Abstract
Gender differences are frequently observed in autobiographical memory (AM). Few studies, however, have investigated the neural basis of potential gender differences in AM. In the present functional MRI (fMRI) study we investigated gender differences in AMs elicited using dynamic visual images vs. verbal cues. We used a novel technology called a SenseCam, a wearable device that automatically takes thousands of photographs. SenseCam differs considerably from other prospective methods of generating retrieval cues because it does not disrupt the ongoing experience. This allowed us to control for potential gender differences in emotional processing and elaborative rehearsal, while manipulating how the AMs were elicited. We predicted that males would retrieve more richly experienced AMs elicited by the SenseCam images vs. the verbal cues, whereas females would show equal sensitivity to both cues. The behavioral results indicated that there were no gender differences in subjective ratings of reliving, importance, vividness, emotion and uniqueness, suggesting that gender differences in brain activity were not due to differences in these measures of phenomenological experience. Consistent with our predictions, the fMRI results revealed that males showed a greater difference in functional activity associated with the rich experience of SenseCam vs. Verbal Cues, than did females.
Gender differences have frequently been observed in autobiographical memory (AM), which refers to memory for events from our personal past. Several studies have documented superior AMs in females compared to males (for a review see Andreano & Cahill, 2009). For example, compared to males, females recall longer and more detailed AMs (e.g., Friedman & Pines, 1991; Pillemer, Wink, DiDonato, & Sanborn, 2003; Pohl, Bender, & Lachmann, 2005; Ross & Holmberg, 1992; Seidlitz & Diener, 1998), are more accurate at dating their AMs (e.g., Skowronski & Thompson, 1990), and are faster to recall AMs (e.g., Davis, 1999). In contrast, other studies have failed to find gender differences in AM (e.g., Rubin, Schulkind, & Rahhal, 1999). The lack of gender differences in behavior does not necessarily imply that there are no gender differences in AM retrieval (e.g., Piefke, Weiss, Markowitsch, & Fink, 2005). However, the bases of potential gender differences during AM retrieval are not well understood. In the present study we employed functional MRI (fMRI) to examine brain activity underlying potential gender differences in AM retrieval.
Reported gender differences in AM have been linked to differences in emotional processing and elaborative rehearsal (for reviews see Andreano & Cahill, 2009; Piefke & Fink, 2005). According to the affective intensity hypothesis female’s superior AM is due to increased emotional processing that subsequently enhances memory recall. For example, females recall more emotional AMs (e.g., Friedman & Pines, 1991; Fujita, Diener, & Sandvik, 1991), more emotional information (e.g., Bauer, Stennes, & Haight, 2003; Bloise & Johnson, 2007), and rate their memories as being more emotional (e.g., Seidlitz & Diener, 1998). Moreover, sex differences have been observed in brain activity during emotional tasks (e.g., Cahill et al., 2001; Canli, Desmond, Zhao, & Gabrieli, 2002). In contrast, the elaborative rehearsal hypothesis suggests that females are better at remembering their life’s experiences because of more frequent and elaborative rehearsal (e.g., Seidlitz & Diener, 1998). Support for this hypothesis is provided by evidence that females discuss events at more detailed length (Friedman & Pines, 1991; Seidlitz & Diener, 1998) and that parents discuss AMs in a more elaborative way with daughters compared to sons (for a review see Fivush & Nelson, 2004).
Alternatively, gender differences in AM might be due to more general cognitive differences in verbal and spatial processing (cf. Andreano & Cahill, 2009; Piefke & Fink, 2005). A substantial body of literature shows that females outperform males on verbal tasks such as phonological fluency, whereas males outperform females on spatial tasks such as mental rotation and navigation (for a review see Andreano & Cahill, 2009). However, previous studies examining gender differences in AM have relied upon verbal cues to elicit AMs and verbal processing strategies to determine the quality of retrieval, in which males might have more disadvantage. Moreover, individual differences in phonological fluency accounts for potential differences in the level of detail in verbal recall of AMs (Addis, Sacchetti, Ally, Budson, & Schacter, In Press). In contrast, functional neuroimaging studies of AM have suggested that there is substantial overlap in the brain regions recruited during navigation (for a meta-analysis see Spreng, Mar, & Kim, 2009) and particular spatial abilities (for reviews see Hassabis & Maguire, 2007; Moscovitch et al., 2005), suggesting that these processes are very important in AM retrieval. Furthermore, episodic memory tests that have controlled for verbal processing have observed less robust gender differences (for a review see Andreano & Cahill, 2009). Thus, it is possible that the reported gender differences in behavioral studies of AM might be the result of potential biases favoring verbal processing in the method of elicitation and evaluation of the retrieved events.
