Abstract
The extracellular domain of the matrix protein 2 (M2e) of influenza viruses is highly conserved among all influenza A subtypes, making it a suitable target for a universal influenza vaccine. In this study, we demonstrated an enhanced immune response and protection of a chimeric M2e vaccine against influenza A viruses using our newly developed vaccine platform, the norovirus P particle, to present the M2e peptide. The 23-amino acid peptide was inserted into one of the surface loops of the P protein, resulting in 24 copies of M2e presented on each P particle. Significantly (P <0.001) increased antibody responses to M2e were observed in mice immunized with the P particle-M2e chimera compared with those immunized with the free M2e peptides. Mice immunized with the P particle-M2e vaccine were fully protected (100% survived) against lethal challenge of a mouse adapted human influenza virus PR8 (H1N1), while only low survival rates (< 12.5%) were found in mice immunized with the free M2e peptides or wild type P particle. In addition, the mouse sera collected after immunization with the P particle-M2e vaccine were able to block the binding of norovirus virus-like particle and P particle to histo-blood group antigen receptors. These results suggest that the P particle-M2e chimera can be used as dual vaccine against both noroviruses and influenza viruses.
Keywords: norovirus, influenza, P particle, M2e, dual vaccine, platform
1. Introduction
Influenza remains one of the most important infectious diseases with significant morbidity and mortality. Seasonal influenza epidemics hospitalize more than 200,000 people each year in the United States and kill 500,000 people worldwide [1, 2]. Although antivirals have been used to treat the influenza infection, vaccination remains the most cost-effective method for controlling the disease. Structurally, influenza A virus contains two exposed, highly immunogenic, but very variable proteins: hemagglutinin (HA) and neuraminidase (NA). New epidemic strains arise every 1 to 2 years because of selected point mutations in these two surface glycoproteins. The annual vaccine targeting these antigens would protect 70% to 90% healthy adults if the strains included in the vaccine match the circulating ones [3]. The major challenge is to predict the strains to use in the vaccine based on global surveillance of influenza epidemics by many laboratories around the world. In addition, it has become a huge burden to the vaccine industry to process and manufacture new vaccines timely before each flu season begins [4, 5].
Influenza A viruses also encodes an integral membrane protein, M2 protein [6, 7], which is 97 amino acids long and abundantly expressed on the surface of influenza A infected cells. The extracellular moiety of the M2 protein (M2e, 24 amino acids) is exposed on the membrane with 19 amino acids spanning the lipid bilayer. M2 forms homo-tetramers in the plasma membrane functioning as an ion channel, which is a target of amantadine and rimantadine, two antivirals against influenza viruses [8]. The M2e domain has been found to be highly conserved in most influenza A strains [9, 10], making it an attractive target for a universal vaccine. However, due to its small size and apparently low immunogenicity [11], a number of approaches, including multiple redundant copies, chemical conjugation and presentation by subviral particles, have been reported to enhance the immunogenicity of M2e for vaccine development [4, 12–14]. In this study, we described an additional M2e vaccine based on a new vaccine platform, the norovirus P particle, which has been demonstrated to be highly effective in presentation of a number of small to large peptides and protein antigens [15].
Noroviruses are the most important cause of epidemic acute gastroenteritis affecting millions of people worldwide [16–21]. The virus is encompassed by a protein capsid that is formed by a single major structural protein, the capsid protein (VP1). The capsid protein is composed of two major domains, the shell (S) domain forming the interior shell and the protrusion (P) domain constituting the arch-like protruding domain of the virus [22]. Expression of the P domain alone results in the P particle in both E. coli and yeast (Pichia pastoris) expression cultures [23, 24]. The P particle is formed by 24 copies of the P monomer. It revealed an octahedral symmetry with a diameter of ~20 nm and a molecular mass of ~840 kDa. The P particle is easily produced, extremely stable, and highly immunogenic. Therefore, it has been proposed as a vaccine candidate for human noroviruses [24]. In addition, it has recently been shown to be a good vaccine platform for antigen presentation. A number of small to large antigens have been successfully inserted into a surface loop on the protrusion of the P particle and immunization with the chimeric P particles in mice revealed significantly increased immune response to the inserted antigen and provided protection against viral challenge[15]. Since each P domain has three surface loops, insertion of a foreign antigen into these loops would result in 24 to 72 copies of the antigen on the surface of a P particle, which could greatly enhance the antigenicity and immunogenicity of the inserted antigens.
