this phase and the side chosen most often out of 9 trials was designated as the dog’s preferred side for that time point. In the PR phase, dogs were rewarded only on their preferred side. This phase was repeated until the dogs reached criterion. Criterion for this phase and the subsequent reversal phases was 8/10 correct choices on two consecutive days or 9–10/10 correct choices on a single day. After passing the PR phase, the dogs progressed immediately to the RL portion of the test, which consisted of 3 reversals. For the first reversal, food rewards were switched to the dog’s non-preferred side, and the dogs underwent daily testing of 10 trials per day until they reached criterion. In the second and third RL phases, food rewards were placed on the dog’s preferred and non-preferred sides respectively. As for the first reversal, the dogs underwent daily testing of 10 trials per day until they reached criterion for each of the second and third RL phases. The number of incorrect choices made prior to reaching criterion was recorded for each dog for each of the RL phases. These totals were averaged for each time point. For example, if at 4 months of age a dog made 12 errors prior to reaching criterion in the first reversal, 10 errors in the second reversal, and 8 errors in the third reversal, the average number of incorrect choices for the 4 month time point would be (12+10+8)/3=10. Time points were taken monthly starting at 4, 5, 6 and 7 months of age.
Dogs were tested 5 days per week. If dogs finished the third reversal before beginning the next monthly time point, there was no testing during the intervening time. This usually resulted in a break from testing of approximately 10 days between each time point. In order to avoid drug-induced effects on cognitive function, dogs undergoing other procedures which required sedation or anesthesia were not tested in the T-maze for at least 12 or 24 hours after recovery, respectively.
The research assistants administering the cognitive test were not blinded to the disease status of the dogs. However, the T-maze apparatus was designed to minimize experimenter/subject interactions while the dogs were inside the T-maze. In addition, the actions required to manipulate the T-maze were methodically detailed in the protocol and research assistants were trained to follow the steps exactly in order to minimize variation in the testing procedure between dogs.
Statistical Analysis
Performance in the cognitive task was recorded as an average of incorrect choices required to reach criterion at each time point for the normal and affected groups. We also separated the incorrect choices into incorrect choices made before the first correct choice in a reversal phase (IBFC) and incorrect choices made after the first correct choice in a reversal phase (IAFC) (Figure 2). Performance was compared at each monthly time point between the two groups and significance was determined using a two-tailed, homoscedastic Student’s t-test.
Fig. 2.
Example of reversal learning data. Tables show total incorrect (all shaded cells), incorrect before first correct (IBFC, grey-shaded cells) and incorrect after first correct (IAFC, black-shaded cells). The dog reaches criterion (10/10 correct) in the 3rd session of this reversal.
ERG b-wave amplitudes in response to scoptopic high-intensity stimuli were measured for four affected and four age-matched normal control dogs. Readings at each time point were averaged within groups and significance was determined using a two-tailed, homoscedastic Student’s t-test.
Results
Clinical Results
Neurological and routine physical examinations were performed weekly beginning at 5 weeks of age for all dogs participating in this study. Clinical signs were reflective of the multifocal nature of this disease. Affected dogs exhibited asymmetric and progressive vision loss under dim light starting between 6 and 7 months of age but were able to maneuver around obstacles without difficulty in ambient light similar to that inside the T-maze. The earliest abnormality detected by neurological examination was an asymmetric reduction in menace response at 8 months of age. Affected dogs exhibited intention and resting tremors and intermittent myoclonic jerks starting at 8 to 9 months of age. The tremors first affected only the head but progressed to include the trunk and limbs starting at approximately 9 months of age. After 9 to 10 months of age, the menace response was absent bilaterally and visual deficits were apparent when challenged with an obstacle course in dim light. Cerebellar ataxia (defined by loss of coordination and dysmetria) was present starting at 9 to 10 months of age. The severity of cerebellar ataxia progressed with age and was worse in the pelvic limbs. Cerebellar ataxia and motor dysfunction were characterized by frequent falling and by gross intention tremors that made prehension difficult. At the onset of cerebellar ataxia, dogs also exhibited asymmetric postural reaction and proprioceptive deficits in the pelvic limbs. As the disease progressed the motor deficits became symmetric and abnormalities affected all limbs. Ocular motor abnormalities consisting of decreased physiologic nystagmus and positional downbeat nystagmus became evident between 9 and 10 months of age. Dull mentation, as reflected in reduced or abnormal responses to a variety of stimuli, was observed starting at approximately 10 months of age. Affected dogs older than 8 to 9 months would startle easily at loud claps. In summary, abnormal neurologic findings included altered cognitive function, cerebellar ataxia and other motor dysfunction, visual impairment and myoclonic jerks.