One solution to examine AM retrieval in a more unbiased way is to use functional neuroimaging to examine covert retrieval of AMs. Gender differences should be observed in the AM retrieval network if females rely more on verbal processing, whereas males rely more on spatial processing during AM recall. Piefke et al. (2005) is the only functional neuroimaging study to our knowledge, which has investigated gender differences in AM retrieval. In this fMRI study, participants were asked to recall AMs elicited via verbal sentences describing particular autobiographical events generated in a pre-scan interview. Females recruited the prefrontal cortex (PFC), a region that is associated with increased control over the recovery of information (for a review see Miller & Cohen, 2001). Piefke et al. (2005) interpreted the greater recruitment of the PFC as reflecting a greater reliance on temporal context when females retrieve AMs. In contrast, males recruited the parahippocampal gyrus, a region associated with bottom-up visuospatial processing (for a review see Burgess, Maguire, & O’Keefe, 2002). Thus, the findings of Piefke et al. (2005) are consistent with the idea that gender differences in AM might be due to differences in retrieval strategy. However, it is not clear whether the use of more spatial cues might attenuate some of these potential gender differences in cognitive strategy during AM retrieval.
To address these issues, we investigated potential gender differences in AMs elicited using verbal cues versus dynamic visual images. We employed a novel technology called a SenseCam (Microsoft Research Cambridge; http://research.microsoft.com/sensecam/) to prospectively collect dynamic, visuospatial cues to elicit AMs during functional MRI (fMRI) scanning. SenseCam is a small wearable digital camera that has electronic sensors (e.g., light, heat, etc.) that can automatically trigger thousands of photographs in a single day (see Figure 1A). This differs considerably from the normal way in which we can use a camera to generate retrieval cues (Cabeza et al., 2004; St. Jacques, Rubin, Labar, & Cabeza, 2008), because it does not disrupt the ongoing experience of events through the act of taking a photograph (for a review see Cabeza & St. Jacques, 2007). Moreover, the SenseCam images can provide effective retrieval cues to elicit everyday AMs, which are less likely to be emotionally intense and elaboratively rehearsed compared to memories that participants retrieve themselves (e.g., Seidlitz & Diener, 1998). Participants wore the SenseCam for a week while it automatically recorded photographs, and they also kept a schedule of their daily activities. Following a week delay, participants came in for their fMRI session, in which they were asked to covertly recall the events depicted in short SenseCam clips versus verbal cues garnered from the daily schedule, then to rate their subjective feeling of re-experience using a reliving rating. We examined the interaction between gender (males, females) and type of cue (SC, VC) on brain activity sensitive to reliving during AM retrieval. Based on reported gender differences in the reliance on spatial vs. verbal processing, we predicted that males would retrieve more richly experienced AMs when cued via the SenseCam images (SC) versus the verbal cues (VC), whereas females would show an equal benefit for both types of cues.
Figure 1.
The experimental design depicting the study conditions.
Methods
Participants
There were twenty-three participants (12 females, and 11 males; age range: 18 – 35 years) who were healthy, right-handed, and without history of neurological or psychiatric episodes (for demographic characteristics see Table 1). Participants gave written informed consent for a protocol approved by the Duke University Institutional Review Board. Participants were tested on subsets of executive function (Intra-Extra Dimensional Set Shifting, Spatial Span, Spatial Working Memory, Rapid Visual Information Processing), visual memory ability (Paired-Associated Learning, Pattern Recognition Memory), and speed of processing (Movement Time and Reaction Time) included in the Cambridge Neuropsychological Test Automated Battery (CANTABeclipse, version 2.0; Cambridge Cognition Ltd.), as well as vocabulary ability using the Shipley Vocabulary Test. Using Bonferroni correction to control for multiple t-tests we found no significant gender differences across these cognitive tasks or in the demographic characteristics of the participants (see Table 1).