The P particle-M2e chimeric vaccine was constructed by insertion of the human influenza A M2e antigen into the loop 2 of the norovirus P particle. Mice developed significantly increased immune responses to M2e after immunization with this chimeric vaccine and 100% survived from a lethal challenge with influenza virus (PR8, H1N1). Furthermore, antibodies induced by the chimeric vaccine blocked norovirus Virus-like Particle (VLP) and P particle binding to Histo-Blood Group Antigens (HBGAs), the receptor of human noroviruses [25, 26], suggesting an opportunity to develop a dual vaccine against both influenza and noroviruses.
2. Materials and Methods
2.1 Recombinant VA387 P particle-M2e construct
The previously made P particle expression vector with a cloning cassette (Spe I and Cla I/EcoR V) [15] was used as the starting construct. This construct is composed of a vector pGEX-4T-1(GST Gene fusion System, GE Healthcare Life Sciences) containing norovirus VA387 [genogroup II, cluster 4 (GII.4)] P domain-encoding sequence and a cystein-containing peptide. M2e peptide (SLLTEVETPIRNEWGCRCNDSSD) of human influenza virus [4] was inserted into the cloning cassette through a primer pair with Spe I and Cla I sites, CTAGTAGTCTTCTAACCGAGGTCGAAACGCCTATCAGAAACGAATGGGGGTGCAGA TGCAACGATTCAAGTGATAT/CGATATCACTTGAATCGTTGCATCTGCACCCCCATTC GTTTCTGATAGGCGTTTCGACCTCGGTTAGAAGACTA. Briefly, the primer pair was denatured at 95°C for 10 minutes, annealed at room temperature for 10 minutes, and then ligated into Spe I/Cla I digested P particle vector. Positive colonies were sequenced to confirm the M2e insertion in the loop-2 of VA387 P protein.
2.2 Expression and purification of recombinant P particle-M2e chimeric proteins
Recombinant P particle-M2e protein was expressed in E. coli (BL21, DE3) with an induction of 0.25 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at room temperature (~23 °C) overnight as described elsewhere [24, 27–29]. Purification of the glutathione S-transferase (GST)-P domain-M2e fusion protein was performed using resin of Glutathione Sepharose 4 Fast Flow (GE Healthcare Life Sciences) according to the manufacturer’s instruction. GST was removed from the target proteins by thrombin (GE Healthcare Life Sciences) cleavage either on bead or in solution (phosphate buffer saline, PBS, pH7.4).
2.3 Gel filtration chromatography
Gel filtration chromatography was carried out through an AKTA FPLC System (GE Healthcare Life Sciences) as described previously [23, 30]. Briefly, the affinity column-purified proteins were loaded on a size exclusion column Superdex 200 (GE Healthcare Life Sciences) powered by an AKTA FPLC system (model 920, GE Healthcare Life Sciences). The molecular weights of the eluted fractions were calibrated by Gel Filtration Calibration Kits (GE Healthcare Life Sciences).
2.4 Vaccination and challenge
Specific pathogen-free female BALB/c mice were purchased from Harlan-Sprague-Dawley (Indianapolis, IN) and immunized at six weeks of age. The animals were housed in a temperature-controlled environment with 12 h light/dark cycles, and received food and water under the control of Veterinary Services of CCHMC (Cincinnati Children Hospital Medical Center). Fifty micrograms of purified chimeric P particle-M2e protein were administrated to the mice (n=8) intranasally without adjuvant or subcutaneously with Montanide ISA720 adjuvant (Seppic Inc, Fairfield, NJ). Mice received three doses separated by a two-week interval. For comparison of immune responses, synthesized free M2e peptide (22.5 micrograms per dose, 5 times higher than chimeric protein in molar amount), wild type VA387 P particle (same dose as chimeric protein) and PBS were immunized to mice (n=8, each group) intranasally as controls. Sera were collected from each mouse prior to the first immunization and two weeks after the third immunization. To evaluate the protective efficacy of the chimeric vaccine, mice were challenged with mouse adapted human influenza virus PR8 (H1N1) strain at a dose of 2 X 106 fluorescent focus forming units per ml (FFU/ml), a challenge dose that was approximately 100 LD50, three weeks after the third immunization. The challenge virus was administered intranasally in a volume of 50 μl, distributing the dose equally into both nostrils. The mice were anesthetized by using Isoflurane (Bulter Animal Health Supply, Dublin, OH, USA) during the virus administration. Survival rates and morbidity of mice following virus challenge were monitored daily for 14 days. Loss of 30% of body weight was used as the endpoint for euthanizing moribund mice.
