Abstract
Interference with cholinergic functions in hippocampus and prefrontal cortex impairs learning and memory for social transmission of food preference, suggesting that acetylcholine (ACh) release in the two brain regions may be important for acquiring the food preference. This experiment examined release of ACh in the hippocampus and prefrontal cortex of rats during training for social transmission of food preference. After demonstrator rats ate a food with novel flavor and odor, a social transmission of food preference group of rats was allowed to interact with the demonstrators for 30 min, while in vivo microdialysis collected samples for later measurement of ACh release with HPLC methods. A social control group observed a demonstrator that had eaten food without novel flavor and odor. An odor control group was allowed to smell but not ingest food with novel odor. Rats in the social transmission but not control groups preferred the novel food on a trial 48 hr later. ACh release in prefrontal cortex, with probes that primarily sampled prelimbic cortex, did not increase during acquisition of the social transmission of food preference, suggesting that training-initiated release of ACh in prelimbic cortex is not necessary for acquisition of the food preference. In contrast, ACh release in the hippocampus increased substantially (200%) upon exposure to a rat that had eaten the novel food. Release in the hippocampus increased significantly less (25%) upon exposure to a rat that had eaten normal food and did not increase significantly in the rats exposed to the novel odor; ACh release in the social transmission group was significantly greater than that of the either of the control groups. Thus, ACh release in the hippocampus but not prelimbic cortex distinguished well the social transmission vs. control conditions, suggesting that cholinergic mechanisms in the hippocampus but not prelimbic cortex are important for acquiring a socially transmitted food preference.
Keywords: acetylcholine, hippocampus, prefrontal cortex, memory formation, learning, social transmission of food preference, memory modulation
INTRODUCTION
The view that acetylcholine (ACh) is an important regulator of memory and neural plasticity is supported by a wide array of findings. For example, systemic administration of cholinergic agonists generally enhance, and antagonists impair, learning and memory in human and non-human subjects (Hasselmo, 2006; Power et al., 2003; Kenney and Gould, 2008; Weinberger, 2007; Gold, 2008; Micheau and Marighetto, 2011). In addition, direct injections of cholinergic drugs into specific brain areas enhance and impair memory in tasks associated with those brain areas (e.g.: Boccia et al., 2009, 2010; Raybuck and Gould, 2010; Pang et al., 2010; Liu and Liang, 2009; Herrera-Morales et al., 2007; Malin and McGaugh, 2006; Power et al., 2003; Rogers and Kesner, 2003; Bunce et al., 2004). Similarly, lesions of cholinergic inputs to specific brain memory systems impair memory for associated tasks, though partial lesions of cholinergic input to the hippocampus results in deficits in only some tasks that are impaired by lesions of the hippocampus per se (Chang and Gold, 2004; Parent and Baxter, 2004).
These lesion and pharmacological studies are supported by neurochemical measurements of ACh release. When measured by in vivo microdialysis, release of ACh is correlated with learning and memory measures, varying across neural systems depending on the particular memory tasks (Gold, 2003, 2004). ACh release in the hippocampus is associated with working memory assessed on a spontaneous alternation task, and glucose enhancement of memory in that task is accompanied by augmentation of training-related ACh release in the hippocampus (Ragozzino et al., 1996, 1998; Stefani and Gold, 2001). ACh release during learning and memory tasks also reflects activation of different brain areas important for those tasks (Chang and Gold, 2003; McIntyre et al., 2003a,b; Ihalainen et al., 2010; Roland and Savage, 2007; Morris et al., 2010), and may contribute to coordination of competition and competition of neural systems during learning (Gold, 2003, 2004; Pych et al., 2005a,b).
The present experiment examines ACh release during learning in a social transmission of food preference (STFP) task. This is a task in which one rat, the observer, interacts with a second rat, the demonstrator, which has recently eaten a food with novel odor. After the interaction and exposure to the demonstrator's breath, the observer rat exhibits a preference for food containing the odor previously consumed by the demonstrator (Galef and Whiskin, 2003). The odor memory is acquired in a single session and can last for weeks after training.
The hippocampus is one brain area implicated in the social transmission of food preference. Acquisition of social transmission of food preference is impaired by lesions of the hippocampus (Winocur, 1990; Bunsey and Eichenbaum, 1995; Alvarez et al., 2001, 2002; Winocur et al., 2001; Clark et al., 2002; Winocur and Moskovitch, 1999). In addition, intra-hippocampal injections of the ACh muscarinic receptor antagonist, scopolamine, immediately after training impairs later memory for social transmission of food preference (Carballo-Marquez et al., 2009). Relatedly, lesions of the medial septum/diagonal band cholinergic input to the hippocampus also impair acquisition of a social transmission of food preference (Berger-Sweeney et al., 2000). Together, these findings suggest that the hippocampus, and more specifically release of ACh in the hippocampus at the time of training, is important for acquiring the food preference.