Table 1.
Demographic Information by Gender
| Females | Males | t (21) | |
|---|---|---|---|
| n | 12 | 11 | |
| Age | 23.08 (2.23) | 24.45 (4.72) | 0.90 |
| Years of Education | 16.17 (1.75) | 15.91 (2.17) | −0.32 |
| Shipley Vocabulary | 33.75 (2.99) | 34.73 (3.44) | 0.73 |
| Executive Tasks | |||
| Spatial Working Memory | |||
| Between errors | 6.75 (9.38) | 10.18 (12.78) | 0.74 |
| Strategy | 24.58 (5.45) | 28.55 (7.54) | 1.45 |
| Spatial Span | 7.58 (1.16) | 8.18 (0.75) | 1.45 |
| Rapid Visual Information Processing | 0.95 (0.03) | 0.98 (0.02) | 2.60 |
| Intra-Extra Dimensional Set Shifting | |||
| Stages Completed | 9.00 (0.00) | 9.00 (0.00) | n/a |
| Total Errors | 9.25 (2.34) | 10.45 (2.50) | 1.19 |
| Memory Tasks | |||
| Paired Associate Learning | 2.17 (2.17) | 5.36 (5.26) | 1.94 |
| Pattern Recognition Memory | 96.53 (3.91) | 96.59 (3.64) | 0.04 |
| Speed Tasks | |||
| Movement time (ms) | 363.89 (75.81) | 351.16 (58.65) | −0.45 |
| Reaction time (ms) | 309.73 (44.75) | 318.56 (57.51) | 0.41 |
Mean (Standard Deviation);
p < .001
Procedure
Prospective Collection
Retrieval cues were prospectively collected across a period of 6 days, in which participants wore the SenseCam and kept a schedule of each day’s activities to be used to parse the SenseCam images into events. The daily schedule was recorded at the end of each day along with a unique identifier to distinguish that particular day from others (e.g., “Today was the day I had lunch with Ben”). Participants were instructed to write about 10–15 brief sentences, one for each major event during the day (e.g., “Had breakfast”, “went to the grocery store …”). Participants were not informed that their written schedules would be later used to test their memory for the events, and debriefing after scanning confirmed that no participant suspected a memory test. Three days were randomly assigned to the SC condition and 3 days, to the VC condition. For each day, 12 events were selected to be tested in the scanner. Participants also viewed SenseCam images from other people’s cameras in separate runs, which were included for additional analyses that were not the focus on the present investigation (St. Jacques, Conway, Lowder, & Cabeza, 2010).
FMRI Scanning
The scanning session took place one week following the last day the SenseCam was worn (mean length of delay = 8 days, SD = 1.2). Participants did not view the photographs prior to fMRI scanning. There were six fMRI runs blocked by condition and presented in an alternating order (i.e., ABABAB), counterbalanced across participants based on a Latin square design. The structure of the runs was similar in each condition (see Figure 1). Each run began with a 10-second title screen (i.e., “Today was the day I…”) and consisted of 12 cues presented in chronological order from that day, for a total of 36 events per condition across 3 runs. The cues were presented for 20 seconds, and participants were instructed to recall the events depicted. The cues in the SC condition consisted of 40 SenseCam pictures depicting a single event and presented at a rate of two pictures per second; the cues in the VC condition consisted of a short description of a single event. To control for differences in the visual input, a static string of letters was presented in the SC condition, whereas participant-specific, Fourier transformed SenseCam images were presented at a rate of two pictures per second in the VC condition. Thus, the visual input between the conditions was identical.