Electroretinography
Electroretinograms were recorded in affected (n=4) and age-matched normal control dogs (n=4) at 4, 6 and 7–8 months of age. Previously published work showed that affected dogs exhibit progressive reductions in scotopic b-wave amplitudes as the disease progresses (Katz et al., 2008). Examination of scotopic, maximum-intensity b-wave amplitudes for dogs in this study confirmed these findings in a larger cohort. The b-wave amplitudes for the control group did not vary significantly with age. We observed significantly reduced b-wave amplitudes (p = 0.015) in the affected group at 4 months of age compared to the control group. The b-wave amplitudes were further reduced between 6 and 8 months in the affected group (Figure 3).
Fig. 3.
Representative electroretinogram tracings. a: a-wave. b: b-wave. Data were collected at 4 months (A), 6 months (B) and 8 months of age (C) for a normal (grey) and affected (black) dog. Maximum, scotopic b-wave amplitudes (μV) were determined for the normal (grey) and affected (black) groups and compared at 4, 6 and 7–8 months of age (D). At each time point, affected dogs displayed a significantly lower (* = p < 0.05) average maximum b-wave response than the normal control group. Error bars represent standard error of the mean.
Cognitive testing
Normal (n=9) and NCL-affected (n=4) dogs were tested in a serial reversal learning task at 4, 5, 6 and 7 months of age. Cognitive ability was quantified by measuring the number of incorrect choices made prior to reaching criterion at each reversal (Figure 4a). There was no detectable difference in performance between the two groups at 4 months of age. Both normal and affected dogs improved in performance over time as signified by declines in the total numbers of incorrect choices with each successive time point. However, improvement in the affected group was slower than that seen in the normal group. The difference between the two groups reached significance at 6 months, with normal dogs making a mean of 3.22 incorrect choices prior to reaching criterion at each reversal while affected dogs made an average of 5.83 incorrect choices (p = 0.0045). This gap in performance increased at 7 months, with normal dogs making an average of 2.26 incorrect choices and affected dogs an average of 5.17 incorrect choices (p = 0.0004).
Fig. 4.
Cognitive performance results obtained using the T-maze testing apparatus. (A) Average incorrect choices required to reach criterion for normal and affected dogs at 4, 5, 6 and 7 months of age. (B) Average incorrect choices made before the first correct choice (IBFC) in each reversal for each time point. (C) Average incorrect choices made after the first correct choice (IAFC) in each reversal for each time point. In the total incorrect and IAFC measures, significant learning deficits were observed in the affected group at 6 and 7 months (asterisks). Error bars represent standard error of the mean.
We also analyzed the performance of the dogs by separating incorrect choices into those made before (IBFC) and after (IAFC) the first correct choice in a reversal. Our results show that the cognitive deficit in the affected dogs was driven primarily by IAFC (Figure 4c). Affected dogs, unlike the normal controls, showed no improvement over time. As with the total incorrect choice measure, the differences between the normal and affected groups reached significance at 6 (p = 0.0037) and 7 (p = 0.0001) months of age. Examining the results for IBFC, we observed a strong improvement over time for both groups and no significant difference between the normal and affected groups at any time point (Figure 4b).