Prelimbic and orbitofrontal regions of prefrontal cortex have also been implicated in learning and memory for social transmission of food preference. However, results for prefrontal cortex are less consistent across methods than are the results for the hippocampus. As found with injections into the hippocampus, post-training scopolamine injections into prelimbic regions of prefrontal cortex impair memory for social transmission of food preference (Carballo-Marquez et al., 2009; Bois-Trelis et al., 2007). Additional evidence for cholinergic contributions to acquisition of social transmission of food preference is found in studies that damage cholinergic inputs to prefrontal cortex. Lesions of the nucleus basalis cholinergic input to neocortex (Berger-Sweeney et al., 2000; Vale-Martinez et al., 2002) impair memory for social transmission of food preference, although the anatomical specificity to prefrontal cholinergic projections is unclear because such lesions would interfere with cholinergic functions through much of neocortex. Also, electrical stimulation of nucleus basalis immediately after social training enhanced memory for a food preference; in parallel, the stimulation resulted in increased c-Fos expression that was especially prominent in most prefrontal cortical regions, including orbitofrontal, prelimbic and infralimbic cortical areas (Boix-Trelis, et al., 2006). Infusions of 192 immunoglobulin G-saporin directly into orbitofrontal cortex also impaired acquisition of a socially transmitted food preference (Ross et al., 2005). In contrast to these findings, however, lesions of orbitofrontal cortex did not interfere with learning and memory for a socially acquired food preference (Smith et al., 2010); the histological descriptions in this report showed damage that extended, in most rats, well beyond orbitofrontal cortex to include prelimbic regions of medial prefrontal cortex.
Thus, both lesions and pharmacological manipulations of hippocampus and its cholinergic functions affect social transmission of food preference. While lesions of orbitofrontal cortex and adjacent areas of prefrontal cortex do not impair learning in this task, manipulations of cholinergic functions in prelimbic and orbitofrontal cortex consistently up- and down-regulate social transmission of food preference. Whether or not activation of cholinergic mechanisms in these brain regions is important for learning the social transmission of food preference is evaluated here by measuring training-related increases in release of the neurotransmitter, with increased release of ACh revealing activation of cholinergic mechanisms. The present experiment employed in vivo microdialysis /high performance liquid chromatography methods to examine ACh release simultaneously in both the hippocampus and prelimbic regions of frontal cortex during training to determine whether ACh actively participates in STFP memory in these brain regions.
METHODS
Animals
Male Fischer-344 × Brown-Norway F1 hybrid rats (3-4 months old) were housed individually and were maintained on a 12:12 (7:00 a.m. on) light-dark cycle with free access to food and water until near the time of behavioral testing. All rats were handled daily beginning before surgery and continuing to the time of behavioral testing. The rats were placed into trained (N=9), social control (N=9), odor control (N=8), and demonstrator (N=6) groups. All rats underwent behavioral testing and microdialysis. However, two rats in the social control group and two rats in the odor control group had technical problems with the probes in frontal cortex. Therefore, while all rats were used for behavioral testing and for hippocampus neurochemistry, the Ns for the frontal cortex chemistry were 7 and 6 for the social and odor control groups, respectively.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign and were in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals; the animal care facilities at the University of Illinois at Urbana-Champaign are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Surgery
All rats except demonstrators were anesthetized with isoflurane and placed in a stereotaxic apparatus with skulls in horizontal orientation. For later insertion of microdialysis probes, guide cannulae were aimed at the ventral hippocampus (5.5 mm posterior to bregma, 5.0 mm lateral to the midline suture and 4.0 mm below the surface of the skull) and at the contralateral medial prefrontal cortex, targeting prelimbic cortex (3.0 mm anterior to bregma, 1.0 lateral, 2.5 mm ventral).
In vivo microdialysis
The dialysis procedures were as described in detail previously (e.g., Chang and Gold, 2003; Chang et al., 2006; Qi and Gold, 2009). On the day of training, the trained, social and odor controls received microdialysis probes (CMA/11, 3 mm) inserted into the guide cannulae approximately 2 hr prior to training. The probes were perfused continuously with aCSF (1.0 μl/min) containing the cholinesterase inhibitor, neostigmine (100 nM). Dialysate samples (10 μl each) were collected every 10 min. Samples collected during the first hour were discarded. The next four samples comprised the baselines for each rat. Rats were then trained while samples continued to be collected every 10 min during the 30 min of training/interaction or odor presentation, and for 50 min thereafter. Samples were stored at -20°C until they were later assessed for ACh content using HPLC methods.
Behavioral Procedures
Rats were allowed to recover from surgery for 7-14 days before testing. At that time, rats began daily handling for 5 min / day to reduce possible stress responses at the time of training. General training procedures were as described before (Countryman and Gold, 2007). Demonstrator and observer rats were housed together in an interaction cage [39 × 51 × 26 cm (L × W × H)] separated into two equal compartments by a wire screen. During this time, each rat had unlimited access to standard food and water for two days and then was food-deprived for the 22 hrs immediately prior to training.
On the day of training, a demonstrator rat was removed from its interaction cage and was placed in a cage in a separate room where it was allowed to eat flavored rat chow (either 1% thyme or 1% turmeric). We previously determined that these flavors and concentrations were preferred equally by untrained rats (Countryman and Gold, 2007). After a 30-min eating session, the demonstrator rat was returned to the interaction cage, where an observer rat (N = 9) could interact with the demonstrator rat for 30 min. The demonstrator rat was then removed and microdialysis samples were collected from the observer rat for an additional 50 min.