2.5 Determination of antibody levels in sera
An enzyme immunoassay (EIA) was used to determine antibody titers of mouse antisera after immunization with the different antigens (see above). Synthesized free M2e peptides or purified wild type VA387 P particles were used as antigens for determination of the antibody against influenza M2e or P particle platform, respectively. The starting dilution of the sera was 1:100 and 2 fold serial dilutions were performed until the OD450 reached the end-point (OD450 of 0.2). Ninety-six well microtiter plates (Dynex Immulon; Dynatech, Franklin, MA) were coated with antigens (100 ng/well) at 4°C overnight. After blocking with 5% nonfat milk in PBS (pH 7.4), antisera at indicated dilutions were added to the coated plates. The bound antibody was detected by the secondary antibody-HRP conjugate as described elsewhere [25]. HRP-conjugated goat anti-mouse IgG, IgG1 and rabbit anti-mouse IgG2a, 2b were purchased from MP Biomedicals (Solon, OH, USA). Sera from animals immunized with wild type P particle or PBS were used as controls. Antigen-specific antibody titers were defined as the endpoint dilution with a cut off signal intensity of 0.2.
2.6 HBGA binding and blocking assays
The saliva-based binding and blocking assays were carried out as described elsewhere [25, 26, 31]. Briefly, boiled human saliva samples with known HBGA phenotypes collected from Cincinnati, Ohio, USA were diluted 1000-fold and used to coat 96-well microtiter plates (Dynex Immulon; Dynatech, Franklin, MA). After blocking with 5% nonfat milk in PBS, Yeast expressed VLPs or E.coli expressed P particles of norovirus (VA387, GII.4) were added. The bound VLPs/P particles were detected using a guinea pig anti-VA387 VLP antiserum (1:3000), followed by the addition of HRP-conjugated goat anti-guinea pig IgG. The blocking effects of the mouse sera collected after immunization with the P particle-M2e chimera on the norovirus VLP/P particle-saliva binding were measured by a pre-incubation of VLP/P particle with diluted sera for 1 hour at 37°C before the VLP/P particle was added to the coated saliva. Equally pooled mouse sera from different groups were used for the blocking assay. The blocking rates were calculated by comparing the optical densities (ODs) measured with and without blocking by the mouse sera of immunized animals. The ODs of unblocked binding of P particles to saliva were 0.3 (type H), 1.1(type A) and 1.2 (type B), respectively. The ODs of unblocked binding of VLPs to saliva were 0.5 (type H), 0.8(type A) and 0.9 (type B), respectively. The sera from PBS immunized animals were used as negative controls.
2.7 Graphics and Statistical analysis
Graphics were made using Microsoft Office Excel 2010 and P values were determined by ANOVA or Chi-square test among data groups by GraphPad Prism 5 for windows (GraphPad Software, San Diego, CA).
2.8 Ethics Statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Cincinnati Children’s Hospital Research Foundation (Animal Welfare Assurance no. A3108-01).
3. Results
3.1 The chimeric P domain-M2e protein forms P particle
The coding sequence of the human influenza A M2e consensus peptide was inserted into Loop-2 of the P domain of a human norovirus strain (VA387, GII.4), which was extensively studied for antigen presentation in our previous studies [15, 23, 24](Fig. 1-A). The chimeric P domain-M2e protein was produced as a GST-fusion protein (~64 KDa, Fig. 1-B) in E. coli with a high yield (> 10mg/liter of culture). Following a thrombin digestion of the GST-fusion protein, the P domain-M2e protein (~ 38 kDa) was released from GST tag and was easily purified by the GST affinity column (Fig 1-B). To confirm the P particle formation, a gel filtration chromatography of the chimeric P domain-M2e protein was performed (Fig. 2-A) and the peak fractions were analyzed by SDS-PAGE (Fig. 2-B). The results showed that the vast majority (>95%) of the chimeric proteins formed a defined peak at ~910 KDa, indicating that the chimeric protein formed P particles like those obtained in our previous studies in presentation of variable small peptide antigens [15]. An estimated 2 mg of purified P particle-M2e protein was obtained from one liter of E. coli culture.