Following the cue presentation, participants indicated the subjective experience associated with each cue. Participants rated the subjective experience of recollection, reliving, which refers to how much they were able to re-experience the event depicted as if it were happening right now or as if they were mentally traveling back to the time when the event occurred. It is important to note that reliving is similar to other subjective measures of recollection, such as the remember/know paradigm (Yonelinas, 2002). For example, in the remember/know paradigm, participants are asked to use introspection to classify items as recollected (vivid re-experiencing of the original event and its context) or merely familiar. Although introspection has its limitations, the results of hundreds of remember/know studies are highly consistent with findings of hundreds of studies using objective measures of recollection, such as source memory (Yonelinas, 2002). However, there are some critical differences between the reliving scale and remember/know paradigm, which make the reliving scale a better measure for AM. First, the reliving scale could be considered a better subjective measure of recollection in AM than remember/know because it does not require the assumption of a dual-process model (Wixted, 2007; Yonelinas, 2002). Second, reliving is a better predictor of recollection in AMs compared to the remember/know scale, which is a better predictor of confidence in AMs (Rubin, Schrauf, & Greenberg, 2003). Ratings were conducted on an 8-point scale from low to high, and were self-paced (up to 6 seconds). Following a response, a fixation cross was presented for a jittered interval between 4 and 8 seconds plus any remaining time from the response period.
Post-Scanning
Immediately following the scanning session, participants viewed the identical SC and VC runs and answered additional questions on a small set of subjective ratings to ensure that the events selected for inclusion in the SC vs. VC conditions were unbiased. More ratings were not included due to time constraints. Participants were asked to rate the events on the following properties: Importance (1 = low to 8 = high), Vividness (1 = low to 8 = high), Emotion (-4 = intense negative to 4 = intense positive), and Uniqueness (1 = low to 8 = high) associated with the event. For more details on the instructions provided to participants, please see the Appendix.
fMRI Methods
Image Acquisition
Scanning was conducted using a 4T GE magnet. Anatomical scanning included a T1-weighted sagittal localizer series and 3D fast spoiled gradient echo recalled (SPGR) structural images were acquired in the coronal plane (2562 matrix, TR = 12.3 ms, TE = 5.4 ms, flip angle = 20°, FOV = 240, 68 slices, 1.9 mm slice thickness). Coplanar functional images were acquired using an inverse spiral sequence (642 image matrix, TR = 2000 ms, TE = 6 ms, FOV = 240, flip angle = 60°, 34 slices, 3.8 mm slice thickness).
fMRI Analyses
Image processing and analyses were performed using Statistical Parameter Mapping software in Matlab (SPM5; Wellcome Department of Imaging Neuroscience). Functional images were corrected for slice acquisition order, realigned to correct for motion artifacts, spatially normalized to a standard stereotactic space, and spatially smoothed using an 8-mm isotropic Gaussian kernel. Evoked hemodynamic responses for the cue was modeled with a boxcar function corresponding to the 20-second phase of presentation, convolved with a canonical hemodynamic response function within the context of the General Linear Model (GLM). Evoked hemodynamic responses for the ratings were modeled with a delta (stick) function, convolved with a canonical hemodynamic response function.
SenseCam Retrieval vs. Verbal Cue Retrieval
To examine brain activity in the SC condition we employed the GLM to examine the neural correlates modulated by reliving using a random-effects parametric approach via the first-order parametric modulation option integrated in SPM5. The parametric approach ensures that potential gender differences reflect memory (i.e., fluctuations in activity tracking reliving) rather than other perceptual differences (e.g., exploration of the visual stimulus). Data analysis was confined to the first 5 seconds of the retrieval blocks (i.e., presentation of the stimulus) to reduce potential differences in the number of retrieval cues provided by the two conditions. Subsequently, we conducted a two sample t-tests to examine the interaction between gender and memory cue (P = .005, > 10 voxel extent threshold) and the main effect of gender across both cuing manipulations, and then inclusively masked this statistical image with the corresponding statistical map for the effect of interest at p = .005 (i.e., SenseCam > Verbal Cues in Males, etc.). Thus, the resulting pattern of group differences in activity had to pass two hurdles: 1) the difference should be significant within one group, and 2) the difference should be significantly larger within one group compared to the other group. Given the a priori role of the MTL in AM (Cabeza & St. Jacques, 2007; Maguire, 2001; McDermott et al., 2009; Spreng et al., 2009; Svoboda et al., 2006) we conducted an additional a priori region of interest (ROI) here at P = .05 using the Talaraich Daemon Atlas (Lancaster, Summerin, Rainey, Freitas, & Fox, 1997; Lancaster et al., 2000), implemented within the PickAtlas software (Maldjian, Laurienti, Kraft, & Burdette, 2003), also using an extent threshold of > 10 voxels.