There were no differences in any of the performance parameters between the homozygous normal dogs and the dogs that were heterozygous for the TPP1 mutation. In addition, the heterozygous dogs exhibit none of the neurologic or ophthalmological signs of NCL. Therefore, the performance data of the heterozygous and homozygous normal dogs were pooled.
The TPP1 mutation did not have any significant effect on the time required for the dogs to navigate the maze at any of the ages at which testing was performed. The Dachshunds almost always required less than 10 seconds to reach the reward box after being released from the start box. These times did not vary significantly with age or genotype.
Discussion
Progressive impairment of cognitive function is one of the hallmarks of the human NCLs (Steinfeld et al., 2002). The Dachshund model of late-infantile NCL recapitulates the human disease with regard to vision loss, retinal degeneration, autofluorescent storage body accumulation, and generalized brain atrophy (Awano et al., 2006; Katz et al., 2008). We have now also documented a progressive cognitive decline in the CLN2 Dachshund, establishing use of a learning task to assess disease progression. This is of particular value as cognitive decline is one of the most debilitating features of human NCL.
Neurologic examination in affected dogs revealed impaired responses to various stimuli and cerebellar ataxia beginning between 6 and 10 months of age. The earliest observable abnormality was a unilaterally decreased menace response. The menace response is mediated by the visual and cerebellar circuitry. Deficits of the menace response can occur with dysfunction of the visual and cerebellar pathways (Robleto & Thompson., 2008; Maggs et al., 2008; de Lahunta & Glass, 2009). Cerebellar signs are often the earliest neurological abnormality in storage diseases. Since clinical signs of cerebellar ataxia and motor dysfunction were not severe until between 9 and 10 months of age, the ability of dogs to navigate the T-maze or their performance on the reversal learning task was not impaired. Even at the latest time point examined, the disease did not affect the time required for the dogs to reach the ends of the reward arms of the maze after they were released from the start box.
Progressive vision loss is a hallmark of the NCLs. Children with late infantile NCL have apparently normal vision at birth, but experience progressive vision loss in early childhood that culminates in blindness (Hainsworth et al., 2009). ERG analyses of affected children younger than 5 years demonstrated profoundly subnormal b-wave amplitudes, whereas a-wave amplitudes were less severely affected (Weleber, 2009). This is similar to what we observed in the Dachshund model (Figure 3). In the NCL affected Dachshunds, the menace response is lost after approximately 7 months of age. In addition the pupillary light reflexes become incomplete at this age and progress to become sluggish a few weeks later. Electroretinography revealed progressive loss of retinal function in affected dogs, with significant reduction in maximum scotopic b-wave amplitudes by 4 months of age. In this group of dogs, abnormalities were restricted to the b-wave portion of the retinal response at all time points, indicating that the inner retina is affected first and most profoundly in this disease. Despite these early abnormalities in ERG responses, dogs remained visual throughout the reversal learning study (~8 months of age) under the light intensities used in the T-maze. It is unlikely that changes in T-maze performance can be attributed to vision loss, as dogs were observed to respond normally to other visual stimuli under typical room light conditions and were able to navigate the maze quickly without hesitation or bumping into the maze walls or doors, even at the latest time point. We have recently developed a video-based system for quantitatively measuring the pupillary light reflex (PLR) in dogs. Recordings of the PLRs from Dachshunds that were homozygous for the TPP1 mutation, as well as PLRs observed demonstrated a robust response to light stimuli for at least two months after the ERG became too small to record (unpublished data). This indicates that the PLR correlates better with visually-mediated behavior than does the ERG.
The reversal learning task was able to detect cognitive decline early in the course of NCL in affected dogs. Testing revealed a significant increase in the number of incorrect choices required to reach criterion for the affected dogs at 6 months of age. The difference in performance between affected and normal dogs increased at 7 months of age. Among the biomarkers analyzed in this study, impaired reversal learning was one of the earliest disease signs that could be detected.