Figure 1.
Production and analysis of the P domain-M2e chimera. (A) Expression construct of the P domain-M2e chimera. A cloning cassette (Spe I/Cla I/EcoR V) was constructed in loop-2 of the VA-387 P domain. The M2e peptide was inserted in loops 2 between Spe I and Cla I sites. pGEX-4T-1 is an expression vector of the GST-Gene Fusion System. The circled C represents a cystein containing peptide (CDCRGDCFC) at the C terminus of the P domain to stabilize P particle formation. (B) Production and purification of the P domain-M2e chimera. SDS PAGE analysis revealed that GST-P domain-M2e fusion protein (GST fusion) is ~64 kDa. Digestion of the fusion protein in solution by thrombin resulted in GST (~26 kDa) and the P domain-M2e chimera (~38 kDa). The P domain-M2e chimera can also be released from the purification beads by a thrombin digestion. M was prestained protein marker (low range, Bio-Rad) with bands from top to bottom representing 112, 92, 51, 36, 29, 20 kDa, respectively.
Figure 2.
P particle formation of the P domain-M2e chimera characterized by a gel filtration chromatography. (A) The elution curve of a gel filtration chromatography of the thrombin-released P-domain-M2 protein using the size exclusion column Sperdex 200. Peaks representing void, P particle-M2e, P dimer-M2e and peak-3 were indicated, respectively. (B) The peak fractions of the gel filtration chromatography were analyzed by SDS PAGE, the fractions representing three peaks are indicated.
3.2 P particle-M2e chimera enhanced immune response to M2e in mice
The immune responses to the P particle-presented M2e were examined in mice (n = 8). Significantly higher titers of M2e-specific IgG, IgG1 and IgG2b were detected in the animals following immunization of the P particle-M2e vaccine either intranasally without an adjuvant or subcutaneously with the SA-720 adjuvant in comparison with mice immunized with the free M2e peptide (P <0.001), while M2e specific IgG2a was not detectable (Fig. 3-A). In fact, only marginal M2e-specific antibody was detected in the animals immunized with free M2e peptide, because free peptide is known as an inefficient immunogen. These data indicated a significant immune enhancement of M2e presented by the P particle. In addition, higher titers of IgG, IgG1, IgG2a and IgG2b against VA387 P particle were also detected in the mice immunized with either the wild type P particle or P particle-M2e chimera (Fig 3-B), confirming the high immunogenicity and antigenicity of the P particle [15, 24]. It was noted that IgG1 was the major component in the IgG subclasses, while a higher IgG2b, compared to IgG2a, was detected after immunization with both P particle and P particle-M2e chimera.
Figure 3.
Antibody response of mice to the P particle-M2e chimera and wild type P particle. Five groups of mice (n=8) were immunized with PBS, wild type P particle, free M2e peptide, and P particle-M2e chimera, either intranasally without adjuvant or subcutaneously with SA-720 adjuvant. Free M2e peptide (A) and wild type P particle (B) were used as antigen for antibody titer determination by EIA. Total IgG, IgG1 and IgG2b titer against M2e were determined using different HRP conjugated secondary antibodies. ***, P <0.001.