Social control rats (N = 9) were treated as the trained rats above, except that the demonstrator rats were presented with unflavored food. Odor control rats (N = 8) were permitted to smell the flavored food but could not eat it; for this condition, the food was placed in a container covered with Plexiglas perforated with holes.
After the training procedure was complete, rats were given access to food for 2 hr, when the food was again removed. Rats received food again for 2 hr on the next day, ending 22 hr before memory testing. Tests of memory were given 48 hr after training. At that time, the observer rats were placed into a cage (42 × 24 × 27 cm) containing two food cups each containing 15 g of food, one cup containing the demonstrated food and one a novel food. The rats were allowed to eat for 1 hour, after which the amount of each remaining food was weighed. The measure of food preference was calculated by: [Demonstrated Food Consumed (g)/Total Food Consumed (g)] × 100].
ACh chemistry
As described before (e.g.: Chang and Gold, 2003; Qi and Gold, 2009), ACh content in each sample was quantified using high performance liquid chromatography and electrochemical detection (Bioanalytical Systems Inc., West Lafayette, IN). Briefly, the assay system included an ion-exchange microbore analytical column, microbore ACh/Ch immobilized enzyme reactor containing acetylcholinesterase and choline oxidase. A Shimadzu LC-10ADvp pump provided relatively pulse-free flow. The working electrode was set at 100 mV vs. a Ag/AgCl reference electrode. The flow rate was 140 μl / min and the injection volume was 6.0 μl, using a 10 μl loop. Each sample required 13 min and the detection limit with this system and procedures was 65 fmol.
Histology
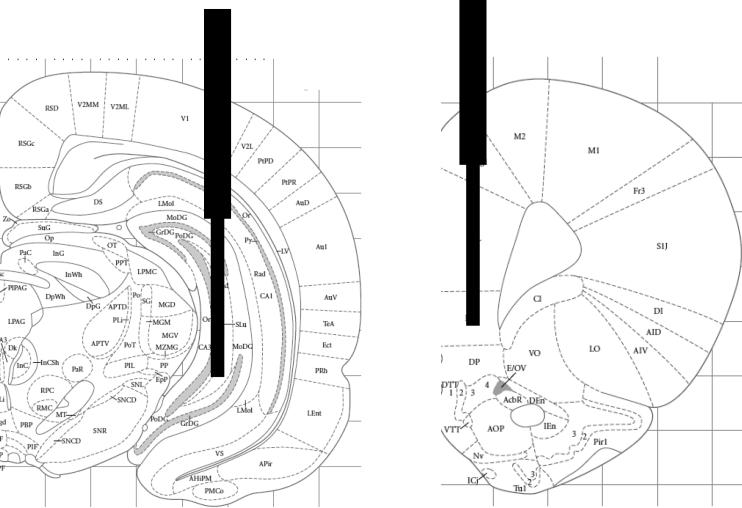
After training, rats received an overdose of sodium pentobarbital. They were perfused with saline followed by 4% paraformaldehyde in a phosphate buffer. Brains were removed and stored in paraformaldehyde for 24 hr, then transferred into a 20% sucrose solution. Coronal sections (40 μm) through the cannulae tracts were collected with cryostat sectioning and were later stained with cresyl violet procedures to confirm microdialysis probe placements. Illustrations of acceptable placements are shown in Figure 1; all placements were confirmed to be within the hippocampus and prefrontal cortex. Because the probes were 3 mm long and ~250 μm in diameter, they sampled a wide area within each brain region, precluding subregional assessments. Each hippocampal probe sampled dentate gyrus, CA3 and CA1 areas. The prefrontal cortex probes sampled ACh release in prelimbic cortex in particular, but probably also sampled cingulate and infralimbic areas as well. It is not clear whether diffusion of ACh from orbitofrontal regions was monitored with these microdialysis probes and placements.
Figure 1.
Illustration of typical placement of microdialysis dialysis probes in hippocampus and medial prefrontal cortex. (Adapted from Paxinos and Watson, 2005: Bregma -5.52 and +3.24 mm).
Statistical analyses
One-way repeated measure ANOVAs were performed to evaluate ACh release in the hippocampal and medial prefrontal cortex. Dunnett's post-hoc test was used to identify time points at which ACh levels differed across conditions. A one-way ANOVA was performed to test differences in average percent of demonstrated food consumed across trained, odor and social conditions. Within groups, one-sample differences from equal preference for the demonstrated vs. novel food ([Demonstrated Food Consumed (g)/Total Food Consumed (g)] × 100]) were used to identify groups that exhibited social transmission of food preference.