Results
Behavioral
The behavioral results indicated that there were no gender differences in the phenomenological experience of memory retrieval measured by reliving, importance, vividness, emotion and uniqueness, or in reaction times to make these subjective ratings (for means and standard deviations see Table 2). We conducted multiple repeated-measures ANOVAs (Gender × Cue) using Bonferroni correction separately on each of the subjective ratings and reaction time measures (p < .005). Both females and males gave higher reliving ratings, F(1,21) = 32.60, p < .001, and vividness ratings, F(1,21) = 78.79, p < .001, for AMs elicited in the SC versus VC conditions. Furthermore, both genders made faster reliving ratings, F(1,21) = 17.80, p < .005, for the SC versus VC conditions. There were no other gender or cueing differences. These results suggest that any gender differences in functional activity cannot be explained by potential gender differences in the phenomenological experience of recall- at least as measured by reliving, emotion, importance, vividness, of the AMs and the uniqueness of the events.
Table 2.
Mean behavioral responses by Gender
| Females | Males | |
|---|---|---|
| SenseCam | ||
| Subjective Ratings | ||
| Reliving | 5.10 (0.48) | 4.97 (0.65) |
| Vividness | 4.75 (0.42) | 4.59 (0.86) |
| Emotional Intensity | 1.91 (0.24) | 1.96 (0.41) |
| Importance | 3.15 (0.70) | 3.53 (0.85) |
| Uniqueness | 4.29 (0.57) | 4.22 (0.77) |
| Reaction Time (s) | ||
| Reliving | 1.12 (0.38) | 1.75 (0.77) |
| Vividness | 1.75 (0.52) | 2.09 (0.71) |
| Emotional Intensity | 1.81 (0.46) | 1.88 (0.46) |
| Importance | 2.09 (0.79) | 3.04 (1.33) |
| Uniqueness | 2.09 (0.55) | 2.30 (0.79) |
| Verbal Cue | ||
| Subjective Ratings | ||
| Reliving | 4.06 (0.60) | 4.25 (0.62) |
| Vividness | 3.67 (0.68) | 3.55 (0.47) |
| Emotional Intensity | 1.93 (0.32) | 1.79 (0.36) |
| Importance | 3.11 (0.70) | 3.37 (0.78) |
| Uniqueness | 4.13 (0.62) | 3.71 (0.67) |
| Reaction Time (s) | ||
| Reliving | 0.95 (0.36) | 1.44 (0.52) |
| Vividness | 1.71 (0.56) | 2.03 (0.65) |
| Emotional Intensity | 1.70 (0.50) | 2.08 (0.78) |
| Importance | 2.06 (0.89) | 3.29 (1.28) |
| Uniqueness | 1.94 (0.47) | 2.37 (1.14) |
Mean (Standard Deviation)
fMRI
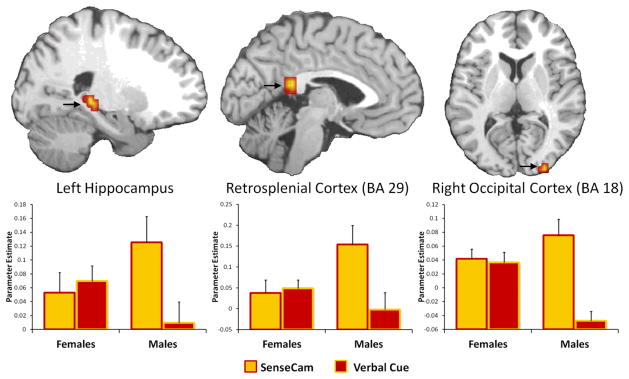
As predicted, we found that males showed greater increase in activity sensitive to reliving AMs elicited in the SC versus VC condition, whereas females showed equivalent sensitivity to reliving in both cuing types (see Table 3, Figure 2). There was greater activity in MTL (left hippocampus), retrosplenial cortex, left inferior frontal gyrus (IFG), and right occipital cortex that was positively correlated with reliving ratings to a greater extent for males vs. females in the SC vs. VC conditions. Further, when we separately examined the influence of gender within each condition, we found that males recruited less activity sensitive to reliving for AMs elicited in the VC condition when compared to females. Specifically, males recruited less hippocampus (x = −26, y = −33, z = 2; t = 2.44, 11 voxels) and IFG (BA 45; x = −45, y = 19, z = 20; t = 4.11, 34 voxels), which overlapped with those showing the gender × cue interaction effects. There were no significant effects of gender that were common to both the SC and VC conditions, suggesting that cue independent effects of reliving were not influenced by gender. In sum, these results suggest that females were equally sensitive to reliving AMs in both conditions, but that males benefited more from the rich visuospatial cues provided by the SenseCam images than the verbal cues.