Analysis of the performance data indicated that the dogs were engaging in two distinct types of learning. The first type, first-order learning, involved the task of recalling the basic information of food reward location from trial to trial within a given session (i.e. short-term memory). The second type, second-order learning, involved recalling experience in the maze from previous sessions and developing strategies for making choices based on those memories (i.e. integration of long-term memories). First order (short–term) learning deficits would affect performance between trials within a session whereas second order (long-term) learning deficits would affect performance from one session to the next. Second-order learning appeared to occur in both groups of dogs as reflected by a progressive reduction in the total number of incorrect choices to reach criterion at each subsequent time point (session). The improvement in performance, illustrated in Figure 4a, shows that the total numbers of incorrect choices for both the normal and affected groups decrease with time.
Given that two types of learning affect performance in serial reversal assessments in the same dogs, we analyzed our data to determine whether both the type of learning that requires long-term memory and that which relies primarily on short-term processing of information were equally impaired in the affected group. We divided the results into incorrect choices made either before (IBFC) or after (IAFC) the first correct choice. These two types of incorrect choices are qualitatively different. The IBFC choices are made before the dog has information about where the reward is located in a given reversal. Second-order learning allows dog to develop strategies that help them make predictions about which side they should choose in subsequent trials based on outcomes of choices from previous trials. Use of second-order learning in this context requires the ability to recall learning acquired over a period of weeks or months. Reductions in IBFC, therefore, are a good indicator that a dog is engaging in effective second-order learning that requires recall of long-term memories. On the other hand, IAFC choices are made shortly after the dog has acquired information about the location of the reward within a reversal session. Incorrect choices made after acquisition of this information reflect deficits in first-order learning (i.e. retaining information between trials within the same session). As illustrated by Figure 4b and c, the deficit in performance of the affected relative to the normal dogs can be completely accounted for by IAFC. Affected dogs performed at the same level as normal dogs for the IBFC measure throughout the study. However, in the IAFC measure, affected dogs showed no improvement over time resulting in significant performance deficits compared to the normal dogs at 6 and 7 months of age. Because the difference in total incorrect choices between the normal and affected groups can be accounted for entirely by the IAFC measure, it appears that the learning deficit displayed by the affected dogs is driven by a reduced ability to engage in first-order learning.
Specific brain regions are known to play an important role in attention, short-term memory storage and long-term memory consolidation (Buschman & Miller, 2007; Treisman & Gelade, 2004; Deadwyler & Hampson, 2004). Based on our cognitive data, we will be focusing our attention on specific brain regions in an effort to detect disease-related brain morphology changes earlier than was previously possible. It will be important in ongoing therapeutic studies to know which brain regions are responsible for specific functional deficits, as it may be possible to target therapies, such as gene therapy, specifically to these regions.
For early-onset NCLs, therapeutic intervention at the earliest stages of disease progression is essential for preservation of cognitive function. As a non-invasive biomarker of early disease progression, we conclude that the reversal learning task will be a valuable tool in evaluating experimental treatments in the Dachshund model. Profound cognitive dysfunction develops relatively slowly in humans and the canine model, so there is a fairly large time window available during which therapeutic intervention could prevent these degenerative changes. Once a therapeutic intervention has been identified as effective in preserving cognitive function in the dog model, established cognitive tests can be administered to human patients undergoing similar investigational therapies.
Acknowledgments
This research was supported by grants R21 NS064554 and 1R01EY018815-01 from the US National Institutes of Health and by grants from the Batten Disease Support and Research Association and Research to Prevent Blindness, Inc. The authors thank Dr. Kristina Narfström for providing the instrumentation and staff training for the ERG assessments. The authors also acknowledge Dr. Norton W. Milgram of the University of Toronto for his assistance with the design of the behavioral studies and for providing the T-maze apparatus.