RESULTS
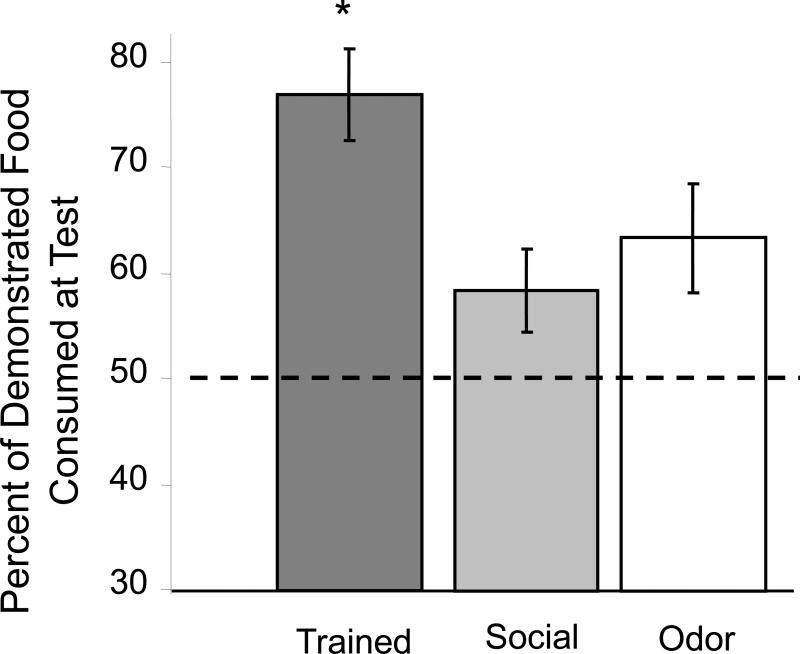
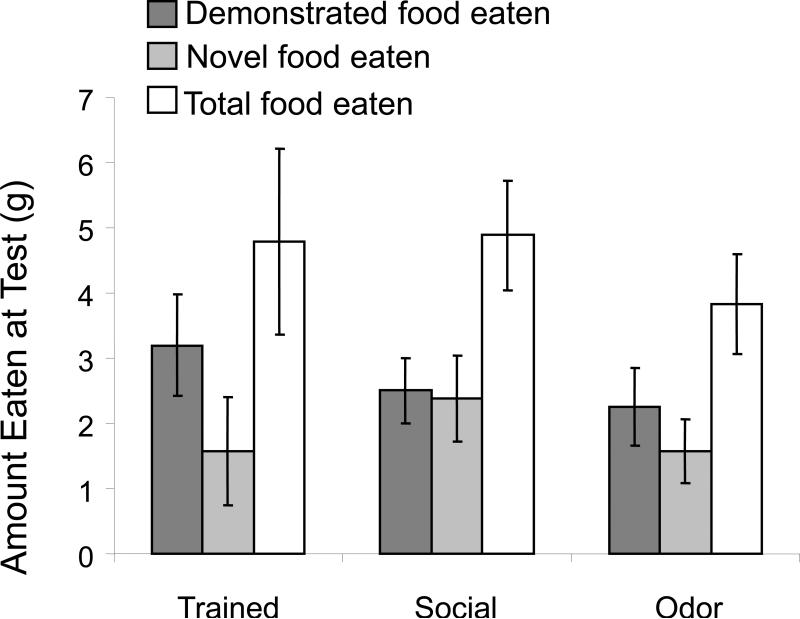
As shown in Figure 2, the trained rats, but not the odor or social control rats, exhibited significant social transmission of food preference responses (one-way ANOVA across treatment conditions: F2,23 = 3.53, p<0.05). When given a choice of the demonstrated food vs. a novel food, trained rats exhibited approximately a 3:1 preference for the demonstrated food (p < 0.05). In contrast, rats in the odor and social control groups did not exhibit significant preferences for either of the food (p's > 0.2). There were no significant differences across groups in the total food eaten (F2,23 = 0.5, p>0.6) (Figure 3). The trained rats consumed 4.8 ± 1.2 grams of total food, as compared to 4.9 ± 0.9 and 4.0 ± 0.7 grams of total food eaten by the social and odor control groups.
Figure 2.
Consumption of demonstrated vs. novel food on memory test. Trained rats consumed significantly more demonstrated food than novel food when tested 48 hr (*p<0.05). Social-control rats and odor-control rats did not exhibit a significant preference for either food (*p<0.05).
Figure 3.
Food consumed on memory test trial. There were no significant differences in total amount of food consumed between groups.
With the microdialysis procedures and neostigmine concentration used here, mean release of ACh at baseline was 20.2 ± 4.9 fmol/10 μl for hippocampus and 42.6 ± 6.6 fmol/10 μl for frontal cortex samples. The hippocampus values are similar to those we have reported before (Chang et al., 2006) and similar to those reported by others who used similar probe sizes, flow rates and neostigmine concentrations to measure ACh release with similar placements in frontal cortex (Kehr et al., 2010; Takase et al., 2009).