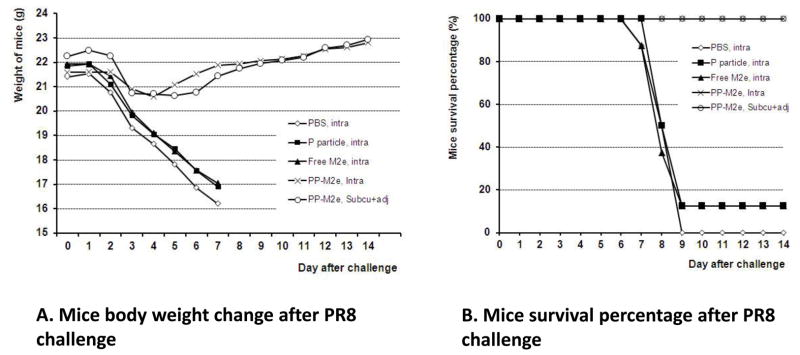
3.3 The P particle-M2e chimeric vaccine protected mice from a lethal challenge of influenza virus
We then studied the protective efficacy of the P particle-M2e chimeric vaccine in mice using a mouse adapted human H1N1 influenza (PR8) challenge model. After three times immunizations with the P particle-M2e vaccine and confirmation of the antibody response to M2e, the mice were challenged with a lethal dose of human H1N1 influenza virus (PR8). The mice vaccinated with the P particle-M2e chimera, either intranasally or subcutaneously, lost 5–10% of the body weight between days 3 and 6 after challenge, then recovered from the virus challenge and gained weight during the rest of the observation period. However, the mice immunized with PBS, free M2e peptide and wild type P particle started to lose weight on day 2 and lost 25–30% body weight by day 7 (Fig. 4-A). Notably, P particle-M2e vaccination provided full protection (100% survival) for the mice against the lethal dose challenge of influenza virus. By contrast, mice in the control groups vaccinated by free M2e peptide, the wild type P particle, or PBS showed only low survival rates (≤12.5%) (Fig. 4-B). The differences of body weight change on day 7 and survival rate between P particle-M2e immunized groups and control groups were statistically significant (P <0.01). Taken together, the P particle-M2e chimeric vaccine successfully protected mice from a lethal challenge of a H1N1 influenza virus.
Figure 4.
The P particle-M2e chimeric vaccine protected mice against a lethal challenge of mouse adapted human H1N1 influenza (PR8). After three time immunizations, mice (n=8) were challenged with PR8 virus intranasally at the dose of 2X106 FFU. PBS, wild type P particle and free M2e peptide were used as negative control. (A) Changes in body weight after virus challenge. (B) Mouse survival rate (%) after virus challenge. The body weight and survival rate were monitored daily for 14 days.
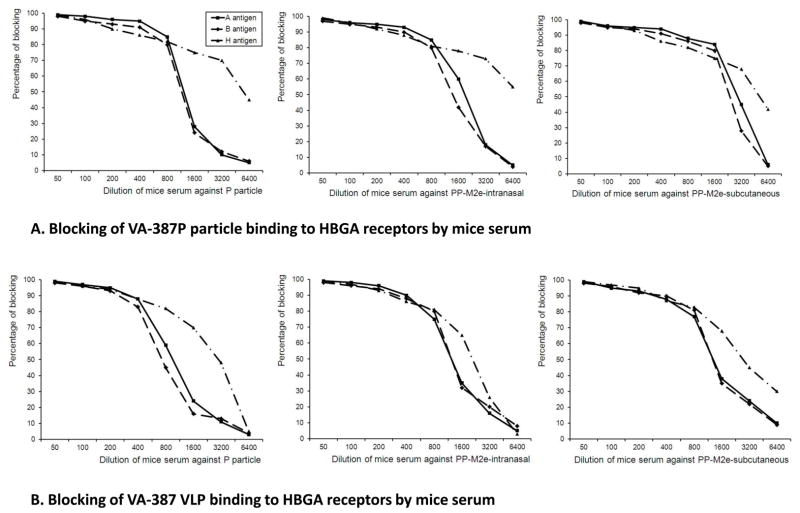
3.4 The P particle-M2e chimera induced antibody blocked norovirus binding to HBGA receptors
The immune response of mice to the P particle platform of the chimeric vaccine was also examined. As indicated in Fig 3-B, the P particle-M2e induced high antibody response against norovirus P particles. These antibodies were able to block norovirus VLP and P particle binding to HBGA receptors (Fig 5). Due to the lack of a cell culture and a small animal model for human noroviruses, the blocking activity of antibody on the binding of norovirus VLP or P particle to HBGAs is referred as an indirect indication of neutralization of noroviruses [15, 20, 31–34]. EIA-based blocking assays showed that the sera resulting from both intranasal and subcutaneous immunization of the P particle-M2e chimeric vaccine strongly blocked the VA387 P particle or VLP binding to type A, B and H antigens in the saliva (Fig. 5-A and B), similar to the sera from the animals given the wild type P particles. No detectable blockage was found from the sera from mice immunized by free M2e peptide. These results indicated that the P particle-M2e chimera could serve as a dual vaccine against both influenza and noroviruses.
Figure 5.