Table 3.
Gender × Cue Effect
| Region | BA | H | x | y | z | t | Voxels |
|---|---|---|---|---|---|---|---|
| SenseCam vs. Verbal Cue | |||||||
| Males > Females | |||||||
| Inferior Frontal Gyrus | 45 | L | −48 | 19 | 17 | 3.34 | 19 |
| * Hippocampus | -- | L | −22 | −33 | 2 | 2.49 | 14 |
| Retrosplenial Cortex | 29 | C | 0 | −39 | 16 | 3.89 | 15 |
| Occipital Cortex | 18 | R | 30 | −94 | 12 | 3.85 | 15 |
| Females > Males | |||||||
| No Significant Voxels | |||||||
| Verbal Cue vs. SenseCam | |||||||
| No Significant Voxels | |||||||
Talaraich Coordinates Reported. BA, Brodmann’s Area; H, Hemisphere.
Region of Interest
Figure 2.
The results of the two-sample t-test showing the effects of gender on cue type for functional activity sensitive to the experience of reliving. Males recruited more richly experienced autobiographical memories cued by the SenseCam than the verbal cues, whereas females showed an equivalent sensitivity to reliving in functional activations for both cueing types. Error bars indicate standard error of the mean.
Discussion
The present study investigated the interaction between gender and the type of cue to elicit AMs during functional scanning. We used a novel technology called a SenseCam to prospectively collect dynamic, visuospatial cues to elicit everyday AMs in the scanning environment. This allowed us to control for potential differences in the emotional intensity and rehearsal of retrieved events. The behavioral results revealed that there were no gender differences in the reliving, emotion, importance, vividness, of the AMs and the uniqueness of the events. Consistent with our predictions, however, the fMRI results revealed that males showed a greater difference in functional activity associated with the rich experience of visuospatial versus verbal cues, than did females.
Males and females might rely on different cognitive strategies during memory retrieval. Previous evidence has indicated an advantage for males on spatial tasks, whereas females show greater advantage on verbal tasks (cf. Andreano & Cahill, 2009; Piefke & Fink, 2005). Consistent with this finding, one previous fMRI study examining gender effects on AM found that males relied more on MTL regions associated with visuospatial processing, whereas females relied more on PFC regions linked to controlled processes, such as temporal context (Piefke, et al., 2005). Substantial evidence has suggested that females have superior AMs compared to males (for reviews see Andreano & Cahill, 2009; Piefke & Fink, 2005). However, the female advantage might be less robust once potential gender differences in these cognitive strategies are controlled (for review see Andreano & Cahill, 2009). Consistent with this idea, we found that males, compared to females, showed greater recruitment of brain regions sensitive to reliving of AMs elicited by visuospatial cues than verbal cues.
The present findings are less amenable to alternative accounts of potential gender differences in AM, which have linked better memory in females to differences in the amount of rehearsal or emotional intensity (for reviews see Andreano & Cahill, 2009; Piefke & Fink, 2005), since we queried for very recent, everyday AMs. Participants did keep a daily schedule of events, which may have involved some minimal rehearsal and perhaps lessened the influence of the SenseCam cues, when compared to the verbal cues. However, potential gender differences in the influence of rehearsal provided by the daily schedule could not easily explain the greater sensitivity to reliving elicited by the SenseCam images in males.