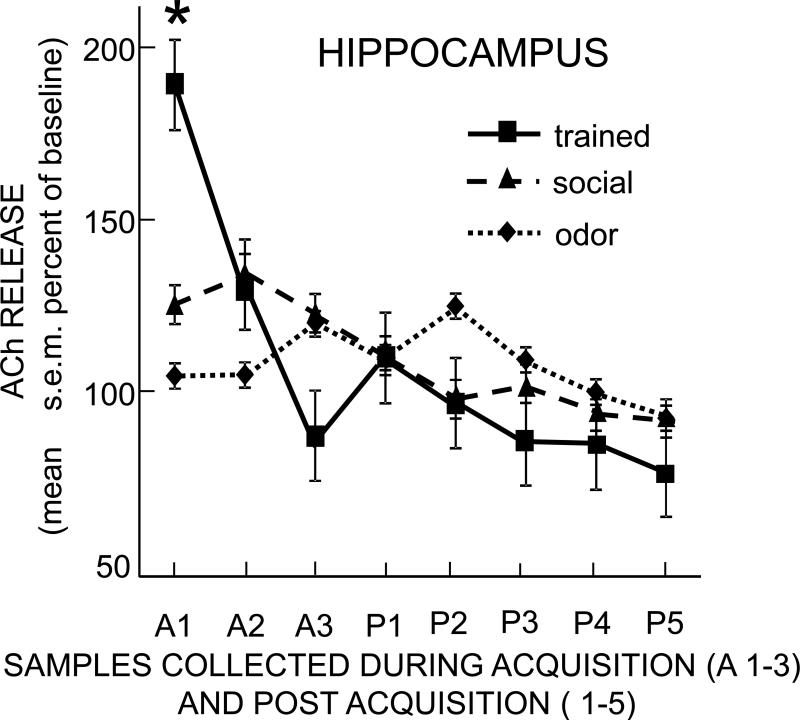
The patterns of ACh release during training in the hippocampus differed from each other by behavioral group (Figure 4), with a significant interaction of time × group (F14,126 = 4.50, p<0.001). In the first acquisition sample, ACh release increased significantly to nearly 200% of baseline in the social transmission of food preference group (p<0.01). There was also a small but significant 25% increase from baseline in ACh release in the social control group (p<0.05), but no significant increase in the odor control group. The increase in release of ACh in the trained group was significantly greater than that of the odor and social controls (ps < 0.05). Thus, exposure to the demonstrator rat that had eaten the novel food resulted in significantly increased ACh release compared to either control group.
Figure 4.
Change in acetylcholine release in the hippocampus during and after training. Each sample was collected during 10 min. Note that the trained rats exhibited a large increase in acetylcholine release at the start of training. Social control rats, but not odor control rats, exhibited a small increase early in training. The increase in release in trained rats was significantly greater than the increases seen in either control group (p's < 0.05 vs. social and vs. odor controls).
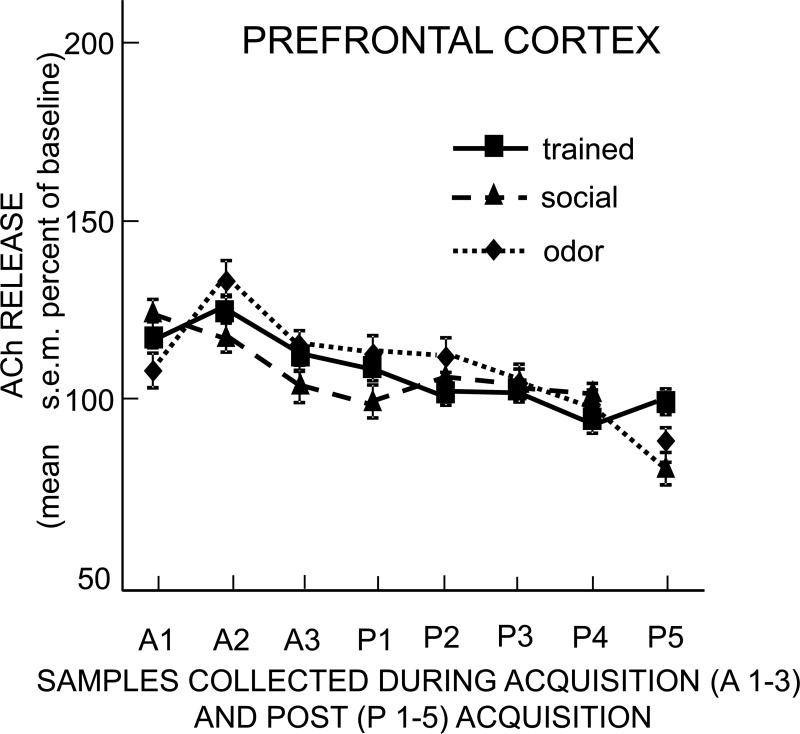
Increases in release of ACh in prelimbic cortex were quite modest, with maximal increases across all groups of about 30% above baseline (Figure 5); these increases in each group were not significant vs. their respective baselines. Moreover, ACh release during the interaction with demonstrators in the trained, odor, and social groups did not differ from each other (time × group: F14,161 = 0.63, p>0.8). Thus, microdialysate samples from frontal cortex exhibited small non-significant increases in ACh release during the trained and control rats’ exposures to a demonstrator rat, but did not reveal differences based on the food eaten by the demonstrator rat. ACh release in the prelimbic region of frontal cortex was therefore not associated with social transmission of food preference.
Figure 5.
Change in acetylcholine release in the prefrontal cortex during and after training. Each sample was collected during 10 min. Note that acetylcholine release did not increase in the trained or in either control group.