Blocking effects of mouse sera on norovirus (VA-387) P particle and VLP binding to HBGA receptors. Sera from the mice after immunization with P particle-M2e intranasally or subcutaneously were examined for blocking activity to P particle or VLP binding to HBGA receptors. Mouse sera after immunization of the wild type P particle served as positive controls and sera from PBS or free M2e immunized mice served as negative controls. (A) Blocking effects of mouse sera on norovirus P particle binding to HBGA receptors. (B) Blocking effects of mouse sera on norovirus VLP binding to HBGA receptors. X-axes, dilution of mouse sera (folds); Y axes, percentage of blocking (%).
4. Discussion
In our previous studies we have demonstrated the potential usefulness of the norovirus P particle as a vaccine platform in presentation of a number of small and large antigens, including the rotavirus surface protein VP8 with 159 amino acids. The resulting P particles -VP8 chimera induced strong immune responses and protected mice against a challenge of a mouse rotavirus [15]. The study described here provides further example of the usefulness of the P particle as a carrier for immune enhancement of small antigens. Insertion of the M2e epitope into one of the surface loops did not affect the P particle formation and the epitope was well presented as indicated by significantly increased immune responses of mice after immunization with the P particle-M2e vaccine compared to the mice immunized with the free M2e peptide. P particle-M2e vaccination completely protected the mice against a lethal challenge of a mouse adapted human influenza virus (PR8), further suggesting that the P particle is a useful platform for antigen presentation for vaccine development.
The norovirus P particle has been proposed as a subunit vaccine candidate against noroviruses owing to the fact that it preserves the surface antigens of noroviruses and that it is easily produced, very stable and highly immunogenic [23, 24]. The availability of the three surface loops of each P domain makes the P particle a unique vaccine platform for highly efficient antigen presentation. It has been reported that significantly higher immune response can be obtained by increasing the copy number of M2e per vaccine molecule through chemical conjugation, synthesis of tandem repeats, or multiple presentation on carriers [4, 12–14]. The norovirus P particle consists of 24 copies of the P domain and thus every P particle -M2e chimera contains 24 copies of M2e on its surface. It would be interesting to explore whether further improvement of the immune enhancement could be reached by taking advantage of all three available surface loops of the P particles. A study to evaluate such multiple presentations, including co-presentation of the M2e epitope with universal T cell helper epitopes, is ongoing in our laboratory.
In this study, we tested vaccination efficiency of the P particle-M2e vaccine through the intranasal route without adjuvant or subcutaneous route with an adjuvant. A high level of immune response and protection in mice against influenza virus infection was observed using either route. Compared to the subcutaneous route with adjuvant, intranasal immunization without adjuvant resulted in higher antibody titers against M2e. This result suggests that the P particle could serve as a good vaccine platform for mucosal immunization against infectious disease like influenza. The significantly higher immune responses induced by the P particle -M2e chimera than that induced by free M2e peptide suggests that the P particle may function as an adjuvant in the P particle-M2e vaccination possibly because of its large size (830 KDa). This property may allow a vaccine without an adjuvant which would be significant for mucosal immunization. This is particularly important for intranasal vaccination because of the safety concern of using an adjuvant with this route.
We also observed a high level of antibody response against the P particle backbone following immunization with the P particle-M2e vaccine and the resulting mouse sera strongly blocked norovirus VLP and P particle binding to HBGA receptors. This suggests a potential dual vaccine of the P particle-M2e chimera against both norovirus and influenza. The concept of utilizing the P particle platform to develop a dual vaccine against both noroviruses and rotaviruses has been recently introduced and are being tested in our laboratory [15]. The significance of P particle-M2e vaccine is that both influenza and noroviruses are highly active and can cause large epidemics in the fall-winter seasons. While it remains unknown whether large-scale seasonal vaccination is needed for prevention of norovirus outbreaks, seasonal immunization against influenza viruses using the P particle-M2e vaccine might impact positively on the norovirus epidemics. This concept may be further extended to develop broadly effective vaccines against multiple infectious diseases in addition to influenza, noroviruses and rotaviruses, using a cocktail of P particle carrying different antigens.
One concern with the P particle-M2e vaccine is the potential interference of pre-existing norovirus-specific antibody in the population. It is known that human noroviruses are widely spread and there is a high prevalence of antibody to noroviruses in the general population. This may decrease the immune responses to this type of vaccine. While a study to address this concern is needed, the results obtained from a similar study on the hepatitis B antigen presented M2e vaccine may provide useful information [5]. It was demonstrated that the pre-existing hepatitis B immunity did not affect the immunogenicity and protective efficacy of hepatitis B presented M2e vaccine in the mouse model.