References
- Awano T, Katz ML, Sohar I, Lobel P, Sanders DN, Khan S, Johnson GC, Giger U, Johnson GS. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile dachshund with neuronal ceroid lipofuscinosis. Molecular Genet Metab. 2006;89:254–260. doi: 10.1016/j.ymgme.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23:809–818. doi: 10.1016/s0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Crystal RG, Sondhi D, Hackett NR. Clinical protocol: Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Human Gene Therapy. 2004;15:1131–1154. doi: 10.1089/hum.2004.15.1131. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42:465–476. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- de Lahunta A, Glass E. Veterinary Neuroanatomy and Clinical Neurology. 3. St. Louis: Elsevier; 2009. Visual System Special Somatic Afferent System. [Google Scholar]
- Hasegawa D, Yayoshi N, Fujita Y, Fujita M, Orima H. Measurement of interthalamic adhesion thickness as a criteria for brain atrophy in dogs with and without cognitive dysfunction (dementia) Vet Radiol Ultrasound. 2005;46:452–457. doi: 10.1111/j.1740-8261.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- Hainsworth DP, Liu GT, Hamm CW, Katz ML. Funduscopic and angiographic appearance in the neuronal ceroid lipofuscinoses. Retina. 2009;29:657–668. doi: 10.1097/IAE.0b013e31819b0542. [DOI] [PubMed] [Google Scholar]
- International Batten Disease Consortium. Isolation of a novel gene underlying Batten disease, CLN3. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Katz ML, Coates JR, Cooper JJ, O’Brien DP, Jeong M, Narfstrom K. Retinal pathology in a canine model of late infantile neuronal ceroid lipofuscinosis. Invest Ophthalmol Vis Sci. 2008;49:2686–2895. doi: 10.1167/iovs.08-1712. [DOI] [PubMed] [Google Scholar]
- Kii S, Uzuka Y, Taura Y, Nakaichi M, Inokuma H, Onishi T. Developmental change of lateral ventricular volume and ratio in Beagle type dogs up to 7 months of age. Vet Radiol Ultrasound. 1998;39:185–189. doi: 10.1111/j.1740-8261.1998.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Kimotsuki T, Nagaoka T, Yasuda M, Tamahara S, Matsuki N, Ono K. Changes of magnetic resonance imaging on the brain in beagle dogs with aging. J Vet Med Sci. 2005;67:961–967. doi: 10.1292/jvms.67.961. [DOI] [PubMed] [Google Scholar]
- Lorenz MD, Coates JR, Kent M. Neurologic history, neuroanatomy and neurologic examination. 5. St. Louis: Elsevier; 2011. [Google Scholar]
- Maggs DJ, Miller PE, Ofri R, Slatter DH. Slatter’s Fundamentals of Veterinary Ophthalmology. St. Louis, MO: Saunders - Elsevier; 2008. [Google Scholar]
- Marmor M, Fulton A, Holder G, Miyake Y, Brigell M, Bach M. Standard for clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas CJ, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol Aging. 2002;23:737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Ranta S, Zhang Y, Ross B, Lonka L, Takkunen E, Messer A, Sharp J, Wheeler R, Kusumi K, Mole S, Liu W, Soares MB, Bonaldo MF, Hirvasniemi A, de la Chapelle A, Gilliam TC, Lehesjoki AE. The neuronal ceroid lipofuscinoses in human EPMR and mnd mutant mice are associated with mutations in CLN8. Nat Genetics. 1999;23:233–236. doi: 10.1038/13868. [DOI] [PubMed] [Google Scholar]
- Robleto K, Thompson RF. Extinction of a classically conditioned response: Red nucleus and interpositus. J Neurosci. 2008;28:2651–2658. doi: 10.1523/JNEUROSCI.4781-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savukoski M, Klockars T, Holmberg V, Santavuori P, Lander ES, Peltonen L. CLN5, a novel gene encoding a putative transmembrane protein mutated in Finnish variant late infantile neuronal ceroid lipofuscinosis. Nat Genetics. 1998;19:286–288. doi: 10.1038/975. [DOI] [PubMed] [Google Scholar]
- Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, Lehesjoki AE, Tyynela J. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129:1438–1445. doi: 10.1093/brain/awl107. [DOI] [PubMed] [Google Scholar]
- Siintola E, Topcu M, Aula N, Lohi H, Minassian BA, Paterson AD, Liu XQ, Wilson C, Lahtinen U, Anttonen AK, Lehesjoki AE. The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am J Hum Genetics. 2007;81:136–146. doi: 10.1086/518902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat DE, Ding L, Wang S, Zhao C, Wang Y, Xin W, Zheng H, Moore DF, Sims KB, Lobel P. Mass spectrometry-based protein profiling to determine the cause of lysosomal storage diseases of unknown etiology. Molec Cell Proteomics. 2009;8:1708–1718. doi: 10.1074/mcp.M900122-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld R, Heim P, von Gregory H, Meyer K, Ullrich K, Goebel HH, Kohlschutter A. Late infantile neuronal ceroid lipofuscinosis: Quantitative description of the clinical course in patients with CLN2 mutations. Am J Med Genet. 2002;112:347–354. doi: 10.1002/ajmg.10660. [DOI] [PubMed] [Google Scholar]
- Steiner R, Koch T, Al-Uzri A. A phase 1 clinical study of human CNS stem cells (HuCNS-SC) in patients with neuronal ceroid lipofuscinosis. Rochester: 11th International Congress on Neuronal Ceroid Lipofuscinosis.2007. [Google Scholar]
- Su MY, Brooks WM. Magnetic resonance imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol Aging. 1998;19:479–485. doi: 10.1016/s0197-4580(98)00081-5. [DOI] [PubMed] [Google Scholar]
- Su MY, Tapp PD, Vu L. A longitudinal study of brain morphometrics using serial magnetic resonance imaging analysis in a canine model of aging. Prog Neuropsychopharm Biol Psychiatry. 2005;29:389–397. doi: 10.1016/j.pnpbp.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW. Size and reversal learning in the Beagle dog as a measure of executive function and inhibitory control in aging. Learning Memory. 2003;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames RA, Robertson ID, Flegel T, Henke D, O’Brien DP, Coates JR, Olby NJ. Development of a morphometric magnetic resonance image parameter suitable for distinguishing between normal dogs and dogs with cerebellar atrophy. Vet Radiol Ultrasound. 2010;51:246–253. doi: 10.1111/j.1740-8261.2009.01655.x. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature–integration theory of attention. Cog Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- Vullo T, Korenman E, Manzo RP, Gomez DG, Deck MDF, Cahill PT. Diagnosis of cerebral ventriculomegaly in normal adult Beagles using quantitative MRI. Vet Radiol Ultrasound. 1997;38:277–281. doi: 10.1111/j.1740-8261.1997.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Sullivan J, Frank J, Stanton M. Serial reversal learning of position discrimination in developing rats. Devel Psychobiol. 2006;48:79–94. doi: 10.1002/dev.20106. [DOI] [PubMed] [Google Scholar]
- Weleber RG. The dystrophic retina in multisystem disorders: The electroretinogram in neuronal ceroid lipofuscinoses. Eye. 1998;12:580–590. doi: 10.1038/eye.1998.148. [DOI] [PubMed] [Google Scholar]
- Wheeler RB, Sharp JD, Schultz RA, Joslin JM, Williams RE, Mole SE. The gene mutated in variant late-infantile neuronal ceroid lipofuscinosis (CLN6) and in nclf mutant mice encodes a novel predicted transmembrane protein. Am J Hum Genetics. 2002;70:537–542. doi: 10.1086/338708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Kida E, Golabek AA, Kaczmarski W, Connell F, Zhong N. Neuronal ceroid lipofuscinoses: Classification and diagnosis. Adv Genetics. 2001;45:1–34. doi: 10.1016/s0065-2660(01)45002-4. [DOI] [PubMed] [Google Scholar]