DISCUSSION
Rats learning a social transmission of food preference task exhibited increased release of ACh in the hippocampus. The increase in release clearly distinguished rats interacting with a demonstrator rat that had recently eaten novel food from rats interacting with demonstrators that had eaten standard rat food or from rats that had smelled but not eaten a novel food. The encounters between the observer rats with demonstrator rats appeared to be similar in the trained and social control groups, with the unusual mouth odor being the main feature distinguishing the experiences. Related to this are possible differences in arousal and the nature of interactions between the rats, although these were not obvious within this experiment. With results showing that the total amount of food eaten was similar across groups, differences in motivation, per se, do not explain the differences in release of ACh in the hippocampus.
The present findings support results obtained with brain lesions revealing an important role for the hippocampus in acquiring a social transmission of food preference (Winocur, 1990; Bunsey and Eichenbaum, 2003; Alvarez et al., 2001; Winocur et al., 2001; Clark et al., 2002; Winocur and Moskovitch, 1999). More specifically, the findings also support an important role for ACh within the hippocampus as a regulator of this type of learning and memory. The present findings are consistent with others showing that lesions of the medial septum/diagonal band cholinergic input to the hippocampus impair social transmission of food preference (Berger-Sweeney et al., 2000; Vale-Martinez et al., 2002) and that immediate post-training infusions of the muscarinic antagonist, scopolamine, into the hippocampus impair memory for the social transmission of food preference (Carballo-Márquez, et al., 2009).
The findings obtained with prefrontal cortex samples, collected simultaneously with the hippocampus samples, did not reveal increases in release of ACh that were associated with the training conditions. These samples showed relatively small increases in ACh release that were comparable in trained and control rats. Therefore, the modest increases are more likely to reflect general arousal of the rat upon exposure to the demonstrator than to reflect specific mechanisms related to learning a social transmission of food preference. These findings are compatible with evidence that lesions of orbitofrontal cortex, which generally extended into the prelimbic areas monitored in the present experiment, have no effect on acquisition of social transmission for food preference (Smith et al., 2010).
The present results raise questions about the interpretations of studies showing that interference with cholinergic functions in prelimbic cortex impair memory for social transmission of food preference tasks. In several studies, injections of the cholinergic toxin, 192 immunoglobulin G (IgG)-saporin into the nucleus basalis region of the forebrain, prior to training resulted in impaired social transmission of food preference (Berger-Sweeney et al., 2000; Vale-Martinez et al., 2002). These lesions resulted in damage to cholinergic innervation in prefrontal cortex but also in other neocortical areas. In addition, injections of scopolamine directly into prelimbic cortex impaired social transmission of food preference (Boix-Trellis et al., 2007). Targeting lesions to orbitofrontal cortex with 192 IgG-saporin infusions directly into that brain area, Ross et al. (2005) attempted to limit cholinergic fiber loss to orbitofrontal cortex. However, the loss of cholinergic function may have extended beyond orbitofrontal cortex to other areas of prefrontal cortex. The Ross et al. (2005) report did not fully describe the extent of the anatomical spread of damage beyond the injection site; the control measure in cingulate cortex was approximate 5 mm from the site of saporin injection vs. the 2-3 mm distance to the main prefrontal area sampled for ACh release in the present experiment. Still, rats with this damage did not exhibit a preference for the demonstrated food, leading to the conclusion that ACh in orbitofrontal cortex is necessary for acquisition of the food preference (Ross et al., 2005). Because the present experiment did not explicitly measure ACh release in orbitofrontal cortex, the results reported here do not bear directly on those findings.
However, the present results show that training-related increases in release of ACh in prelimbic cortex do not accompany learning of a social transmission of food preference. While a positive correlation would not show necessary involvement of training-related increases in ACh release in social transmission of food preference, absence of a correlation does indicate that training-related increases in ACh release do not accompany, and therefore are not involved, in this task. With these results, the evidence from loss of function studies in prelimbic cortex should not be taken as strong support for critical, necessary or essential roles for ACh activation in acquisition of social transmission of food preference (Berger-Sweeney et al., 2000; Boix-Trellis et al., 2007). When these prior findings are viewed together with the present results, it appears that the presence of ACh at baseline levels of release and receptor activation may be necessary for acquisition of the food preference, even as the evidence indicates that training-related release of ACh is not necessary. In this regard, the present results leave open the possibility that baseline cholinergic “tone,” i.e. the low levels of cholinergic activity at baseline, is necessary for optimal functioning of prefrontal cortex during acquisition of a socially transmitted food preference.
In contrast to the results obtained with ACh release in prefrontal cortex, the present findings obtained with neurochemistry of the hippocampus clearly support the view that activation of cholinergic mechanisms in hippocampus is important in regulating learning and memory for social transmission of food preference.
HIGHLIGHTS.
Acetylcholine release increases in the hippocampus during social transmission of food preference training.
The increase was not seen in social or odor controls.
These findings support the view that cholinergic mechanisms in the hippocampus are important for this type of learning.
In the same rats, acetylcholine release in prelimbic regions of prefrontal cortex did not increase during training.
Activation of acetylcholine release in prelimbic cortex does not appear to be critical for social transmission of food preference.
ACKNOWLEDGMENTS
This research was supported by research grants from the National Institute on Aging (AG07648), the National Institute on Drug Abuse (DA016951 and DA024129), NSF (IOS 08-43157), and the Alzheimer's Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Gold et al.