In the present study we showed that gender differences were directly linked to memory experience by examining brain activity that was parametrically modulated by participant ratings of reliving. Males showed greater sensitivity to the experience of reliving when cued by the visuospatial cues in a number of brain regions. First, there was greater sensitivity to reliving in the hippocampus, a region associated with spatial memory (for a review see Burgess, et al., 2002), and to recollection processes (for a review see Diana, Yonelinas, & Ranganath, 2007). Furthermore, functional neuroimaging studies of AM have shown that the amount of details associated with retrieval modulates activity in the left hippocampus (e.g., Addis, McIntosh, Moscovitch, Crawley, & McAndrews, 2004), and patient evidence links this region to recollection of AMs (e.g., Steinvorth, Corkin, & Halgren, 2006). Second, males showed greater sensitivity to reliving when cued by the visuospatial cues in the retrosplenial cortex. Previous functional neuroimaging studies have found the retrosplenial cortex to be sensitive to the amount of reliving associated with AM retrieval (Daselaar et al., 2008), and damage here can result in amnesia (e.g., Valenstein et al., 1987).. Third, there was greater sensitivity to reliving in males in the occipital cortex. The occipital cortex is associated with the visual imagery that is critical to AM retrieval (Greenberg & Rubin, 2003), and has been linked to the rich elaboration of AMs (Conway, Pleydell-Pearce, & Whitecross, 2001; Daselaar et al., 2008). In sum, males and females showed a differential effect of reliving AMs cued by visuospatial versus verbal cues, with males showing a greater effect visuospatial compared to verbal cues and females showing equal sensitivity to both cues.
Conclusion
The present findings demonstrate the potential of using SenseCam to prospectively generate everyday AMs, which was particularly advantageous to the current investigation of gender differences in functional neuroimaging of AMs. We found that males had a differential effect on brain activity recruited when retrieving richly re-experienced AMs elicited by the SenseCam images, whereas females showed an equal effect to both SenseCam and verbal cues. The findings emphasize the importance of using unbiased measures to elicit and evaluate AMs when examining gender differences.
Acknowledgments
The authors would like to thank Matthew W. Lowder for help with participant testing. This research was supported by a grant from Microsoft Research Cambridge and the National Institute of Health RO1 AG23770, both awarded to RC.
Appendix
After viewing each event you will be making four different ratings:
Importance. You will be rating how important, or significant, this event is to your life. Some events are more personally meaningful than others because they impart an important message or represent an anchor, critical juncture, or turning point. Other events are relatively insignificant and do not carry any sort of personal meaning to you. Rate each event on a scale of 1 (not at all important) to 8 (very important).
Vividness. You will be rating how vivid each memory is. You may find that some memories are fuzzier than others, with bits and pieces missing, and it takes a great deal of effort to consciously reconstruct the event. On the other hand, some memories are full and detailed, with virtually no missing information. Rate each memory on a scale of 1 (vague; unclear; not at all vivid) to 8 (completely clear; very vivid).
Emotion. You will be rating the emotional content associated with recalling the event. This scale is a little different from the others in that we are trying to capture two types of information. First you will decide whether retrieving this memory represents negative emotions or positive emotions for you. If the memory is negative, you will be using the keys on the left; if it is positive, you will be using the keys on the right. Then, we are also interested in the intensity of that emotion. Some of your memories may be extremely sad, while others may be just somewhat sad. Similarly, some of your memories may be extremely happy and exciting, whereas others may just be pleasing or calming. Rate each memory on a scale from −4 (intense negative) to 4 (intense positive). Memories that carry virtually no emotional content should be rated −1 or 1. We are interested the emotion associated with retrieving the event now in the present, rather than the emotion that might have been attached to the event during the time it occurred.
Uniqueness. You will be rating how unique each event is to your life. There are certain events we all do regularly, as part of a daily routine, that may seem mundane or commonplace. Alternatively, there are other events we rarely experience that we may regard as different or special. Rate the uniqueness of each event on a scale from 1 (something you do every day) to 8 (something completely unique that you rarely do).