In looking at the antibody responses, we examined different IgG subclasses in addition to total IgG to determine the major IgG class of antibody in the sera. The IgG1 titer was found to reach a similar level as the total IgG (Fig. 3A and B), which means a strong Th2 type response to both P particle and M2e has been elicited and IgG1 might play an important role in the protective immunity. Lower IgG2a and higher IgG2b against the P particle were also detected in the mice immunized with both wild type P particle and P particle-M2e chimera. M2e-specific IgG2a was not detectable, while IgG2b against M2e was detected at a low titer, indicating the Th1 immune response was relatively low. It is reported that the M2e-specific serum antibodies are crucial for protection against influenza infection, although the role of the T-cell mediated responses cannot be ruled out. Both murine anti-M2e IgG1 and IgG2a/2b isotypes protected mice from influenza A virus challenge [35]. In our study, the M2e-specific IgG2a and IgG2b could be enhanced by adding a universal T-cell epitope to generate a vaccine that can induce a balanced Th1/Th2 type immune response [36, 37].
In conclusion, we have described a vaccine that significantly improves the immunogenicity of M2e and provides a fully protective immunity in mice against a lethal influenza A virus challenge. The vaccine is generated by insertion of the human influenza M2e epitope into norovirus P particle through genetic engineering. The chimeric P particles are easily produced using the E.coli expression system. The M2e epitope is well presented on the surface of the particles, which may be similar to those on the influenza virus or on infected cells. The vaccine can be administered subcutaneously with an adjuvant or intranasally without adjuvant. The antibodies can block the norovirus binding to HBGA receptors. It is possible that the vaccine can be further improved by the inclusion of a universal T-helper or insertion of more copy numbers in the same or different loops of the P particle.
Acknowledgments
The research described in this article was supported by the National Institute of Health, the National Institute of Allergy and Infectious diseases (R01 AI37093, R01 AI055649 and R01 AI089634), and the Department of Defense (PR033018) to X.J. This study was also supported by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 1UL1RR026314-01 to M. T. In addition, the Infectious Disease Grant to M. T. sponsored by Nipert Foundation contributed to this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Grandea AG, 3rd, Olsen OA, Cox TC, Renshaw M, Hammond PW, Chan-Hui PY, et al. Human antibodies reveal a protective epitope that is highly conserved among human and nonhuman influenza A viruses. Proc Natl Acad Sci U S A. 2010;107:12658–63. doi: 10.1073/pnas.0911806107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R, Song A, Levin J, Dennis D, Zhang NJ, Yoshida H, et al. Therapeutic potential of a fully human monoclonal antibody against influenza A virus M2 protein. Antiviral Res. 2008;80:168–77. doi: 10.1016/j.antiviral.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–63. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 5.De Filette M, Martens W, Smet A, Schotsaert M, Birkett A, Londono-Arcila P, et al. Universal influenza A M2e-HBc vaccine protects against disease even in the presence of pre-existing anti-HBc antibodies. Vaccine. 2008;26:6503–7. doi: 10.1016/j.vaccine.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–33. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 7.Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–72. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto LH, Lamb RA. Controlling influenza virus replication by inhibiting its proton channel. Mol Biosyst. 2007;3:18–23. doi: 10.1039/b611613m. [DOI] [PubMed] [Google Scholar]
- 9.Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991;65:5491–8. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Zou P, Ding J, Lu Y, Chen YH. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 2005;7:171–7. doi: 10.1016/j.micinf.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Zhang M, Mozdzanowska K, Zharikova D, Hoff H, Wunner W, et al. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virol J. 2006;3:102. doi: 10.1186/1743-422X-3-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26:201–14. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 13.De Filette M, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283:11382–7. doi: 10.1074/jbc.M800650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao G, Lin Y, Du L, Guan J, Sun S, Sui H, et al. An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses. Virol J. 2010;7:9. doi: 10.1186/1743-422X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan M, Huang P, Xia M, Fang PA, Zhong W, McNeal M, et al. Norovirus P particle, a novel platform for vaccine development and antibody production. J Virol. 2011;85:753–64. doi: 10.1128/JVI.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan M, Jiang X. Norovirus-host interaction: implications for disease control and prevention. Expert Rev Mol Med. 2007;9:1–22. doi: 10.1017/S1462399407000348. [DOI] [PubMed] [Google Scholar]