Acetylcholine release in the hippocampus and prefrontal cortex during acquisition of a socially transmitted food preference.
REFERENCES
- Alvarez P, Lipton PA, Melrose R, Eichenbaum H. Differential effects of damage within the hippocampal region on memory for a natural, nonspatial Odor-Odor Association. Learning and Memory. 2001;8:89–86. doi: 10.1101/lm.38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Wendelken L, Eichenbaum H. Hippocampal formation lesions impair performance in an odor-odor association task independently of spatial context. Neurobiology of Learning and Memory. 2002;78:470–476. doi: 10.1006/nlme.2002.4068. [DOI] [PubMed] [Google Scholar]
- Andre JM, Leach PT, Gould TJ. Nicotine ameliorates NMDA receptor antagonist-induced deficits in contextual fear conditioning through high-affinity nicotinic acetylcholine receptors in the hippocampus. Neuropharmacology. 2011;60:617–625. doi: 10.1016/j.neuropharm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Sweeney J, Stearns NA, Frick KM, Beard B, Baxter MG. Cholinergic basal forebrain is critical for social transmission of food preference. Hippocampus. 2000;10:729–738. doi: 10.1002/1098-1063(2000)10:6<729::AID-HIPO1010>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Boccia MM, Blake MG, Baratti CM, McGaugh JL. Involvement of the basolateral amygdala in muscarinic cholinergic modulation of extinction memory consolidation. Neurobiology of Learning & Memory. 2009;91:93–97. doi: 10.1016/j.nlm.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia MM, Blake MG, Krawczyk MC, Baratti CM. Hippocampal alpha7 nicotinic receptors modulate memory reconsolidation of an inhibitory avoidance task in mice. Neuroscience. 2010;171:531–543. doi: 10.1016/j.neuroscience.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Boix-Trelis N, Vale-Martinez A, Guillazo-Blanch G, Costa-Miserachs D, Marti-Nicolovius M. Effects of nucleus basalis magnocellularis stimulation on a socially transmitted food preference and c-Fos expression. Learning and Memory. 2006;13:783–793. doi: 10.1101/lm.305306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix-Trelis N, Vale-Martinez A, Guillazo-Blanch G, Marti-Nicolovius M. Muscarinic cholinergic receptor blockade in the rat prelimbic cortex impairs the social transmission of food preference. Neurobiology of Learning and Memory. 2007;87:659–668. doi: 10.1016/j.nlm.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Bunce JG, Sabolek HR, Chrobak JJ. Intraseptal infusion of the cholinergic agonist carbachol impairs delayed-non-match-to-sample radial arm maze performance in the rat. Hippocampus. 2004;14:450–459. doi: 10.1002/hipo.10200. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5:546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- Carballo-Marquez A, Vale-Martinez A, Guillazo-Blanch G, Marti-Nicolovius M. Muscarinic receptor blockade in ventral hippocampus and prelimbic cortex impairs memory for socially transmitted food preference. Hippocampus. 2009;19:446–455. doi: 10.1002/hipo.20530. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: Changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. Journal of Neuroscience. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Impaired and spared cholinergic functions in the hippocampus after lesions of the medial septum/ventral diagonal band with 192 IgG-saporin. Hippocampus. 2004;14:170–179. doi: 10.1002/hipo.10160. [DOI] [PubMed] [Google Scholar]
- Chang Q, Savage LM, Gold PE. Microdialysis measures of functional increases in ACh release in the hippocampus with and without inclusion of acetylcholinesterase inhibitors in the perfusate. Journal of Neurochemistry. 2006;97:697–706. doi: 10.1111/j.1471-4159.2006.03765.x. [DOI] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Zola SM, Squire LR. Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. Journal of Neuroscience. 2002;22:4663–4669. doi: 10.1523/JNEUROSCI.22-11-04663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman RA, Gold PE. Rapid forgetting of social transmission of food preferences in aged rats: relationship to hippocampal CREB activation. Learning and Memory. 2007;14:350–358. doi: 10.1101/lm.524907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG, Jr., Whiskin EE. Socially transmitted food preferences can be used to study long-term memory in rats. Learning and Behavior. 2003;31:160–164. doi: 10.3758/bf03195978. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiology of Learning and Memory. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Gold PE. Coordination of multiple memory systems. Neurobiology of Learning and Memory. 2004;82:230–242. doi: 10.1016/j.nlm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Gold PE. Memory enhancing drugs. In: Byrne J, editor. Memory Systems (H. Eichenbaum, Ed.), vol. 3 of Learning and Memory: A Comprehensive Reference. Elsevier Science; Oxford: 2008. pp. 555–576. [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Current Opinion in Neurobiology. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Morales W, Mar I, Serrano B, Bermudez-Rattoni F. Activation of hippocampal postsynaptic muscarinic receptors is involved in long-term spatial memory formation. European Journal of Neuroscience. 2007;25:1581–1588. doi: 10.1111/j.1460-9568.2007.05391.x. [DOI] [PubMed] [Google Scholar]