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage. 2004;23(4):1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL. Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2009.05.018. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16(4):248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Stennes L, Haight JC. Representation of the inner self in autobiography: women’s and men’s use of internal states language in personal narratives. Memory. 2003;11(1):27–42. doi: 10.1080/741938176. [DOI] [PubMed] [Google Scholar]
- Bloise SM, Johnson MK. Memory for emotional and neutral information: gender and individual differences in emotional sensitivity. Memory. 2007;15(2):192–204. doi: 10.1080/09658210701204456. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, et al. Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience. 2004;16(9):1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques PL. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, et al. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem. 2001;75(1):1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci U S A. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE. The neuroanatomy of autobiographical memory: A slow wave cortical potential study of autobiographical memory. Journal of Memory & Language. 2001;45:493–524. [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex. 2008;18(1):217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Davis PJ. Gender differences in autobiographical memory for childhood emotional experiences. Journal of Personality and Social Psychology. 1999;76:498–510. doi: 10.1037//0022-3514.76.3.498. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Fivush R, Nelson K. Culture and language in the emergence of autobiographical memory. Psychological Science. 2004;15(9):573–577. doi: 10.1111/j.0956-7976.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- Friedman A, Pines A. Sex differences in gender-related childhood memories. Sex Roles. 1991;25:25–32. [Google Scholar]
- Fujita F, Diener E, Sandvik E. Gender differences in negative affect and well-being: the case for emotional intensity. J Pers Soc Psychol. 1991;61(3):427–434. doi: 10.1037//0022-3514.61.3.427. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rubin DC. The neuropsychology of autobiographical memory. Cortex. 2003;39(4–5):687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends Cogn Sci. 2007;11(7):299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerin JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon, a database server for Talairach Atlas Labels. Neuroimage. 1997;5(4):S633. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talaraich atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroantomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. (WFU Pickatlas, version 1232.1233) [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Fink GR. Recollections of one’s own past: the effects of aging and gender on the neural mechanisms of episodic autobiographical memory. Anat Embryol (Berl) 2005;210(5–6):497–512. doi: 10.1007/s00429-005-0038-0. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp. 2005;24(4):313–324. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillemer DB, Wink P, DiDonato TE, Sanborn RL. Gender differences in autobiographical memory styles of older adults. Memory. 2003;11(6):525–532. doi: 10.1080/09658210244000117. [DOI] [PubMed] [Google Scholar]
- Pohl RF, Bender M, Lachmann G. Autobiographical memory and social skills of men and women. Applied Cognitive Psychology. 2005;19:745–759. [Google Scholar]
- Ross M, Holmberg D. Are wives memories for events in relationships more vivid than their husband’s memories? Journal of Social and Personal Relationships. 1992;9:585–604. [Google Scholar]
- Rubin DC, Schrauf RW, Greenberg DL. Belief and recollection of autobiographical memories. Mem Cognit. 2003;31(6):887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Schulkind MD, Rahhal TA. A study of gender differences in autobiographical memory: Broken down by age and sex. Journal of Adult Development. 1999;6(1):61–71. [Google Scholar]
- Seidlitz L, Diener E. Sex differences in the recall of affective experiences. J Pers Soc Psychol. 1998;74(1):262–271. doi: 10.1037//0022-3514.74.1.262. [DOI] [PubMed] [Google Scholar]
- Skowronski JJ, Thompson CP. Reconstructing the dates of events: Gender differeces in accuracy. Applied Cognitive Psychology. 1990;4:371–381. [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Rubin DC, Labar KS, Cabeza R. The Short and Long of It: Neural Correlates of Temporal-order Memory for Autobiographical Events. J Cogn Neurosci. 2008 doi: 10.1162/jocn.2008.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Conway MA, Lowder MW, Cabeza R. Watching my mind unfold vs. yours: An fMRI study using a novel camera technology to examine neural differences in self-projection of self vs. other perspectives. 2010. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: An fMRI study of recent and remote memory retrieval. Neuroimage. 2006:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev. 2007;114(1):152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]