- 17.Tan M, Farkas T, Jiang X. Molecular Pathogenesis of Human Norovirus. In: Yang DC, editor. RNA Virus: Host Gene Responses to Infection. 1. Singapore: World Scientific; 2009. pp. 575–600. [Google Scholar]
- 18.Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13:285–93. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Tan M, Jiang X. Norovirus gastroenteritis, increased understanding and future antiviral options. Curr Opin Investig Drugs. 2008;9:146–51. [PubMed] [Google Scholar]
- 20.Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6:e1000983. doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan M, Jiang X. Virus-Host Interaction and Celluar Receptors of Caliciviruses. In: Hansman G, Jiang X, Green K, editors. Caliciruses. Norwich: Caister Academic Press; 2010. pp. 111–30. [Google Scholar]
- 22.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–90. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 23.Tan M, Jiang X. The p domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J Virol. 2005;79:14017–30. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan M, Fang P, Chachiyo T, Xia M, Huang P, Fang Z, et al. Noroviral P particle: structure, function and applications in virus-host interaction. Virology. 2008;382:115–23. doi: 10.1016/j.virol.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang P, Farkas T, Marionneau S, Zhong W, Ruvoen-Clouet N, Morrow AL, et al. Noroviruses Bind to Human ABO, Lewis, and Secretor Histo-Blood Group Antigens: Identification of 4 Distinct Strain-Specific Patterns. J Infect Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 26.Huang P, Farkas T, Zhong W, Tan M, Thornton S, Morrow AL, et al. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol. 2005;79:6714–22. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan M, Meller J, Jiang X. C-terminal arginine cluster is essential for receptor binding of norovirus capsid protein. J Virol. 2006;80:7322–31. doi: 10.1128/JVI.00233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan M, Xia M, Cao S, Huang P, Farkas T, Meller J, et al. Elucidation of strain-specific interaction of a GII-4 norovirus with HBGA receptors by site-directed mutagenesis study. Virology. 2008;379:324–34. doi: 10.1016/j.virol.2008.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan M, Xia M, Chen Y, Bu W, Hegde RS, Meller J, et al. Conservation of carbohydrate binding interfaces: evidence of human HBGA selection in norovirus evolution. PLoS One. 2009;4:e5058. doi: 10.1371/journal.pone.0005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan M, Hegde RS, Jiang X. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J Virol. 2004;78:6233–42. doi: 10.1128/JVI.78.12.6233-6242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia M, Farkas T, Jiang X. Norovirus capsid protein expressed in yeast forms virus-like particles and stimulates systemic and mucosal immunity in mice following an oral administration of raw yeast extracts. J Med Virol. 2007;79:74–83. doi: 10.1002/jmv.20762. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Xia M, Tan M, Huang P, Zhong W, Pang XL, et al. Genetic and phenotypic characterization of GII-4 noroviruses that circulated during 1987 to 2008. J Virol. 2010;84:9595–607. doi: 10.1128/JVI.02614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LoBue AD, Lindesmith L, Yount B, Harrington PR, Thompson JM, Johnston RE, et al. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine. 2006;24:5220–34. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 34.Tan M, Jiang X. Norovirus-host interaction: multi-selections by the human HBGAs. Trends in Microbiology. 2011 doi: 10.1016/j.tim.2011.05.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. 2011;186:1022–31. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 36.Nardin EH, Calvo-Calle JM, Oliveira GA, Nussenzweig RS, Schneider M, Tiercy JM, et al. A totally synthetic polyoxime malaria vaccine containing Plasmodium falciparum B cell and universal T cell epitopes elicits immune responses in volunteers of diverse HLA types. J Immunol. 2001;166:481–9. doi: 10.4049/jimmunol.166.1.481. [DOI] [PubMed] [Google Scholar]
- 37.Krikorian D, Panou-Pomonis E, Voitharou C, Sakarellos C, Sakarellos-Daitsiotis M. A peptide carrier with a built-in vaccine adjuvant: construction of immunogenic conjugates. Bioconjug Chem. 2005;16:812–9. doi: 10.1021/bc049703m. [DOI] [PubMed] [Google Scholar]