- Ihalainen J, Sarajarvi T, Kemppainen S, Keski-Rahkonen P, Lehtonen M, Tanila H. A novel delayed non-match to sample object recognition task that allows simultaneous in vivo microdialysis. Journal of Neuroscience Methods. 2010;189:210–215. doi: 10.1016/j.jneumeth.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Kehr J, Hu X-J, Yoshitake T, Wang F-H, Osborne P, Stenfors C, □gren SO. European Neuropsychopharmacology. 2010;20:487–500. doi: 10.1016/j.euroneuro.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Molecular Neurobiology. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TL, Liang KC. Posttraining infusion of cholinergic drugs into the ventral subiculum modulated memory in an inhibitory avoidance task: interaction with the bed nucleus of the stria terminalis. Neurobiology of Learning & Memory. 2009;91:235–242. doi: 10.1016/j.nlm.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiology of Learning and Memory. 2003a;79:177–183. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Cooperation between memory systems: Acetylcholine release in the amygdala correlates positively with good performance on a hippocampus-dependent task. Behavioral Neuroscience. 2003b;117:320–326. doi: 10.1037/0735-7044.117.2.320. [DOI] [PubMed] [Google Scholar]
- Micheau J, Marighetto A. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behavioural Brain Research. 2011;221:424–429. doi: 10.1016/j.bbr.2010.11.052. [DOI] [PubMed] [Google Scholar]
- Morris KA, Chang Q, Mohler EG, Gold PE. Age-related memory impairments due to reduced blood glucose responses to epinephrine. Neurobiology of Aging. 2010;31:2136–2145. doi: 10.1016/j.neurobiolaging.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang MH, Kim NS, Kim IH, Kim H, Kim HT, Choi JS. Cholinergic transmission in the dorsal hippocampus modulates trace but not delay fear conditioning. Neurobiology of Learning and Memory. 2010;94:206–213. doi: 10.1016/j.nlm.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learning and Memory. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. (CD ROM) Elsevier, Academic Press; Oxford, UK: [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiology of Learning and Memory. 2003;80:178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Gold PE. Acetylcholine release in hippocampus and striatum during training on a rewarded spontaneous alternation task. Neurobiology of Learning and Memory. 2005a;84:93–101. doi: 10.1016/j.nlm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE. Acetylcholine release in the hippocampus and striatum during place and response training. Learning and Memory. 2005b;12:564–572. doi: 10.1101/lm.33105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Gold PE. Intrahippocampal infusions of anisomycin produce amnesia: contributions of increased release of norepinephrine, dopamine and acetylcholine. Learning and Memory. 2009;16:308–314. doi: 10.1101/lm.1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: Augmentation by glucose. Proceedings of the National Academy of Sciences. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and of memory by intrahippocampal glucose injections. Journal of Neuroscience. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiology of Learning and Memory. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval. Neurobiology of Learning and Memory. 2003;80:332–342. doi: 10.1016/s1074-7427(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. Blunted hippocampal, but not striatal, acetylcholine efflux parallels learning impairment in diencephalic-lesioned rats. Neurobiology of Learning and Memory. 2007;87:123–132. doi: 10.1016/j.nlm.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, McGaughy J, Eichenbaum H. Acetylcholine in the orbitofrontal cortex is necessary for the acquisition of a socially transmitted food preference. Learning and Memory. 2005;12:302–306. doi: 10.1101/lm.91605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, East BS, Colombo PJ. The orbitofrontal cortex is not necessary for acquisition or remote recall of socially transmitted food preferences. Behavioural Brain Research. 2010;208:243–249. doi: 10.1016/j.bbr.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. Intra-hippocampal infusions of K-ATP channel modulators influence spontaneous alternation performance: relationships to acetylcholine release in the hippocampus. Journal of Neuroscience. 2001;21:609–614. doi: 10.1523/JNEUROSCI.21-02-00609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase K, Kimura F, Yagami T, Mitsushima D. Sex-specific 24-h acetylcholine release profile in the medial prefrontal cortex: simultaneous measurement of spontaneous locomotor activity in behaving rats. Neuroscience. 2009;159:7–15. doi: 10.1016/j.neuroscience.2008.12.039. [DOI] [PubMed] [Google Scholar]
- Vale-Martinez A, Baxter MG, Eichenbaum H. Selective lesions of basal forebrain cholinergic neurons produce anterograde and retrograde deficits in a social transmission of food preference task in rats. European Journal of Neuroscience. 2002;16:983–998. doi: 10.1046/j.1460-9568.2002.02153.x. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hearing Research. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G. Anterograde and retrograde amnesia in rats with dorsal hippocampal or dorsomedial thalamic lesions. Behavioural Brain Research. 1990;38:145–154. doi: 10.1016/0166-4328(90)90012-4. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Anterograde and retrograde amnesia after lesions to frontal cortex in rats. Journal of Neuroscience. 1999;19:9611–9617. doi: 10.1523/JNEUROSCI.19-21-09611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, McDonald RM, Moscovitch M. Anterograde and retrograde amnesia in rats with large hippocampal lesions. Hippocampus. 2001;11:18–26. doi: 10.1002/1098-1063(2001)11:1<18::AID-HIPO1016>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]